Open Journal of Stomatology
Vol.3 No.2(2013), Article ID:32053,7 pages DOI:10.4236/ojst.2013.32031
Antifungal activity of chemotype essential oils from rosemary against Candida albicans
![]()
1Division of Oral Care and Rehabilitation, Department of Control of Physical Functions, Science of Physical Functions, Kyushu Dental University, Kitakyushu, Japan
2Department of Oral Health Management, School of Oral Health Sciences, Kyushu Dental University, Kitakyushu, Japan
3Division of Infections and Molecular Biology, Department of Health Promotion, Science of Health Improvement, Kyushu Dental University, Kitakyushu, Japan
Email: *matsu.44.yum.44@gmail.com
Copyright © 2013 Yusuke Matsuzaki et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 13 March 2013; revised 26 April 2013; accepted 8 May 2013
Keywords: Candida albicans; Essential Oil; Rosemary; Rosmarinus officinalis; Chemotype; Antifungal Activity
ABSTRACT
Oral candidiasis is caused by Candida albicans. The symptoms including pain in the oral cavity affect our eating function. While some antifungal agents are commonly used for the treatment of candidiasis, occasional consumption of the effective dose cannot be tolerated due to side effects. There is also a possibility of induction of antifungal resistance, thus the development of replacement agents has been awaited. We aim to explore antimicrobial activities of essential oils. We evaluated the antifungal activities against C. albicans of essential oils from seven aromatic plants from three manufacturers, and of three chemotype essential oils from rosemary (Rosmarinus officinalis). As a result, we found that the antifungal activity was increased several times by the addition of Tween 80. All the tested essential oils showed stable antifungal activity, however, the variation was observed among the manufacturers of rosemary and eucalyptus. Rosemary has three chemotypes; CINEOL, CAMPHOR and VERBENON. They derived from a same plant species, but contain different chemical components. The CINEOL, dose-dependently decreased the number of C. albicans in the time-kill assay. Hence we concluded that the components of rosemary essential oil would have an effect on its antifungal activity. A chemotype is the first to consider in measuring antifungal activities of rosemary oil.
1. INTRODUCTION
Candida albicans is present on the skin and mucosal surfaces as a human microbial flora [1]. C. albicans is associated with immune dysfunction in HIV infection and other diseases such as hypothyroidism, diabetes, acute leukemia, mucosal irritation, oral cancer, xerostomia, or the use of broad-spectrum antibiotics or steroids, and the use of dentures with poor hygiene and trauma, in young and elderly patients [2-4]. C. albicans causes opportunistic infections such as oral candidiasis, oesophageal candidiasis and vaginal candidiasis. It is known that oral and oesophageal candidiasis cause oral cavity pain, tongue pain, taste disturbance, odynophagia or dysphagia etc. [4-6]. Since symptoms of oral and oesophageal candidiasis appear in oral regions, eating function is greatly deteriorated. The reduction of food intake leads to undernutrition and impaired quality of life, and sometimes causes life-threatening risks.
Oral candidiasis is normally treated with antifungal agents such as nystatin, miconazole, fluconazole, itraconazole, imidazole, triazole and amphotericin B. However, occasional consumption of the effective dose cannot be tolerated due to side effects [7,8]. In addition, these agents may induce antifungal resistance in C. albicans [9-12]. As oral candidiasis is intractable and recurrent, development of substitutes for the antimicrobials is expected. It is known that some parts and extracts of medical and aromatic plants have antimicrobial activities [13]. Essential oils are natural materials extracted from flowers, leaves, peels, barks, roots, seeds, resins of aromatic plants, and are volatile and hydrophobic aromatic substances containing effective compounds in high density [14]. Since each essential oil has peculiar fragrance and effects, the antimicrobial activity has been examined as the possible substitute for medicine [15,16] and food preservatives [17,18]. Though there have been many reports on the antimicrobial activities of essential oils, the results have been diverse and the evaluation methods have been inconsistent. It is known that components of essential oils from aromatic plants of the same scientific name could be different according to the plants’ habitats, or parts and methods for extraction. That variation is called “chemotype” [19]. Chemotype occurs when plants such as rosemary (Rosmarinus officinalis), thyme (Thymus vulgaris) and basil (Ocimum basilicum) etc. grow under different condition, including soils and climates [19,20]. In the previous study, Janssen et al. have described that the methods in the antimicrobial activity studies of essential oils have been diverse and often insufficient [21]. However, the vagueness in the antimicrobial properties of essential oils is still remaining in many reports.To eliminate ambiguities over the antifungal properties of essential oils presented in the previous studies, we evaluated the antimicrobial activities against C. albicans of seven essential oils of the same extracting method and habitat, and of three rosemary chemotypes with or without a surfactant, using microbiologic techniques.
2. MATERIALS AND METHODS
2.1. Essential Oils and Other Reagents
Seven essential oils from aromatic plants, lemongrass (Cymbopogon citrates), eucalyptus (Eucalyptus globules), tea tree (Melaleuca alternifolia), peppermint (Mentha piperita), sweet marjoram (Origanum majorana), geranium (Pelagonium graveolens) and rosemary (Rosmarinus officinalis) were obtained from three manufacturers (LIBRA NATUTHERAPY, Hyogo, Japan; Flavor Life, Tokyo, Japan; TREE OF LIFE, Tokyo, Japan). The three chemotypes of rosemary were CINEOL, CAMPHOR and VERBENON (LIBRA NATUTHERAPY, Hyogo, Japan). The characteristic properties of seven essential oils and three chemotypes were shown in Tables 1 and 2. Chemical compositions of rosemary chemotypes analyzed by gas chromatography mass spectrometry (GC-MS) were kindly provided by LIBRA NATUTHERAPY (Table 3). 1,8-cineole (99% purity), α-pinene (98% purity) and camphor (96% purity) were purchased from SigmaAldrich (St. Louis, MO, USA).
Seven essential oils and three rosemary chemotypes, α-pinene,1,8-cineole or camphor in various concentrations, were dissolved in RPMI 1640 with L-glutamine and 0.165 M MOPS without sodium bicarbonate (RPMI 1640-buffered MOPS) (Lonza, Allendale NJ, USA). Camphor was dissolved in ethanol with density 1 µg/ml. Polyoxethylene (20) Sorbitan Monooleate (Tween 80), a surfactant, was purchased from Wako Pure Chemical Industries (Osaka, Japan).
2.2. Fungal Strains and Culture Conditions
C. albicans ATCC 18804 was maintained in YEAST MOLD BROTH (YM broth) (Becton, Dickinson, Franklin Lakes, NJ, USA) at 28˚C for 48 hours. The yeast colonies were adjusted before inoculation, and the final inoculum for broth microdilution assay was approximately 5 - 6 × 105 CFU/ml.
2.3. Minimal Inhibitory Concentration (MIC) Assay and Minimal Fungicidal Concentration (MFC) Assay to C. albicans
The minimal inhibitory concentration (MIC) was measured by the two-fold serial broth dilution assay, based on the Clinical and Laboratory Standards Institute (CLSI) reference protocol M27-A3 with modification. The experiments were performed using sterile 96-well plates. One hundred µl of RPMI 1640-buffered MOPS were added to each well, but not to the first row of wells, 200 µl of 40 µl/ml seven essential oils, three rosemary chemotypes, α-pinene, 1,8-cineole or camphor with or without 20 µl/ml Tween 80 were added to the first row, and serially diluted from the first row by taking 100 µl into the second row. The microdilution was continued to the last row and 100 µl from the last row were discarded 100 µl of C. albicans was suspended in RPMI 1640-buffered
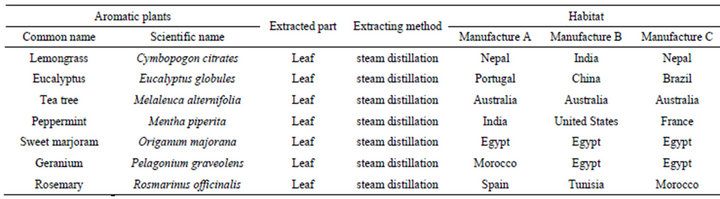
Table 1. The characteristic properties of various essential oils from aromatic plants.
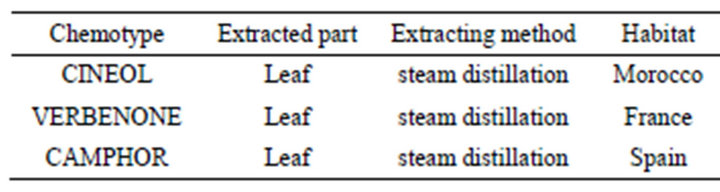
Table 2. The characteristic properties of chemotype essential oils of rosemary.
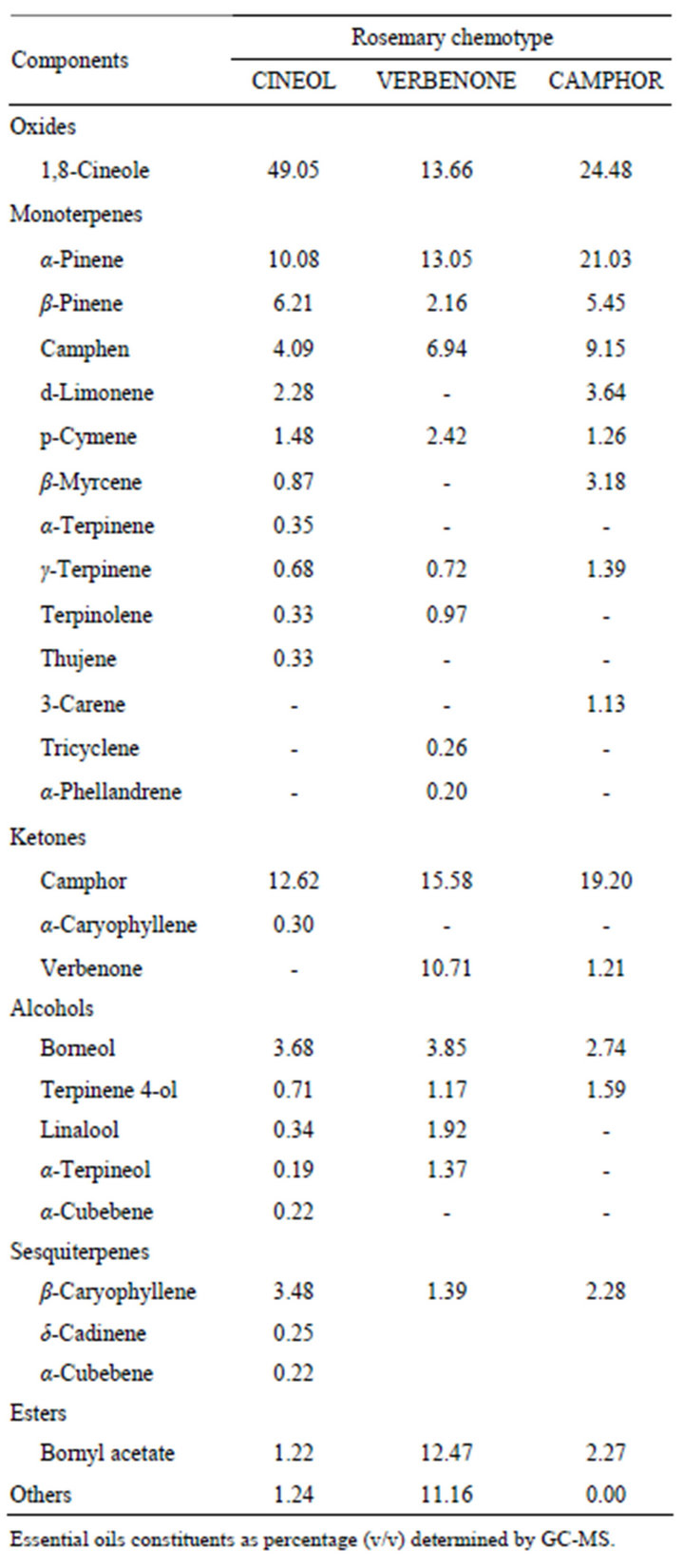
Table 3. Chemical composition of chemotype essential oils from rosemary.
MOPS and adjusted 1 - 5 × 103 CFU/ml was added to each well. The plates were cultured at 35˚C for 48 hours and the MICs were determined. The MIC was defined as the lowest concentration to prevent visible growth of C. albicans. The concentration range of essential oils, rosemary chemotypes, 1,8-cineole, α-pinene or camphor, was 20 - 0.02 µl/ml. To determine the minimal fungicidal concentration (MFC), the fluids from all wells showing inhibition in MIC assay were subcultured in 10 ml of YM broth at 28˚C for 48 hours. The MFC was defined as the lowest concentration that completely prevented the development of the inoculums.
As a control, an inoculum was added to essential oilfree culture medium, and the growth of C. albicans was observed. Also we confirmed that microbes did not grow in the culture broth with an essential oil and Tween 80 and no inoculum. All experiments were performed in at least triplicate.
2.4. Time-Kill Assay
To confirm the antifungal activity of CINEOL with Tween 80, time-kill procedures by Klepser with modification were performed [22]. C. albicans was subcultured in YM broth at 28˚C for 48 hours and prepared at 5 - 6 × 105 CFU/ml in fresh YM broth. RPMI-1640 buffered MOPS containing 0.5%, 1%, and 2% CINEOL with Tween 80, resuspended with the same amount of inoculation. The concentration of CINEOL with Tween 80 used in the assay was equivalent to that in the MIC assay. At predetermined time points (0, 20, 40 minutes, 1, 2, 3, 4, 5 and 6 hours) during incubation, total volume of test solution was added to YM broth and centrifuged at 2040 × g for 10 minutes at 4˚C to remove CINEOL. The pellets were properly diluted and spread on the surface of YM agar plates. The plates were incubated at 28˚C for 48 hours to determine CFU/ml. Each experiment was performed in at least triplicate.
3. RESULTS
3.1. Antifungal Activity against C. albicans of Various Essential Oils from Aromatic Plants
Since essential oils contain hydrophobic components, we examined the antifungal activity of various essential oils with or without Tween 80. Essential oils without Tween 80 showed high MICs against C. albicansor no antifungal activity at tested concentrations. By addition of Tween 80, 2 - 16 times lower MICs were obtained, and antifungal activity was observed in all oils. The MICs against C. albicans in the presence of Tween 80 varied in eucalyptus and rosemary oils, whereas the values were similar in the other essential oils among the three manufacturers (Table 4).
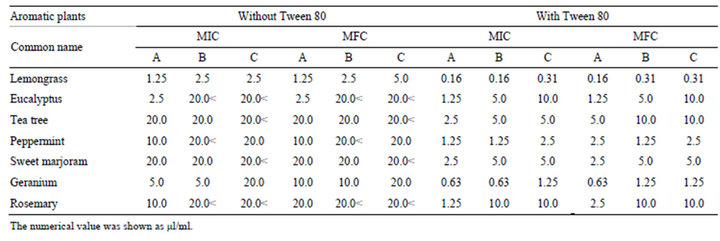
Table 4. The MICs and MFCs values of various essential oils against C. albicans.
To examine whether the antifungal activity of essential oils depends on bacteriostatic or germicidal action, we investigated the minimal fungicidal concentration (MFC). The MFCs of each essential oil except for tea tree were about the same with MICs against C. albicans with or without Tween 80 (Table 4). The MFCs against C. albicans varied in eucalyptus and rosemary oils with Tween 80 among the three manufacturers.
3.2. The Habitats and Chemical Components of Three Rosemary Chemotypes
The parts and method for extraction were common in the three chemotypes, however, the habitats were different (Table 2). Three chemotypes were analyzed by GM-CS, and 26 components in total were identified (Table 3). The major components of three chemotypes, CINEOL, VERBENONE, and CAMPHOR, were 1,8-cineole, α-pinene, and camphor. The contents and ratios of these components were different in the three chemotypes. CINEOL, one of the rosemary chemotypes, includes 1,8-cineole (49.05%), α-pinene (10.08%) and camphor (12.62%). VERBENONE includes 1,8-cineole (13.66%), α-pinene (13.05%) and camphor (15.58%). CAMPHOR includes 1,8-cineole (24.48%), α-pinene (21.03%) and camphor (19.20%).
3.3. Antifungal Activity of Three Rosemary Chemotype
The MICs against C. albicans of CINEOL, VERBENON and CAMPHOR without Tween 80 were 5 µl/ml, 10 µl/ml, and 10 µl/ml, respectively. The MICs with Tween 80 were 1.25 µl/ml, 2.5 µl/ml, and 5.0 µl/ml, respectively. Antifungal activity increased 2 - 4 times by adding of Tween 80. The MFCs against C. albicans without Tween 80 were 20.0 µl/ml, however, with Tween 80, the MFC and MIC values against C. albicans were the same (Table 5). By addition of Tween 80, 4 - 16 times lower MFCs were obtained.
3.4. Antifungal Activity of 1,8-Cineole, α-Pinene, Camphor
The MICs of α-pinene, 1,8-cineole and camphor, major components of rosemary chemotypes, with Tween 80 against C. albicans were 0.63 µl/ml, 5.0 µl/ml, and 5.0 µl/ml, respectively. Antifungal activity increased 4 - 8 times by adding of Tween 80. The MFCs of α-pinene and 1,8-cineole were the same as the MICs against C. albicans, while the MFC of camphor was twice higher than the MIC. Alpha-pinene showed the lowest MIC and MFC among the rosemary chemotypes (Table 6).
3.5. Time-Kill Assay
C. albicans proliferated in culture medium without CINEOL, however by the addition of 0.5%, 1%, and 2% CINEOL, the number of C. albicans was dose-dependently lowered. The decrease was the greatest in the 2% CINEOL culture medium, with no viable C. albicans detected in two hours. In the 1% medium, no live C. albicans was detected in four hours, and in the 0.5% medium, C. albicans was not completely killed in six hours (Figure 1).
4. DISCUSSION
In this study, we confirmed that antifungal activities against C. albicans of seven essential oils with a standard method. The antifungal activities against C. albicans varied among the essential oils from the three manufacturers, though the parts and methods for extraction were the same. Among the seven essential oils from three manufacturers, sweet marjoram and tea tree were from the same habitats (Australia and Egypt respectively), however, the other five plants were from different habitats. Since essential oils are natural materials from various plants, it is suggested that the components of essential oils could be different according to the parts and methods for extraction and the habitats of the plants etc. [23,24].
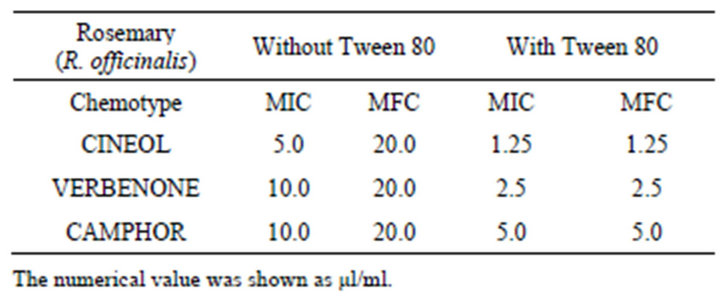
Table 5. The MICs and MFCs values of three chemotype essential oils of rosemary against C. albicans.
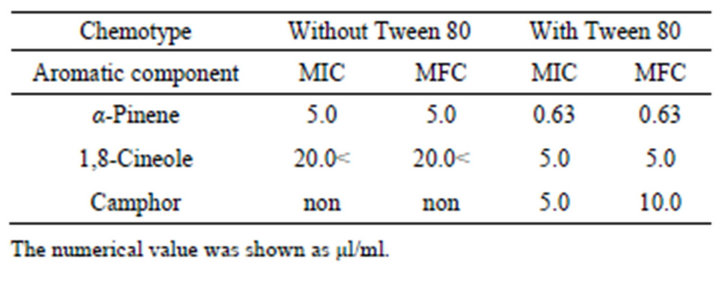
Table 6. The MICs and MFCs values of main aromatic components against C. albicans.
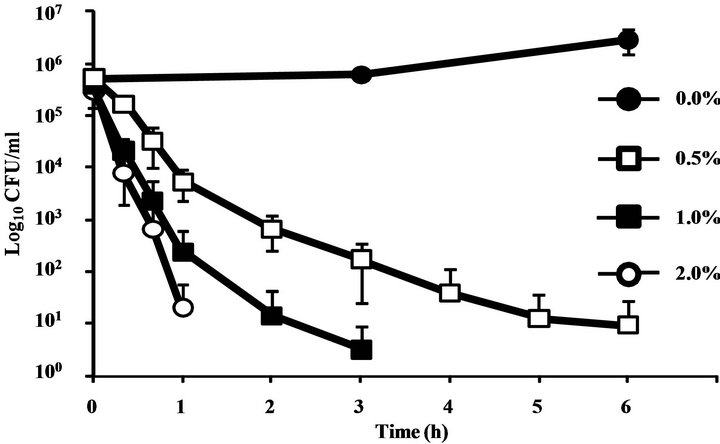
Figure 1. Time-kill curve plots for C. albicans following an exposure to CINEOL. In control suspensions containing no essential oil (●), after the treatment with CINEOL at four times the MIC (□), eight times the MIC (■) and sixteen times the MIC (○). The MIC of CINEOL was 0.125% (v/v). The organism was suspended in RPMI 1640-buffered MOPS. Each symbol indicated the means ± SEs of at least three replicates.
We investigated the antifungal activities against C. albicans of essential oils of common extraction parts and methods, however, we were not able to explain the differences in antifungal activity related to the plants’ habitats. Therefore, we focused on the components of the three rosemary chemotypes.
Typical components of rosemary are 1,8-cineole, α- pinene and camphor [25] and the relatively stable ratio of these components defines each chemotype. It is known that the rosemary oils are widely divided into two chemotypes by the ratio of major components; one with more than 40% of 1,8-cineole and the other with almost the same percentage of 1,8-cineole, α-pinene and camphor [26]. That classification was consistent with that of the oils used in our study. We considered that the remarkable difference between the components in chemotypes might have an influence on the antifungal activity. The CINEOL containing more than 49% of 1,8-cineole, showed the highest antifungal activity of the three chemotypes, however, it was also found that the antifungal activity against C. albicans was low with the 1,8-cineole alone. We found that VERBENONE and CAMPHOR contained 1,8- cineole, α-pinene and camphor in similar ratio by a component analysis. The antifungal activity was the highest in α-pinene, but not in camphor or 1,8-cineole. The result accorded with the report by Zuzarte et al., which claimed that the antifungal activity against C. albicans of α-pinene was higher than those of 1,8-cineole and camphor [27]. On the other hand, the results from some studies examining antimicrobial activity against C. albicans of rosemary oil are conflicting [28,29]. In addition, it was reported that α-pinene has enantiomer, (+)-α-pinene and (−)-α-pinene in the latest report [30]. Biological activities were observed in (+)-α-pinene, but not in (−)-α-pinene. In other studies, it was reported that the enantiomeric distribution of the α-pinene in rosemary depends on the plant’s geographical origins [31]. Therefore we could not explain the difference in the antifungal activities of the chemotypes, even by the major components. There are various kinds of components included in an essential oil, each of which is present in a small amount [32,33]. In determining an antimicrobial mechanism, we cannot ignore the existence of these small but significant minor components. These results explain the difficulty of elucidating active components of essential oils with antifungal activity.
Essential oils contain both water-soluble and hydrophobic components [34-36]. In this study, the addition of Tween 80 enhanced antifungal activity of the essential oils against C. albicans several times. It is known that the components solubilized by Tween 80 increased antimicrobial activity against C. albicans. Alpha-pinene and camphor are typical fat soluble components [34,35]. Without surfactants such as Tween 80, α-pinene and camphor do not dissolve in culture media. It is suggested that the presence of surfactants has a major influence on the results of antimicrobial studies. For example, since oxygen dose not dissolve in culture media covered with insoluble ingredients, the growth of aerobes or facultative anaerobes, such as some fungi and bacteria would be consequently affected. Furthermore, since an essential oil cannot be suspended when it separated from its aqueous layer, the preparation of precise concentration is difficult and that affects its antimicrobial activity. Thus, it was found that the use of the surfactant, Tween 80, is necessary for antimicrobial activities of the essential oils.
In recent studies, however, there have been some reports on antimicrobial activity in the absence of surfactants. In consideration of the safety, we selected Tween 80, which is not toxic for human and is actually used as a food additive [37]. There are several reports on the effects of surfactants and solvents on antimicrobial activities of essential oils. Remmal et al. showed that the agar dispersion is more excellent when Tween 80 and ethanol added in investigating the antibacterial activities against grampositive and negative bacteria of essential oils from oregano (Origanum vulgare) and clove (Syzygium aromaticum) [36]. Inouye et al. reported that the addition of Tween 80 inhibits the antifungal activities against Aspergillus fumigatus of perillaldehyde and citral, which are the components of essential oils dissolve in dimethyl sulfoxide [38]. Though it seems to contradict our results, it remains unknown whether it is caused by the difference in strains or by the influence of dimethyl sulfoxide. Further studies to explore the surfactants or solvents suitable for the study of antimicrobial activity will be necessary.
Though we compared parts and methods for extraction, plants’ habitats, and antifungal activities against C. albicans of seven essential oils, the variation of the components from lot to lot could not be examined. Also, whether the components of essential oils may be affected by the status of use and preservation remains uncertain. Janssen et al. have mentioned that the procedures of antimicrobial assays, concentrations, or presence of surfactants are not standardized in many reports on antimicrobial activities of essential oils [21]. To obtain a stable evaluation on the antimicrobial activities of essential oils, standardization of antimicrobial activity and normalization of the essential oil components for quality maintenance would be necessary.
In this study, we evaluated antifungal activities against C. albicans of various essential oils and found that the activity would be enhanced by the addition of Tween 80. Rosemary essential oil acts against C. albicans, and the activity would be varied depending on its chemical compositions. Therefore, in examining the antifungal activity of rosemary essential oil, the chemotypes need to be considered first.
REFERENCES
- Décanis, N., Savignac, K. and Rouabhia, M. (2009) Farnesol promotes epithelial cell defense against Candida albicans through toll-like receptor 2 expression, interleukin-6 and human β-defensin 2 production. Cytokine, 45, 132-140. doi:10.1016/j.cyto.2008.11.011
- Epstein, J.B. and Polsky, B. (1998) Oropharyngeal candidiasis: A review of its clinical spectrum and current therapies. Clinical Therapeutics, 20, 40-57. doi:10.1016/S0149-2918(98)80033-7
- Ellepola, A.N.B. and Samaranayake, L.P. (2000) Antimycotic agents in oral candidosis: An overview: I. Clinical variants. Dental Update, 27, 111-112, 114-116.
- Pappas, P.G., Kauffman, C.A., Andes, D., Benjamin Jr., D.K., Calandra, T.F., Edwards Jr., J.E., Filler, S.G., Fisher, J.F., Kullberg B.J., Zeichner, L.O., Reboli, A.C., Rex, J.H., Walsh ,T.J. and Sobe, J.D. (2009) Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clinical Infectious Diseases, 48, 503-535. doi:10.1086/596757
- Terai, H. and Shimahara, M. (2005) Atrophic tongue associated with Candida. Journal of Oral Pathology & Medicine, 34, 397-400. doi:10.1111/j.1600-0714.2005.00324.x
- Terai, H. and Shimahara, M. (2010) Glossodynia from Candida-associated lesions, burning mouth syndrome, or mixed causes. Pain Medicine, 11, 856-860. doi:10.1111/j.1526-4637.2010.00861.x
- Runyoro, D.K., Matee, M.I., Ngassapa, O.D., Joseph, C.C. and Mbwambo, Z.H. (2006) Screening of Tanzanian medicinal plants for anti-Candida activity. BMC Complementary and Alternative Medicine, 6, 11. doi:10.1186/1472-6882-6-11
- Zeng, H., Tian, J., Zheng, Y., Ban, X., Zeng, J., Mao Y. and Wang, Y. (2011) In vitro and in vivo activities of essential oil from the seed of Anethum graveolens L. against Candida spp. Evidence-Based Complementary and Alternative Medicine, 2011, 659704. doi:10.1155/2011/659704
- Furletti, V.F., Teixeira, I.P., Obando-Pereda, G., Mardegan, R.C., Sartoratto, A., Figueira, G.M., Duarte, R.M.T., Rehder, V.L.G., Duarte, M.C.T. and Höfling, J.F. (2011) Action of Coriandrum sativum L. essential oil upon oral Candida albicans biofilm formation. Evidence-Based Complementary and Alternative Medicine, 2011, 985832. doi:10.1155/2011/985832
- Pietrella, D., Angiolella, L., Vavala, E., Rachini, A., Mondello, F., Ragno, R., Bistoni, F. and Vecchiarelli, A. (2011) Beneficial effect of Mentha suaveolens essential oil in the treatment of vaginal candidiasis assessed by real-time monitoring of infection. BMC Complementary and Alternative Medicine, 11, 18. doi:10.1186/1472-6882-11-18
- Chifiriuc, C., Grumezescu, V., Grumezescu, A.M., Saviuc, C., Lazăr, V. and Andronescu, E. (2012) Hybrid magnetite nanoparticles/Rosmarinus officinalis essential oil nanobiosystem with antibiofilm activity. Nanoscale Research Letters, 7, 209.
- Ramage, G., Milligan, S., Lappin, D.F., Sherry, L., Sweeney, P., Williams, C., Bagg J. and Culshaw, S. (2012) Antifungal, cytotoxic, and immunomodulatory properties of tea tree oil and its derivative components: Potential role in management of oral candidosis in cancer patients. Frontiers in Microbiology, 3, 220.
- Cowan, M.M. (1999) Plant products as antimicrobial agents. Clinical Microbiology Reviews, 12, 564-582.
- Deans, S.G. and Ritchie, G. (1987) Antibacterial properties of plant essential oils. International Journal of Food Microbiology, 5, 165-180. doi:10.1016/0168-1605(87)90034-1
- Pattnaik, S., Subramanyam, V.R., Bapaji, M. and Kole, C.R. (1997) Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios, 89, 39-46.
- Soković, M., Tzakou, O., Pitarokili, D. and Couladis, M. (2002) Antifungal activities of selected aromatic plants growing wild in Greece. Food/Nahrung, 46, 317-320.
- Lixandru, B.E., Drăcea, N.O., Dragomirescu, C.C., Dră- gulescu, E.C., Coldea, I.L., Anton, L., Dobre, E., Rovinaru, C. and Codiţă, I. (2010) Antimicrobial activity of plant essential oils against bacterial and fungal species involved in food poisoning and/or food decay. Roumanian Archives of Microbiology and Immunology, 69, 224- 230.
- Hyldgaard, M., Mygind, T. and Meyer R.L. (2012) Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Frontiers in Microbiology, 3, 12.
- Stewart, E. (2005) The chemistry of essential oils made simple: God’s love manifest in molecules. Care Publications, Marble Hill.
- Mills, S.Y. (2003) The scientific foundation for herbal medicinal products. 2nd Edition, European Scientific Cooperative on Phytotherapy, Exeter.
- Janssen, A.M., Scheffer, J.J.C. and Baerheim, S.A. (1987) Antimicrobial activities of essential oils. A 1976-1986 literature review on possible applications. Pharmaceutisch weekblad, Scientific Edition, 9, 193-197. doi:10.1007/BF02029329
- Klepser, M.E., Ernst, E.J., Lewis, R.E., Ernst, M.E. and Pfaller, M.A. (1998) Influence of Test conditions on antifungal time-kill curve results: Proposal for standardized methods. Antimicrobial Agents and Chemotherapy, 42, 1207-1212.
- Kelmanson, J.E., Jäger, A.K. and van Staden, J. (2000) Zulu medicinal plants with antibacterial activity. Journal of Ethnopharmacology, 69, 241-246. doi:10.1016/S0378-8741(99)00147-6
- Zanetti, S., Cannas, S., Molicotti, P., Bua, A., Cubeddu, M., Porcedda, S., Marongiu, B. and Sechi, L.A. (2010) Evaluation of the antimicrobial properties of the essential oil of Myrtus communis L. against clinical strains of Mycobacterium spp. Interdisciplinary Perspectives on Infectious Diseases, 2010, 931530. doi:10.1155/2010/931530
- Flamini, G., Cioni, P.L., Morelli, I., Macchia, M. and Ceccarini, L. (2002) Main agronomic-productive characteristics of two ecotypes of Rosmarinus officinalis L. and chemical composition of their essential oils. Journal of Agricultural and Food Chemistry, 50, 3512-3517. doi:10.1021/jf011138j
- Özcan, M.M. and Chalchat, J.C. (2008) Chemical composition and antifungal activity of rosemary (Rosmarinus officinalis L.) oil from Turkey. International Journal of Food Sciences and Nutrition, 59, 691-698. doi:10.1080/09637480701777944
- Zuzarte, M., Gonçalves, M.J., Cavaleiro, C., Canhoto, J., Vale-Silva, L., Silva, M.J., Pinto, E. and Salgueiro, L. (2011) Chemical composition and antifungal activity of the essential oils of Lavandula viridis L’He´r. Journal of Medical Microbiology, 60, 612-618. doi:10.1099/jmm.0.027748-0
- Fu,Y., Zu, Y.G., Chen, L.Y., Shi, X.G., Wang, Z., Sun, S. and Efferth, T. (2007) Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytotherapy Research, 21, 989-994. doi:10.1002/ptr.2179
- Jiang, Y., Wu, N., Fu, Y.J., Wang, W., Luo, M., Zhao, C.J., Zu, Y.G. and Liu, X.L. (2011) Chemical composition and antimicrobial activity of the essential oil of rosemary. Environmental Toxicology and Pharmacology, 32, 63-68. doi:10.1016/j.etap.2011.03.011
- Rivas da Silva, A.C., Lopes, P.M., Barros de Azevedo, M.M., Costa, D.C.M., Alviano, C.S. and Alviano, D.S. (2012) Biological activities of α-pinene and β-pinene enantiomers. Molecules, 17, 6305-6316. doi:10.3390/molecules17066305
- Coleman 3rd, W.M. and Lawrence, B.M. (2000) Examination of the enantiomeric distribution of certain monoterpene hydrocarbons in selected essential oils by automated solid-phase microextraction-chiral gas chromatography-mass selective detection. Journal of Chromatographic Science, 38, 95-99. doi:10.1093/chromsci/38.3.95
- Rasooli, I., Fakoor, M.H., Yadegarinia, D., Gachkar, L., Allameh, A. and Rezaei, M.B. (2008) Antimycotoxigenic characteristics of Rosmarinus officinalis and Trachyspermum copticum L. essential oils. International Journal of Food Microbiology, 122, 135-139. doi:10.1016/j.ijfoodmicro.2007.11.048
- Mahboubi, M. and Kazempour, N. (2009) The antimicrobial activity of essential oil from perovskia abrotanoides Karel and its main components. Indian Journal of Pharmaceutical Sciences, 71, 343-347. doi:10.4103/0250-474X.56016
- Knobloch, K., Pauli, A., Iberl, B., Weis, N. and Weigand, H. (1988) Mode of action of essential oil components on whole cells of bacteria and fungi in plate tests. In: Schreier, P., Ed., Bioflavor '87, Walter de Gruyther, Berlin, 287-299.
- Knobloch, K., Pauli, A., Iberl, B., Weigand, H. and Weis, N. (1989) Antibacterial and antifungal properties of essential oil components. Journal of Essential Oil Research, 1, 119-128. doi:10.1080/10412905.1989.9697767
- Remmal, A., Bouchikhi, T., Tantaoui-Elaraki, A. and Ettayebi, M. (1993) Inhibition of antibacterial activity of essential oils by Tween 80 and ethanol in liquid medium. Journal de Pharmacie de Belgique, 48, 352-356.
- Edris, A.E. and Malone. C.F. (2012) Preferential solubilization behaviours and stability of some phenolic-bearing essential oils formulated in different microemulsion systems. International Journal of Cosmetic Science, 34, 441-450. doi:10.1111/j.1468-2494.2012.00737.x
- Inouye, S., Tsuruoka, T., Uchida, K. and Yamaguchi, H. (2001) Effect of sealing and Tween 80 on the antifungal susceptibility testing of essential oils. Microbiology and Immunology, 45, 201-208.
NOTES
*Corresponding author.

