World Journal of Cardiovascular Surgery
Vol.3 No.2(2013), Article ID:33389,7 pages DOI:10.4236/wjcs.2013.32012
Late Anatomic Findings after “Rescue CABG” for Peri-Operative Ischemia Following Aortic Root Replacement
1Section of Cardiac Surgery, Yale University School of Medicine, New Haven, USA
2Department of Surgery, St. Louis University School of Medicine, St. Louis, USA
3Department of Diagnostic Imaging, Yale University School of Medicine, New Haven, USA
Email: john.elefteriades@yale.edu
Copyright © 2013 Aarthi Ramarathnam et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 19, 2013; revised April 19, 2013; accepted April 28, 2013
Keywords: Aneurysm; Aortic Root; Ischemia
ABSTRACT
Background: Acute myocardial ischemia, seen in about 2% of aortic root replacements (ARR), is acutely life-threatening, manifesting as failure to wean from bypass, ventricular fibrillation, or unstable hemodynamics. The exact precipitating anatomic cause is usually not apparent at the time of surgery. In this report, we take advantage of late computed tomographic (CT) angiograms of long-term survivors of peri-operative ischemia after ARR to determine what abnormalities of the coronary button reattachments produced the peri-operative ischemia. Methods: The database of the Aortic Institute at Yale-New Haven was reviewed to identify all patients undergoing ARR over a 15-year period. Operative records, patient charts, and CT angiograms of patients who had peri-operative ischemia were reviewed in detail, including analysis by an imaging specialist. Results: 271 patients underwent ARR, 220 with mechanical and 51 with biological valved conduits. Hospital mortality was 2.95%. Clinical follow-up ranged from 1 to 182 months. Survival in discharged patients was 97.7% at 5 years and 95.2% at 7 years. Peri-operative ischemia was seen in 4 of 271 patients (1.5%). All four affected patients survived—with interventions including supplemental coronary bypass grafts (4 patients), intra-aortic balloon pump placement (2 patients), and left ventricular assist device insertion (1 patient). Late CT angiograms revealed severe but non-obstructive left main calcification serving as a focal point for coronary angulation in 2 patients, angulation without calcification in 1 patient, and totally normal anatomy in 1 patient. Conclusions: Myocardial ischemia after ARR is rare but acutely life-threatening. Prompt recognition and treatment by supplemental coronary artery bypass grafting preserves life and leads to good late survival. Intramural calcification (non-obstructive) of the distal left main coronary artery predisposes to angulation after coronary button creation and should be a “red flag” for this potential problem.
1. Introduction
Aortic root replacement (ARR) is recognized as the historic standard therapy for patients with diseases of the aortic root, particularly those with aortic root aneurysms and aortic dissections [1,2]. As a result of years of refinement, the ARR procedure now can be performed with low peri-operative risk, excellent long-term survival and freedom from complications.
However, aortic root replacement procedures still carry some peri-operative risk, with experienced centers noting contemporary operation-related mortality of 2% to 6% [3-9]. In a previous paper, we highlighted the potential problems related to attachment of the coronary artery buttons that can produce acute ischemia in the operating room or shortly thereafter, thus jeopardizing immediate survival [8]. This occurred in 2.2% of our patients, and had been reported with similar frequency in other series as well [9-11]. Misalignment of the coronary buttons, including issues with length, lie, and torsional orientation, can precipitate acute ischemia in this setting leading to potentially lethal complications, including myocardial ischemia, ventricular arrhythmias, myocardial infarction and pump failure. These specific potential technical issues are illustrated by line drawings in our prior paper [8].
Although different treatments have been advised to remedy such complications, we highlighted the effectiveness of rescue coronary artery bypass grafting (CABG) to treat myocardial ischemia during and immediately after ARR [8]. “Rescue CABG” is defined as intraoperative or immediate postoperative unplanned revascularization (via CABG) of the right or left coronary systems.
In our earlier paper, 3 patients out of 139 ARR procedures experienced peri-operative ischemia, presumably due to problems with coronary button alignment, and thus underwent rescue CABG operations [8]. In the short term, we found that rescue CABG operations on patients suspected of myochardial ischemia were (uniformly) life saving. We found supplemental CABG simple and effective, and perhaps more efficacious in this setting than direct revision of the buttons themselves.
In the present paper, we look from the same perspective at a larger group of ARR patients. Also, the time that has passed for the earlier patients who underwent rescue CABG affords the opportunity to examine their intercurrent imaging procedures to make assessments of the actual coronary button anatomy—whereas before we only speculated peri-operatively about the potential precipitating anatomic factors. Because these patients have all survived, they have now undergone late 64-slice CT angiography (for aortic follow-up). From these scans, we have valuable information at hand to determine if there is, in fact, any issue with the coronary button alignment in these patients and what role the supplemental coronary grafts seem to have played. From such analysis, we hope to glean preventive and therapeutic lessons regarding the rare but life-threatening phenomenon of early coronary ischemia after ARR.
2. Patients and Methods
Between January 1995 and March 2010, 271 consecutive patients underwent ARR at Yale-New Haven Hospital. Of those patients, 248 underwent the surgical procedure for aortic root aneurysms and 23 for aortic dissection. Four patients of the aortic root aneurysm cohort received concomitant planned coronary artery bypass grafting (CABG) procedures for coronary artery disease; these are not included in the rescue CABG group.
Mechanical valved conduits were utilized in 220 patients (81.5%) and biological in 51 patients (18.4%). The biological valved conduits were constructed on the table by sewing a porcine or bovine pericardial valve (Edwards LifeSciences) within a Dacron graft (Hemashield) Hospital mortality was 2.95%.
During the longer study interval of the present report, one additional patient (beyond the three from the original series) required rescue CABG.
Clinical follow-up ranged from 1 to 182 mos. Survival in discharged patients was 97.7% at 5 years and 95.2% at 7 years.
One additional patient required rescue CABG since our original report, making for a current 4/271 (1.5%) requirement for rescue CABG. The four patients (1.5%) who received rescue CABG operations during the entire experience were followed up via chart reviews and regular office visits. Follow-up on these patients ranged from 1 to 182 months. High-quality 64-slice CT scans were reviewed in detail by a cardiac imaging specialist (DC). Morphology and patency of coronary buttons and the supplementary “rescue” grafts were assessed on the CT scan representing the longest follow-up for each patient.
3. Results
Four patients of 271 ARR (1.5%) showed evidence of intra-operative or immediate post-operative ischemia— three via difficulty to wean from cardiopulmonary bypass (CPB) and one via ventricular fibrillation on arrival in ICU. One patient had required endarterectomy of the coronary button wall, extending into the left main. All 4 patients underwent supplemental CABG (vein or IMA) and one patient required a temporary LVAD. All 4 patients did well and survived long-term. All four patients remain alive and well, 19, 61, 72, and 154 months after their original ARR.
Late CT angiographic follow-up on these 4 patients revealed that 2 patients had normal button anatomy, and 2 showed angulation. In one patient, in particular, angiography indicated heavy calcification at the distal left main coronary artery, which served as a fulcrum for angulation. All supplemental vein and IMA grafts were patent. The patient with angulation of the calcified left main underwent successful late coronary stenting three years after the original ARR. Case-specific details and images are provided below (see Figures 1-4 and Table 1).
Case #1. In the first case, a 56-year-old male underwent ARR with a 23-mm St. Jude valved conduit to treat a 6.0-cm aortic root aneurysm. The aortic root was extremely calcified, including the region around the right and left coronary artery buttons. The calcification was so severe that there was no possibility of sewing through the button tissue. Reimplantation of the coronary arteries required extensive formal endarterectomy of the perimeter of the coronary buttons, with endarectomy extending into the body of the left main coronary artery itself. At the conclusion of the procedure, upon closing the chest, the patient showed signs of severe hemodynamic deterioration and ventricular arrhythmias. Cardiopulmonary bypass was immediately resumed and the left anterior descending artery (LAD) was grafted with a reversed saphenous vein graft. An intra-aortic balloon pump was

Figure 1. (a) Late post-op CT scan demonstrating calcium in left main coronary artery, beyond surgical endarterectomy site, 72 months following aortic root replacement; (b) Late post-operative CT scan demonstrating widely patent saphenous vein graft, 72 months following aortic root replacement; (c) Pre-operative coronary angiogram showing widely patent left main coronary artery, not demonstrating extensive calcification seen in operating room and on subsequent CT scan; calcium, although extensive, did not affect lumen at this time.
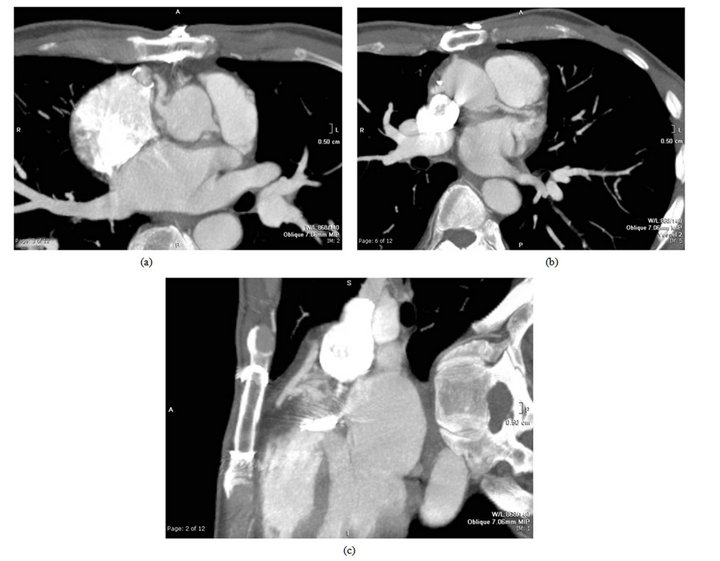
Figure 2. (a) Late post-operative CT scan showing angulation in right coronary artery just beyond coronary button; (b) Left main coronary (small, as noted in operating room) lies without angulation; (c) The saphenous vein graft to the RCA is widely patent (as is the saphenous vein graft to the LAD, not shown).
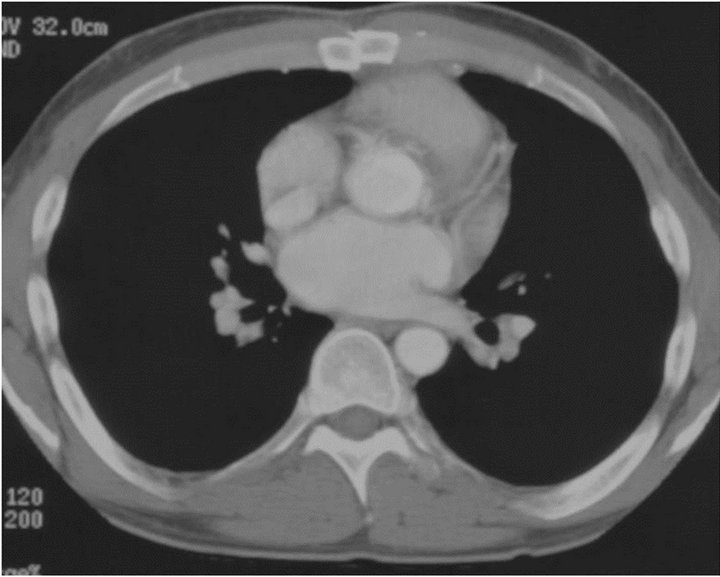
Figure 3. Late CT angiogram showing patent right and left coronary artery buttons, without discrete irregularity. LIMA is patent. (This is a scan from the 1990s and does not achieve the technical quality of today’s images).
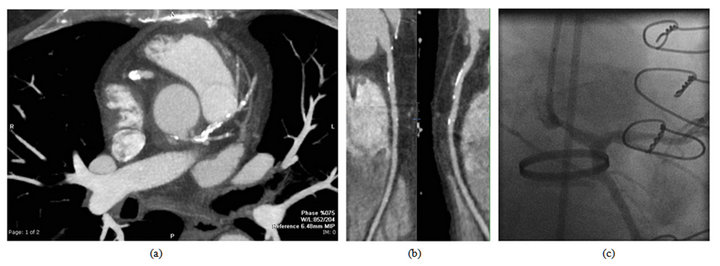
Figure 4. (a) Late CT angiogram after aortic root replacement. Note extreme calcification of left main and LAD. Note stenosis just proximal to severe left main calcification; (b) “Splay-out” view of right coronary button and right coronary artery button, showing a smooth course, without narrowing or angulation, but scattered non-obstructive coronary calcification; (c) Late post-operative coronary angiography, showing narrowing of left main coronary artery artery beyond button, at site of severe calcification seen on CT scan (Figure 4(a)).
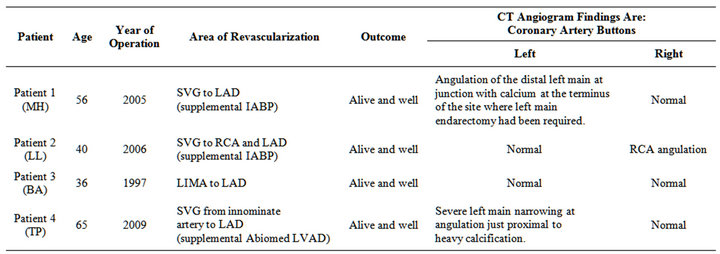
Table 1. Summary of patient histories and CT angiogram findings for those individuals needing rescue CABG.
placed for cardiac support. The patient could then successfully be separated from cardiopulmonary bypass. Transesophageal echocardiogram had revealed anterolateral hypokinesis which improved with reperfusion via the vein graft. The intra-aortic balloon pump was removed, and the patient did well post-operatively. He continues well now at 72 months post-procedure.
The radiographic images (see Figure 1) indicate that there remained extensive calcium at the limit of the coronary button endarectomy; we postulate that there was early angulation after button attachment, with the calcium serving as a fulcrum. It is of interest that the preoperative coronary arteriogram showed no luminal compromise despite the extensive intra-mural calcification that was later documented. The saphenous vein graft remains widely patent.
Case #2. In the second case, a 40-year-old male underwent ARR to treat a 7.0-cm aortic root aneurysm. A 25-mm St. Jude valved conduit was placed. The coronary arteries were markedly displaced and the left main ostium was particularly small. The operation was performed successfully and the patient was weaned from CPB without difficulty. Shortly after the arrival in the intensive care unit, the patient’s rhythm lapsed into ventricular fibrillation. He was transferred to the operating room and placed on CPB. Suspecting coronary insufficiency, we performed rescue CABG to the LAD and the proximal right coronary artery using saphenous vein grafts. Following vein graft reperfusion and placement of an ntraaortic balloon pump, the patient was weaned from bypass without any difficulty. He remains alive and well 61 months post-procedure.
The patient’s recent CT angiogram (see Figure 2) has shown a mild angulation in the proximal native RCA beyond the button, which likely explains the early ischemia. The left button is fine. The vein grafts to the LAD and RCA are widely patent.
Case #3. The third patient was a 36-year-old male being operated on for a symptomatic Marfanoid 4.2 cm aortic root aneurysm. He underwent composite graft replacement with a 27-mm St. Jude valved conduit. He was able to be weaned very briefly from CPB, but hemodynamics deteriorated and he was placed back on the heartlung machine. He had demonstrated anterior hypokinesis, and accordingly, a left internal mammary graft was placed on the LAD, and the patient was weaned easily and effectively from CPB. He remains alive and well 154 months post-procedure.
Post-operative CT angiography (see Figure 3) demonstrated patent coronary buttons without discernible irregularities, as well as a patent left internal mammary graft to the LAD.
Case #4. This 65-year-old severely obsese male (117 kg, BSA 34) presented urgently with shortness of breath.
He was found to have a 6.3 cm ascending aortic aneurysm with aortic regurgitation. Cardiac catheterization showed a 50% right coronary artery lesion, but no other significant narrowings. Pulmonary artery pressures were high (45/25). The heart was hypertrophied and fibrotic, and mobilization of the coronary arteries was difficult. There was extensive fungating calcium at the mouth of the left main coronary artery that was debrided extensively as part of the mobliization of the coronary artery button. The left coronary artery button was anastomosed in standard fashion to the main graft. For anatomic reasons, the right button was reattached to the main graft via a short Dacron graft. The patient was weaned without difficulty from cardiopulmonary artery bypass. During chest closure, a very focal area of hypocontractility was noted on echocardiography in the mid anterior wall; wall motion elsewhere was normal. Bypass was resumed and a saphenous vein graft placed to the LAD (with the proximal taken from the innominate artery, rather than from the main Dacron graft). A similar focal wall motion abnormality was seen once again upon chest closure. An Abiomed left ventricular assist device was required to wean from cardiopulmonary bypass. The patient did well post-operatively, and the assist device was weaned and removed before hospital discharge. The vein graft was functioning well at the time of Abiomed explant and later chest closure. He has continued well in the long-term (29 months). Late CT scanning (see Figure 4) showed a narrowing at the site of severe calcification in the left main coronary artery. The severe intra-mural left main coronary calcification had not shown on the pre-operative coronary angiography, but was clearly the cause of the peri-operative hemodynamic instability at the time of ARR. The patient underwent a supplementary left main coronary artery stent procedure one and one-half years post-operatively, when he had the onset of ischemic symptoms and the saphenous vein graft from the innominate artery to the LAD was found to be closed.
4. Discussion
Aortic root replacement has evolved and matured since the introduction in 1968 by Bentall & DeBono [1] and Ross & colleagues [12] and the popularization by Cabrol [13]. The advent of direct coronary button reimplantation has enhanced safety and durability of the procedure [2,3]. The overall mortality associated with ARR is now in the range of 2% - 6% in recent studies [3-9]. In series presented in this clinical report, this procedure was very safe, with an overall mortality of only 2.95%. However, the design and construction of coronary artery buttons, despite delicacy and precision in technique, are not without potential pitfalls. We have described and illustrated the technical concerns and tips in our earlier report [8]. Tension, buckling, and torsion can impair coronary button flow. Problems with the buttons may manifest as failure to wean from cardiopulmonary bypass, as regional wall motion abnormalities on the intra-operative echocardiogram, or as ventricular fibrillation. In our earlier report, we indicated the utility of “Rescue CABG” operations immediately after aortic root replacement in patients suspected of myocardial infarction; our experience demonstrated that rescue CABG can be a life-saving procedure.
Coronary button related myocardial ischemia is rare after composite graft replacement of the aortic root. In this series, we encountered this problem in 4/271 (1.5%) of patients. In this report, we look at this problem and these particular patients “with a microscope” to see what lessons can be gleaned—particularly with the aid of late post-operative CT angiography.
It is important to be cognizant of this rare problem— early ischemia post-ARR—so as to recognize it, and to act promptly to avoid prolonged ischemia and subsequent myocardial damage and death. Prompt recognition and targeted grafting can be life-saving, as demonstrated in this series. Echocardiography can guide localization of ischemic areas and targeted grafting. With prompt recognition and rescue coronary grafting, long-term prognosis seems to be excellent in these patients.
From detailed review of these rare cases requiring rescue coronary artery bypass grafting, supplemented by late CT coronary arteriography, we glean the following anatomic lessons:
• Calcium in and around the aortic root and the proximal coronaries is a potential risk factor for coronary artery button problems and should raise a red flag even from the pre-operative evaluation. Calcified buttons and calcified right or left main coronary arteries, even if widely patent angiographically, pose a specific risk when the buttons are reapproximated to the new root graft (especially by predisposing to angulation, with the calcium serving as a fulcrum). As seen in these cases, there can be extremely severe intramural calcification of the proximal coronary arteries without significant luminal narrowing on conventional coronary angiography.
• Use of the intra-aortic balloon pump appears to be of benefit in restoring stability—as a supplement to directed coronary grafting to areas of apparent ischemia.
• Vein grafts appear to remain patent when constructed in this setting—not only when there is a persistent anatomic irregularity but also when the button is normal in late follow-up.
• In some instances, no irregularity of the lie or other physical characteristics of the buttons is discernible on late imaging; we presume that in such cases the cause of peri-operative deterioration was not anatomically-based ischemia, or that an angulation ameliorated with time. (Of course, air embolism and coronary spasm can masquerade as anatomically-based ischemia, and protamine reaction and poor myocardial protection can also produce hemodynamic instability.)
We present these “dirty laundry” cases for various reasons: (1) To encourage recognition of coronary button related ischemia in the rare instances when it does occur. Delay or failure in recognition can be lethal. (2) To draw attention to the success of rescue CABG operations in patients with suspected ischemia, post-ARR operations. (3) To utilize long-term post-operative radiographic information from CT angiograms to enhance our understanding of the anatomy and pathophysiology of coronary button ischemia. (4) To emphasize the long-term efficacy of the rescue CABG operation. In addition to a high level of precision and care in coronary button reattachment, surgeons must be aware of the potential for coronary insufficiency and look to correct such events immediately. In our experience, the rescue CABG operation was immensely beneficial in both the short and long term treatment of patients.
We advise surgeons to be aware of a precarious preexisting anatomic circumstance: Calcification of the left main just before its bifurcation. The presence of calcification near the bifurcation of the left main coronary artery beyond the button sets the stage for potential angulation where the soft proximal artery meets the calcified portion just beyond.
In conclusion, rescue CABG is a life-saving operation that can help correct potentially life-threatening myocardial ischemia early after ARR. The radiologic images provided in this report help to understand the genesis of such ischemic events and lead directly to the observations and suggestions enumerated above. In the case that coronary ischemia is suspected, immediate rescue CABG is likely to be life-saving, with excellent long-term survival being salvaged from an acutely life-threatening— and often anatomically based—situation.
REFERENCES
- H. Bentall and A. De Bono, “A Technique for Complete Replacement of the Ascending Aorta,” Thorax, Vol. 23, No. 4, 1968, pp. 338-339. doi:10.1136/thx.23.4.338
- B. P. van Putte, S. Ozturk, S. Siddiqi, M. A. Schepens, R. H. Heijman and W. J. Morshuis, “Early and Late Outcome after Aortic Root Replacement with a Mechanical Valve Prosthesis in a Series of 528 Patients,” The Annals of Thoracic Surgery, 2011, Epub.
- I. E. Platis, G. S. Kopf, M. L. Dewar, R. K. Shaw and J. A. Elefteriades, “Composite Graft with Coronary Button Reimplantation: Procedure of Choice for Aortic Root Replacement,” International Journal of Angiology, Vol. 7, No. 1, 1998, pp. 41-45. doi:10.1007/BF01616275
- B. Lima, C. G. Hughes, A. Lemaire, et al., “Short-Term and Intermediate-Term Outcomes of Aortic Root Replacement with St. Jude Mechanical Conduits and Aortic Allografts,” The Annals of Thoracic Surgery, Vol. 82, No. 2, 2006, pp. 579-585. doi:10.1016/j.athoracsur.2006.03.068
- S. Westaby, T. Katsumata and G. Vaccari, “Aortic Root Replacement with Coronary Button Re-Implantation: Low Risk and Predictable Outcome,” European Journal Cardio-Thoracic Surgery, Vol. 17, No. 3, 2000, pp. 259- 265. doi:10.1016/S1010-7940(00)00347-X
- I. Hatzaras, G. J. Koullias, M. Tranquilli, H. Achneck and J. A. Elefteriades, “Mid-Term Thromboembolic and Bleeding Complications Are Minimal after Composite Graft Replacement of the Aortic Root,” International Journal of Angiology, Vol. 14, 2005, pp. 118-112. doi:10.1007/s00547-005-2050-x
- H. E. Achneck, J. A. Rizzo, M. Tranquilli and J. A. Elefteriades, “Safety of Thoracic Aortic Surgery in the Present Era,” The Annals of Thoracic Surgery, Vol. 84, 2007, pp. 1180-1185. doi:10.1016/j.athoracsur.2007.05.038
- A. Shahriari, M. Eng, M. Tranquili and J. A. Elefteriades, “Rescue Coronary Artery Bypass Grafting (CABG) after Aortic Composite Graft Replacement,” Journal of Cardiac Surgery, Vol. 24, No. 4, 2009, pp. 392-396. doi:10.1111/j.1540-8191.2008.00762.x
- L. Svensson, “Management and Complications of Aortic Dissection,” American Heart Association Scientific Sessions, New Orleans, 2004.
- G. J. Byrne, A. N. Karavas, M. Leacche, et al., “Impact of Concomitant Coronary Artery Bypass Grafting on Hospital Survival after Aortic Root Replacement,” The Annals of Thoracic Surgery, Vol. 79, No. 2, 2005, pp. 511-516. doi:10.1016/j.athoracsur.2004.07.063
- E. H. Kincaid, A. R. Cordell, J. W. Hammon, et al., “Coronary Insufficiency after Stentless Aortic Root Replacement: Risk Factors and Solutions,” The Annals of Thoracic Surgery, Vol. 83, No. 3, 2007, pp. 964-968. doi:10.1016/j.athoracsur.2006.09.021
- R. M. Donaldson and D. N. Ross, “Replacement of the Ascending Aorta and Aortic Valve with a Composite Graft: Results and Follow-Up in 71 Patients,” The Journal of Thoracic and Cardiovascular Surgery, Vol. 29, No. 4, 1981, pp. 195-199. doi:10.1055/s-2007-1023476
- C. Cabrol, A. Pavie, I. Gandjbakhch, et al., “Complete Replacement of the Ascending Aorta with Reimplantation of the Coronary Arteries,” The Journal of Thoracic and Cardiovascular Surgery, Vol. 79, 1980, pp. 388-401.

