Advances in Biological Chemistry
Vol.4 No.2(2014), Article ID:44859,12 pages DOI:10.4236/abc.2014.42021
Differential Deterrent Activity of Natural Products Isolated from Allophylus edulis (Sapindaceae)
Martina Díaz1,2,3, Lucía Castillo1, Carmen E. Díaz2, Ricardo Guillermo Álvarez2, Azucena González-Coloma3, Carmen Rossini1*
1Laboratorio de Ecología Química, Facultad de Química, Universidad de la República, Montevideo, Uruguay
2Instituto de Productos Naturales y Agrobiología, Consejo Superior de Investigaciones Científicas, La Laguna, Tenerife, Spain
3Instituto de Ciencias Agrarias, Consejo Superior de Investigaciones Científicas, Madrid, Spain
Email: *crossini@fq.edu.uy
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 1 March 2014; revised 3 April 2014; accepted 11 April 2014
ABSTRACT
The phytochemical study of Uruguayan specimens of Allophylus edulis (Sapindaceae) yielded the isolation of various natural products being some of them reported for the first time in this species. Although most of them are ubiquitous in the plant kingdom, some revealed in this study to have anti insect properties. Two sesquiterpenes (6,7-Epoxicaryophyllene and spathulenol), two phytosterols (sitosterone and sitosterol), a pentacyclic triterpene (lupeol) and a clerodane diterpene were isolated. 6,7-Epoxycaryophyllene, lupeol and sitosterol showed to be deterrent against the aphid Myzus persicae and the coleopteran Epilachna paenulata. Moreover, the crude ethanolic extract of twigs of A. edulis showed to be deterrent against both insect species being some of its fractions also active against another aphid, Rhopalosiphum padi. Various active compounds against different insect models have been isolated from the twigs extract of A. edulis. The results evidenced synergic as well as antagonistic effects in the mixture, summed to differential activity against the insects, a desirable attribute when developing botanical pesticides.
Keywords:Anti Insect; Allophylus edulis; Myzus persicae; Rhopalosiphum padi; Epilachna paenulata

1. Introduction
The use of synthetic pesticides in agricultural production yields effective results in the short term, but it has several drawbacks, such as the development of resistance and the adverse environmental effects. Among other approaches to substitute such products in Integrated Pest Management programs (pheromones, monitoring, and organic production), the developing of new pesticides from natural resources such as undamaged native plants (“botanical pesticides”) is becoming more and more comprised. These pesticides will be intrinsically less toxic and more environmentally friendly ways of control [1] .
In particular this study focuses on Allophylus edulis (Sapindaceae), commonly called Chal Chal, that occurrs in the Uruguayan and Brazilian native flora as well as in Bolivia, Argentina and the Guayanas (http://micol.fcien.edu.uy/flora/Allphyllus-edulis.html). Products obtained from A. edulis were evaluated against four pest species chosen to represent different feeding modes (chewing and sucking) and diet breadth (oligophagy and polyphagy). The four insect species, Spodoptera littoralis (Lepidoptera: Noctuidae), Epilachna paenulata (Coleoptera: Coccinelidae), Myzus persicae and Rophalosiphum padi (Hemiptera: Aphididae), are important agricultural pests, either in conventional or organic production [2] .
Various species belonging to the genus Allophylus have been studied on their biological activity. For instance, A. rubifolius and A. africanus revealed to act as antioxidants; the latter also exhibited antibacterial properties [3] [4] . Cytotoxicity is another property that has been described in species of this genus. In a study of A. timoriensis, its extracts showed to be cytotoxic to carcinogenic lung and colon cells [5] .
A. edulis has been scarcely studied. However, there are some reports about its antihepatotoxic activity [6] , its inhibitory action of the angiotensin converting enzyme [7] and its antiulcerogenic [8] and genotoxic activities [9] . The infusion of leaves is popularly used as throat anti-inflammatory agent and for intestinal disorders, as well as being considered antidiabetic [10] . As far as we know, anti-insect activity has only been reported by our group for the species A. edulis when tested against the aphid M. persicae and the coleopteran E. paenulata. In that occasion no fractionation neither isolation of pure compounds had been pursued [11] . Regarding isolated compounds from this species, there are reports on the presence of cyanolipids in seeds [12] [13] , of the methyl inositol quebrachitol in twigs [14] , of phenolic compounds (responsible for the inhibitory action of the angiotensin converting enzyme) in branches and leaves [7] , as well as of C-glycosil flavones (with antihepatotoxic activity) in leaves [6] .
In this study the phytochemical characterization of the ethanolic extract from twigs of A. edulis has been pursued following a bioguided fractionation procedure, resulting in the isolation of various active compounds against different insect models.
2. Materials and Methods
2.1. General
IR spectra were obtained in a IR Prestige-21 Shimadzu Spectrometer in NaCl.
The NMR spectra were recorded using an AMX-500 Spectrometer (400 and 500 MHz for 1H and 100, 125 and 150 MHz for 13C) in CDCl3 in the case of 1 to 4. In the case of 5 and 6 spectra were recorded using a Bruker DPXAdvance Spectrometer (400 MHz for 1H and 100 MHz for 13C) in CDCl3 as well.
In the cases of 1 to 3 mass spectrometric analyses were performed in a Micromass Autospec spectrometer with direct sample introduction and electronic impact ionization. Low resolution mass spectra were obtained in a Shimadzu 2010 Plus Gas Chromatograph coupled to a Shimadzu QP2010 Mass Spectrometer, with direct sample introduction and electronic impact ionization. In the cases of 4 and 6 analyses were performed using a Bruker micrOTOF-Q II mass spectrometer (Bruker Daltonics, Billerica, MA, USA), equipped with ESI. The instrument was operated at a capillary voltage of 4.5 kV with an end plate offset of −500 V, a dry temperature of 180˚C using N2 as dry gas at 4.0 L/min and a nebulizer pressure of 0.4 bar.
High Performance Liquid Chromatography was developed in a Shimadzu LC-20AT Prominence, equipped with a SPD-M20A Prominence diode array detector and Silica Phase columns. A Macherey Nagel Nucleosil-100 (7 mm, 250 × 16 mm) column was used, with DCM/MeOH as mobile phase, and a flow of 8 mL/min, in the case of 6; and a Beckman Ultrasphere Si-100 (5 mm, 250 × 10 mm) column, with hex/AcOEt as mobile phase, a flow of 8 mL/min, in the cases of 1-4. Flash and Liquid Chromatographies were developed in silica gel MN Kieselgel 60 M (0.04 - 0.063 mm/230 - 400 mesh). For flash columns a FMI Lab Pump, RP-SY and a IWAKI Air Pump, AP-115S were used. Fractions were monitored by thin layer chromatography developed in aluminium Silica gel 60 G/UV254 plates (Macherey-Nagel), and spots were visualized by heating after spraying with Oleum reagent [H2O:acetic:acid:sulfuric acid (16:80:4)] or Anisaldehide/H2SO4 (95 mL EtOH, 4 mL H2SO4, 1 mL anisaldehyde) [15] .
2.2. Extraction and Isolation
Vegetal material was collected in the countryside of Canelón Chico (Latitude: −34.4836111˚, Longitude: −56.3238889˚), Uruguay. Plant material was identified by Prof. Eduardo Alonso-Paz (Cátedra de Botánica, Facultad de Química) and a voucher specimen was deposited at the Herbarium of Facultad de Química, Montevideo, Uruguay [11] . Plant material was then dried (48 h, 40˚C) and ground with a IKA MF10 grinder. The extract was obtained by maceration in ethanol of 764 g of dried twigs, extracting the vegetal material three times for 24 h, yielding 5.1%. The extract (36 g) was fractionated by vacuum liquid chromatography (VLC) in silica gel (using a 13 cm height and 6 cm diameter silica column). Fractions were eluted with 2 - 3 dead volumes (200 mL) of mixtures of hex/AcOEt and AcOEt/MeOH, increasing the polarity over fractions. Fourteen fractions were obtained. Some of them were further separated in flash chromatography and preparative HPLC (see above) obtaining six isolated compounds (1-6).
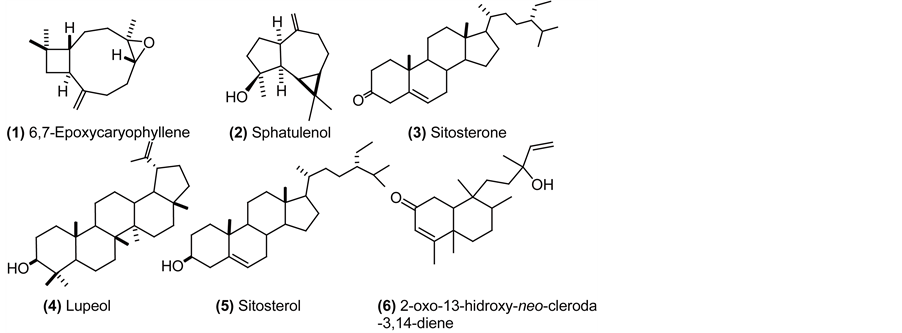
6,7-Epoxicaryophyllene (1): colorless oil (hexane); 1H NMR (CDCl3, 500 MHz) δ 0.96 (1H, m, H-8), 0.98 (3H, s, H-12), 1.01 (3H, s, H-13), 1.20 (3H, s, H-14), 1.32 (1H, m, H-5), 1.42 (1H, m, H-9), 1.65 (3H, m, H-1, H-9), 1.76 (1H, t, J = 10 Hz, H-10), 2.09 (2H, m, H-8, H-4), 2.25 (1H, m, H-5), 2.34 (1H, m, H-4), 2.60 (1H, dd J = 10, 18.5 Hz, H-2), 2.88 (1H, dd, J = 10.6, 4.3 Hz, H-6), 4.86 (1H, m, H-15), 4.97 (1H, d, J = 0,8 Hz, H-15). 13C NMR (CDCl3, 125 MHz) δ 17.2 (CH3, C-14), 21.7 (CH3, C-13), 27.4 (CH2, C-9), 30.0 (CH2, C5), 30.1 (CH3, C-12), 30.4 (CH2, C-4), 34.2 (C, C-11), 39.3 (CH2, C-8), 39.9 (CH2, C-1), 8.9 (CH, C-2), 51.0 (CH, C-10), 60.0 (C, C-7), 63.9 (CH, C-6), 112.9 (CH2, C-15), 152.0 (C, C-3). HREIMS m/z 220.1794 (calcd. for C15H24O, 220.18271).
Sphatulenol (2): colorless oil (hexane); 1H NMR (CDCl3, 500 MHz) δ 0.47 (1H, dd, J = 11.3, 9.5 Hz, H-1), 0.71 (1H, ddd, J = 11.3, 9.5, 6.1 Hz, H-2), 1.01 (1H, m, H-3), 1.04 (3H, s, H-13), 1.06 (3H, s, H-12), 1.28 (3H, s, H-15), 1.32 (1H, m, H-10), 1.57 (1H, m, H-8), 1.63 (1H, m, H-7), 1.77 (1H, m, H-8), 1.91 (1H, m, H-7), 1.98 (1H, m, H-3), 2.04 (1H, m, H-4), 2.20 (1H, m, H-6), 2.42 (1H, dd, J = 13.5, 7.0 Hz, H-4), 4.66 (1H, d, J = 1 Hz, H-14), 4.69 (1H, bs, H-14). 13C NMR (CDCl3, 125 MHz) δ 16.5 (CH3, C-13), 20.4 (C, C-11), 24.9 (CH2, C-3), 26.2 (CH3, C-15), 26.9 (CH2, C-7), 27.6 (CH, C-2), 28.8 (CH3, C-12), 30.1 (CH, C-1), 39.0 (CH2, C-4), 41.9 (CH2, C-8), 53.5 (CH, C-6), 54.5 (CH, C-10), 81.1 (C, C-9), 106.4 (CH2, C-14), 153.6 (C, C-5). HREIMS m/z 220,2002 (calcd. for C15H24O, 220, 18271).
Sitosterone (3): amorphous solid (hexane); 1H NMR (CDCl3, 500 MHz) δ 0.71 (3H, s, H-18), 0.83 (6H, m, H-26, H-27), 0.85 (3H, m, H-29), 0.92 (5H, m, H-21, H-9, H-24), 1.02 (2H, m, H-14, H-7), 1.12 (2H, m, H-15, H-17), 1.17 (4H, m, H-23, H-22, H12), 1.18 (3H, s, H-19), 1.25 (2H, m, H-28), 1.33 (2H, m, H-2, H-22), 1.36 (1H, m, H-20), 1.43 (1H, m, H-11), 1.51 (2H, m, H-11. H-8), 1.61 (1H, m, H-15), 1.67 (1H, m, H-25), 1.70 (1H, m, H-1), 1.84 (1H, m, H-6), 1.86 (2H, m, H-16), 2.02 (2H, m, H-1; H-12), 2.28 (1H, m, H-6), 2.39 (2H, m, H-2, H-7), 5.72 (1H, s, H-4). 13C NMR (CDCl3, 100 MHz) δ 12.13 (CH3, C-18), 12.15 (CH3, C-29), 17.6 (CH3, C-19), 18.8 (CH3, C-21), 19.2 (CH3, C-26), 20.0 (CH3, C-27), 21.2 (CH2, C-11), 23.3 (CH2, C-28), 24.4 (CH2, C-15), 26.3 (CH2, C-23), 28.4 (CH2, C-16), 29.4 (CH, C-25), 32.3 (CH, C-6), 33.1 (CH2, C-7), 34.1 (CH2, C-22), 34.2 (CH2, C-2), 35.8 (CH, C-8), 35.9 (CH2, C-1), 36.3 (CH, C-20), 38.8 (C, C-10), 39.8 (CH2, C-12), 42.6 (C, C-13), 46.1 (CH, C-24), 54.1 (CH, C-9), 56.1 (CH, C-14), 56.2 (CH, C-17), 123.9 (CH2, C-4), 171.8 (C, C-5), 199.8 (C, C-3). HREIMS m/z 412,3970 (calcd. for C29H48O 412.3705).
Lupeol (4): amorphous solid (hexane); 1H NMR (CDCl3, 500 MHz) δ 0.68 (1H, d, J = 9.2 Hz, H-5), 0.76 (3H, s, H-24), 0.79 (3H, s, H-28), 0.83 (3H, s, H-25), 0.94 (3H, s, H-27), 0.97 (3H, s, H-23), 1.03 (3H, s, H-26), 1.07 (2H, m, H-12), 1.28 (1H, m, H-9), 1.32 (1H, m, H-21), 1.36 (1H, m, H-18), 1.38 (1H, m, H-22), 1.39 (3H, m, H-6, H-7), 1.42 (2H, m, H-11), 1.48 (2H, m, H-16), 1.52 (1H, m, H-6), 1.57 (2H, m, H-2), 1.59 (2H, m, H-15), 1.66 (2H, m, H-1), 1.68 (4H, bs, H-13, H-30), 1.92 (1H, m, H-21), 2.01 (1H, m, H-22), 2.38 (1H, td, J = 11.1, 5.8 Hz, H-19), 3.19 (1H, dd, J = 11.4, 4.9 Hz, H-3), 4.57 (1H, dd, J = 2.5, 1.3 Hz, H-29), 4.68 (1H, d, J = 1.3 Hz, H-29). 13C NMR (CDCl3, 125 MHz) δ 14.7 (CH3, C-27), 15.6 (CH3, C-24), 16.1 (CH3, C-25), 16.3 (CH3, C-26), 18.2 (CH3, C-28), 18.5 (CH2, C-6), 19.5 (CH3, C-30), 21.1 (CH2, C-11), 25.2 (CH2, C-12), 27.58 (CH2, C-2), 27.6 (CH2, C-15), 28.2 (CH3, C-23), 29.9 (CH2, C-21), 34.5 (CH2, C-7), 35.8 (CH2, C-16), 37.3 (C, C-10), 38.7 (CH2, C-1), 38.2 (CH, C-13), 39.0 (C, C-4), 40.2 (CH2, C-22), 41.0 (C, C-8), 43.0 (C, C-14), 43.2 (C, C-17), 48.2 (CH, C-19), 48.5 (CH, C-18), 50.6 (CH, C-9), 55.5 (CH, C-5), 79.2 (CH, C-3), 109.5 (CH2, C-29); 151.1 (C, C-20). HRESIMS m/z 426.3821 [M]+ (calcd. for C30H50O: 426.38615).
Sitosterol (5): amorphous solid (hexane); 1H NMR (CDCl3, 400 MHz) δ 0.69 (3H, s, H-18), 0.80-0.85 (9H, m, H-27, H-29, H-26), 0.92 (5H, m, H-21, H-24, H-9), 1.01 (3H, s, H-19), 1.02 -1.04 (2H, m, H-17, H-22), 1.09 (1H, m, H-1), 1.12 (1H, m, H-14), 1.17 (3H, m, H-23, H-12), 1.25 (2H, m, H-28), 1.31 (1H, m, H-22), 1.36 (1H, m, H-20), 1.50 (5H, m, H-2, H-7, H-8, H-11), 1.59 (2H, m, H-15), 1.68 (1H, m, H-25), 1.85 (3H, m, H-1, H-2, H-16), 1.96 (1H, m, H-7), 2.02 (1H, m, H-12), 2.28 (2H, m, H-4), 3.51 (1H, m, H-3), 5.3 (1H, m, H-6). 13C NMR (CDCl3, 100 MHz) δ 12.0 (CH3, C-29), 12.1 (CH3, C-18), 18.9 (CH3, C-21), 19.2 (CH3, C-26), 19.6 (CH3, C-27), 20.0 (CH3, C-19), 21.2 (CH2, C-11), 23.2 (CH2, C-28), 24.5 (CH2, C-15), 26.2 (CH2, C-23), 28.4 (CH2, C-16), 29.3 (CH, C-25), 31.8 (CH2, C-2), 32.0 (CH, C-8), 32.1 (CH2, C-7), 34.1 (CH2, C-22), 36.3 (CH, C-20), 36.6 (C, C-10), 37.4 (CH2, C-1), 39.9 (CH2, C-4), 42.4 (CH2, C-12), 42.5 (C, C-13), 46.0 (CH, C-24), 50.3 (CH, C-9), 56.2 (CH, C-17), 56.9 (CH, C-14), 72.0 (CH, C-3), 121.9 (CH, C-6), 140.9 (C, C-5). EIMS m/z 414 [M]+ 399, 381, 354, 329, 303, 273, 255, 231, 213.
2-oxo-13-hidroxy-neo-cleroda-3,14-diene (6): colorless oil (hexane); IR (NaCl) 3441, 2934, 2850, 1730, 1660, 1454, 1381, 1261, 1022, 902, 848, 802 cm-1; 1H NMR (CDCl3, 400 MHz) δ 0.8 (3H, s, H-20), 0.82 (3H, d, H-17), 1.10 (3H, s, H-19), 1.25 (3H, s, H-16), 1.29 (2H, m, H-11), 1.32 (2H, m, H-6), 1.48 (5H, m, H-7, H-12, H-8), 1.83 (1H, m, H-10), 1.87 (3H, d, J = 1.3 Hz, H-18), 2.33 (2H, m, H-1), 5.06 (1H, dd, J = 10.7, 1.2 Hz, H-15), 5.20 (1H, dd, J = 17.3, 1.2 Hz, H-15), 5.70 (1H, d, J = 1.3 Hz, H-3), 5.80 (1H, dd, J = 10.8, 17.6 Hz, H-14); 13C NMR (CDCl3, 100 MHz) δ 15.9 (CH3, C-17), 18.2 (CH3, C-20), 18.5 (CH3, C-19), 19.1 (CH3, C-18), 27.0 (CH2, C-7), 27.9 (CH3, C-16), 31.3 (CH2, C-11), 35.0 (CH2, C-6), 35.01 (CH2, C1), 35.7 (CH2, C-12), 36.0 (CH, C-8), 38.5 (C, C-9) 40.0 (C, C-5), 45.8 (CH, C-10), 73.3 (C, C-13), 112.2 (CH2, C-15), 125.7 (CH, C-3), 145.1 (CH, C-14), 172.0 (C, C-4), 200.0 (C, C-2). HRESIMS m/z 304.23995 (calcd. for C20H32O2: 304.24022).
2.3. Insects
Epilachna paenulata Germar (Coleoptera: Coccinellidae) is an olyphagous insect specialized in Cucurbitaceae. Adults were maintained on squash (Cucurbita pepo L.) under controlled conditions of temperature (20˚C ± 2˚C) and photophase (14L:10D).
Spodoptera littoralis Boisduval (Lepidoptera: Noctuidae) is a generalist species. The laboratory colony was kept on an artificial diet [16] at 25˚C ± 1˚C, >70% relative humidity, with a photoperiod of (16L:8D) in a growth chamber.
Rhopalosiphum padi L. (Hemiptera: Aphididae) is a grass specialist species. These aphids were reared on Hordeum vulgare L. foliage and maintained at 20˚C ± 1˚C, >70% relative humidity, and with a photoperiod of (16L:8D) in a growth chamber.
Myzus persicae Sulzer (Hemiptera: Aphididae) is a generalist species. These aphids were reared on bell pepper foliage (Capsicum annuum L.) at 25˚C ± 1˚C, >70% relative humidity, with a photoperiod of (16L:8D) in a growth chamber.
2.4. Aphid Settling Bioassay
The activity on aphid settling was tested in choice experiments as described elsewhere [16] . The extracts were tested in plastic boxes (3 × 3 × 1.5 cm) lined at the bottom with 2% agar (20 replicates per extract). Two leaf pieces (ca. 1 cm2) cut from the appropriate host plant (C. annuum and H. vulgare for M. persicae and R. padi, respectively) were placed on the agar and treated either with the extract at 100 μg/cm2 (in the case of pure compounds a 50 μg/cm2 dose was used) or the same amount of solvent (MeOH). Ten aphids were placed in the box and the percentage of aphids settled on each surface was recorded after 24 hours of exposure. A Preference Index was also calculated for each replicate as PI = [(%C − %T)/(%C + %T)], where %T and %C are the percentages of aphids settled on the treated and control leaf pieces, respectively. In this manner, a PI < 0 indicates settling stimulation and a PI > 0 indicates settling deterrence.
2.5. Feeding Deterrence Bioassay
The antifeedant (phagodeterrent) activity was evaluated in choice-bioassays in Petri dishes (9 cm × 1 cm) lined at the bottom with a layer of agar (2%). Insects were offered four leaf discs (1 cm2) of the appropriate host plant (C. pepo for E. paenulata and C. annuum for S. littoralis). Two of the discs (T) were coated with 100 μg of the extract or 50 μg of pure compounds (10 μL of a 10% or 5% MeOH solution respectively), and the other two (C) were treated with 10 μL of MeOH. Insects were tested individually as adults for E. paenulata (10 - 15 replicates per extract) and as larvae for S. littoralis (5 - 6 replicates per extract). To measure deterrent activity, a visual score of area consumed (0%, 12.5%, 25%, 37.5%, 50%, 62.5%, 75%, 87.5% or 100%) was assigned for all discs within the plate, and a preference index (PI) was determined for each replicate using the formula PI = (C - T)/(C + T), where C and T are the amounts consumed for the control and treatment of the leaves respectively [17] . In this manner, a PI < 0 indicates a feeding stimulant effect and a PI > 0 indicates an antifeedant (deterrent) effect.
2.6. Statistics
Bioassay data were analyzed by Wilcoxon Rank tests [18] . Antisettling activity determination was based on the number of aphids settled on the leaf treated with solvent (control) compared to the number settled on the leaf treated with the substance tested (treatment). Replicates with fewer than five aphids settled in total (on both leaves) were not considered. Antifeedant (deterrent) activity determination was based on the proportions of the consumption of the leaf treated with solvent (control) compared to the proportions of the consumption of the leaf treated with the substance tested (treatment). Replicates where no consumption was detected were not considered.
3. Results and Discussion
The ethanolic twigs extract of A. edulis had showed significant deterrence against M. persicae as well as against E. paenulata (data from our previous report [11] ). However, many of the fractions obtained from the VLC showed to be active also against R. padi, the other aphid tested in this study (Table 1). Indeed, there were more fractions active against aphids (13 out of 14) than against chewers (10 out of 14). On the other hand, as a general trend, when activity was found, fractionation caused a decrease in activity against aphids and an increased effect
Table 1. Anti-insect activity of fractions eluted from the Vacuum Liquid Chromatography (VLC) of the ethanolic crude extract from twigs of A. edulis. Results are shown as mean ± standard error (SE).
aPI = (C − T)/(C + T), where C and T are the amounts consumed for the control and treatment leaves respectively for the chewers insects; and PI = [(%C − %T)/(%C + %T)], where %T and %C are the percentages of aphids settled on the treated and control leaf pieces, respectively for the aphids used. bAnti insect activity for the twig extract was previously reported [11] . *and bold results denote significant difference between C and T (p < 0.05, Wilcoxon 2 tail rank test). The activity is deterrent when PI > 0, and stimulant when PI < 0). #Denotes significant difference between C and T (deterrent, p < 0.05, Wilcoxon 1 tail rank test). NT: not tested.
on chewers compared to the whole twig extract, with the extreme in fraction 11 in which an antifeedant effect against S. littoralis was found when no activity was detected in the crude extract from which fraction 11 was obtained. These observations may point to some kind of adding or even synergistic effect in the mixture when tested against aphids, and on the contrary, an antagonistic effect in the case of the chewers.
The first fraction eluted with hex/AcOEt (8:2), was deterrent against M. persicae and E. paenulata but settling stimulant on R. padi (PI = −0.4 ± 0.1, p < 0.05, Table 2). This settling stimulating effect was neither present in the crude extract nor recovered for the compounds isolated from this fraction (that is, compounds 1 - 4, Table 2 and Table 3); indeed compound 1 isolated from this fraction was deterrent against this species. Deterrent activity of 1 was also found against the other aphid, M. persicae and E. paenulata.
Compound 2 was also isolated from this same fraction. From the analysis of mono and bidimensional NMR spectra and comparing them with bibliography it was concluded that 1 corresponded to 6,7-Epoxicaryophyllene [19] and 2 to spathulenol (Spectra as well as a brief description of elucidation are included in the supplementary material) [20] . Being both of them sesquiterpenes usually found in plant species but reported here for the first time in A. edulis.
These compounds have shown anti insect activity against different species, according to previous reports. Spathulenol (2) which was not tested in this study owing to the insufficient quantity obtained, has been already reported as a component of active essential oils against A. aegyptii larvae [21] [22] ; and adults of Rhyzopertha
Table 2. Anti insect activity of pure compounds isolated by flash chromatography and HPLC from the VLC fractions. For comparison purposes the activity of the VLC fractions from which the isolated compounds were obtained are included before such compounds. Results are shown as mean ± standard error (SE).
aSee Table 1 for details on PI. *and bold results denote significant difference between C and T (deterrent, p < 0.05, Wilcoxon 2 tail rank test). SE: Standard error. NT: not tested.
Table 3. Compounds isolated from the different fractions eluted from the VLC.
dominica (Coleoptera: Bostrichidae), Tribolium castaneum (Coleoptera: Tenebrionidae) (repellence and fumigant toxicity) and Sitophilus zeamais (Coleoptera: Curculionidae) (contact and fumigant toxicity) [23] -[25] . In these cases the individual activity of such compound was not tested, however there is a study of repellence against Aedes aegypti and Anopheles stephensi (Diptera: Culicidae) in which the pure compound showed significative activity [26] . Regarding 6,7-Epoxicaryophyllene (1) which showed activity against both aphid species as well as against E. paenulata (Table 2), there are many previous reports of plant essential oils that contain it and have shown activity against different insects. Some examples include the larvicidal activity of essential oils against different mosquitoes species (A. aegypti [27] [28] , Culex pipiens [29] , A. stephensi, Anopheles culicifacies, A. albopictus, Culex quinquefasciatus [30] [31] , Anopheles anthropophagus) [32] ; the fumigant toxicity against Ephestia kuehniella and Ectomyelois ceratoniae (Lepidoptera: Pyralidae) [33] ; the feeding and oviposition deterrence against larvae and adults respectively of S. frugiperda [34] ; the contact toxicity against Drosophila melanogaster L. (Diptera: Drosophilidae) and S. zeamais [35] [36] ; and the fumigant toxicity against Sitophilus granarius [37] . In the case of the aphids here tested, similar activity against R. padi and M. persicae was found for this compound (Table 2). Previous reports describe the activity of this compound on the settling of R. padi [19] and M. persicae [16] . In the case of the chewers, 6,7-Epoxicaryophyllene (1) probed to be feeding deterrent against E. paenulata but not against S. littoralis (Table 2). 1 accounts for 0.7% of its original fraction (fraction 2). However, it showed almost the same activity than the fraction against M. persicae and E. paenulata, indicating that other active compounds are also present in Fraction 2. As far as we are concerned, no previous studies had reported the activity of this compound against E. paenulata or S. littoralis.
Compound 3, also isolated from fraction 2 (Table 2) showed in its mass spectra a molecular ion m/z 412 which suggested the molecular formula C29H48O. In the 13C NMR spectra, 29 signals were observed, confirming along with 1H NMR signals and by comparison with bibliography data, that 3 corresponded to sitosterone [38] , described in this study for the first time in this plant species. Sitosterone was tested in this study against M. persicae and S. littoralis, not being active against any of them. As far as we know, there are no reports of activity of this compound against insects in previous literature.
Compound 4, also isolated from fraction 2 (Table 2) showed in its mass spectra a molecular ion m/z 426 which suggested the molecular formula C30H50O. By comparing NMR data with bibliography it was concluded that 4 corresponded to the pentacyclic triterpene lupeol [39] isolated for the first time in this vegetal species and revealing activity against M. persicae and E. paenulata.
In the case of lupeol (4), our results show deterrent activity against M. persicae and E. paenulata. There is a previous study in which its inactivity against M. persicae and S. littoralis is reported [40] . The divergence in the result on M. persicae found with the present work may be due to different aphid instars used in the tests. Within the frame of phytochemical and biological activity studies of plant extracts, this compound has been isolated from many plant species [41] -[49] . Moreover it has been reported to be component of a fraction active against the leaf miners Ctenopsteutis obliquana (Lepidoptera: Tortricidae) in feeding deterrence bioassays. However, when tested as a pure compound, it did not show activity against those larvae [50] .
The consecutive fraction [second fraction eluted with hex/AcOEt (8:2)] showed activity against E. paenulata (PI = 0.8 ± 0.3, p < 0.05), being inactive against the other insect models. From this fraction pure 5 was isolated. It showed in its mass spectra a molecular ion m/z 414 consistent with the molecular formula C29H50O. In such spectra there were signals of the ions m/z 273 and 255, corresponding to the steroid tetracyclic fragment. The loss of the side chain originates the fragment m/z 273 which after the elimination of H2O generates the ion m/z 255. The NMR signals of 5, were very similar to those of 3 except for the presence of an hydroxyl moiety in C3 instead of a carbonyl. With bibliography data it was possible to confirm that 5 corresponded to sitosterol [51] [52] , which in this study revealed to be active against M. persicae and E. paenulata. Noteworthy is the fact that the oxidation state in C3 is the only difference between 3 and 5, which may indicate a correlation of the moiety in such position with the activity of these sterols against insects. Similar correlations have previously been reported involving the activity of phytosterols against insects [53] .
At least a hundred different sterols have been isolated from plants, being them of vital importance for their growing processes [53] . Sitosterol is probably the most abundant and common sterol in plants [54] -[57] . Furthermore, sterols play a critical role in all the organisms of the animal kingdom, owing to their basic role as components of cell membranes and in some cases as hormone precursors (including molting hormones). However, herbivorous insects do not have the capacity of synthesising cholesterol. That is why they depend on the ingestion of phytosterols which could be metabolized to cholesterol [54] . In spite of that fact, in many cases anti insect activity has been found on some plant sterols. In those cases of phytosterols that have their function in the basic metabolism of plants and present also defensive activity against insect, it would be interesting to reveal if plants that produce them do so in different concentrations, deposit them differentially in their organs and/or are able to increase the biosynthesis in response to damage (assuming dose-dependent effect). In this direction, our results show (Table 2) that isolated sterols exhibited in the case of 3 decreased activity and in the case of 5 increased activity compared to the fraction from where they were isolated.
The next fraction [eluted with hex/AcOEt (1:1), fraction 4 in Table 1] was active against both aphids and the coleopteran. After its fractionation, 6 was isolated from it. In its mass spectra a molecular ion m/z 304 was observed which suggested the molecular formula C20H32O2. The signals observed in the mass spectra, 1H and 13C NMR revealed that 6 is 2-oxo 13-hidroxy-neo-cleroda-3,14-diene, a diterpene belonging to the clerodane series [58] ; isolated for the first time in Stachys rosea (Labiatae) [59] , being the present study the first report of this compound in the Sapindaceae family. However, some clerodanes have recently been reported on this family, particularly in the species Dodonaea viscosa [60] [61] . Owing to the little quantity obtained of this compound, no bioassays were developed assessing its activity. Concerning the activity of this compound against insects, to our knowledge, there is in literature only one report of its activity against E. paenulata [62] . Moreover, the group of the clerodanes has been described mainly from Lamiaceae and Asteraceae, and they have exhibited a wide range of anti-insect properties as it has been reviewed previously [63] -[65] . Seeing that this compound was isolated in the present study from a fraction (fraction 4; Table 2) active against E. paenulata and M. persicae, it could be suggested that 6 is at least in part, responsible of such activity.
4. Conclusions
In summary, A. edulis is here reported to produce various natural products with anti insect activity which seems to present in some cases synergic effects and in other antagonic effects. Besides, these products exhibited differential activity against the insects tested, a desirable attribute when developing botanical pesticides.
Further studies on the optimization of the extraction of bioactive compounds from this vegetal species should be developed, in order to complete the description of the anti insect activity of natural products from this plant.
Acknowledgements
The authors would like to acknowledge Professor E. Alonso Paz for the identification of the vegetal material and Dr. Gabriela M. Cabrera (Departamento de Química Orgánica, UMyMFOR-CONICET, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Argentina) for the acquisition of the mass spectra. The awards of postgraduate scholarships from LATU-FQ and ANII are specially acknowledged by the author M. D. The work developed in Spain was supported by PEDECIBA, CSIC and OPCW.
References
- Philogène, C., Regnault-Roger, C. and Vincent, C. (2005) Botanilcals: Yesterday’s and Today’s Promises. In: Regnault-Roger, C., Philogène, B.J.R. and Vincent, C., Eds., Biopesticides of Plant Oirigin, Intercept Ltd, Paris, 1-15.
- Bentancourt, C.M. and Scatoni, I.B. (1999) Guía de Insectos y Ácaros de Importancia Agrícola y Forestal en el Uruguay.
- Marwah, R.G., Fatope, M.O., Al Mahrooqi, R., Varma, G.B., Al Abadi, H. and Al-Burtamani, S.K.S. (2007) Antioxidant Capacity of Some Edible and Wound Healing Plants in Oman. Food Chemistry, 101, 465-470. http://dx.doi.org/10.1016/j.foodchem.2006.02.001
- Sofidiya, M.O., Jimoh, F.O., Aliero, A.A., Afolayan, A.J., Odukoya, O.A. and Familoni, O.B. (2012) Evaluation of Antioxidant and Antibacterial Properties of Six Sapindaceae Members. Journal of Medicinal Plants Research, 6, 154- 160.
- Bradacs, G., Maes, L. and Heilmann, J. (2010) In Vitro Cytotoxic, Antiprotozoal and Antimicrobial Activities of Medicinal Plants from Vanuatu. Phytoterapy Research, 24, 800-809.
- Hoffmann-Bohm, K., Lotter, H., Seligmann, O. and Wagner, H. (1992) Antihepatotoxic C-Glycosylflavones from the Leaves of Allophyllus edulis var. edulis and gracilis. Planta Medica, 58, 544-548. http://dx.doi.org/10.1055/s-2006-961546
- Arisawa, M., Morinaga, Y., Nishi, Y., Ueno, H., Suzuki, S., Hayashi, T., Shimizu, M., Yoshizaki, M., Morita, N. and Berganza, L.H. (1989) Chemical and Pharmaceutical Studies on Medicinal Plants in Paraguay. Constituents of Angiotensin Converting Enzyme Inhibitory Fraction from “Cocu,” Allophylus edulis Radlk. Shoyakugaku Zasshi, 43, 78-80.
- Dharmani, P., Mishra, P.K., Maurya, R., Chauhan, V.S. and Palit, G. (2005) Allophylus serratus: A Plant with Potential Anti-Ulcerogenic Activity. Journal of Ethnopharmacology, 99, 361-366. http://dx.doi.org/10.1016/j.jep.2005.01.011
- Yajia, M.E., Marti, D.A., Bidau, C.J., Amat, A.G., Riglos, A.G. and Silvestroni, A. (1999) Genotoxicity Evaluation of Allophylus edulis (Camb.) Radlk. (Sapindaceae) Aqueous Extract. Second World Congress on Medicinal and Aromatic Plants for Human Welfare Wocmap-2: Pharmacognosy, Pharmacology, Phytomedicines, Toxicology, 501, 31-35.
- Körbes, V.C. (1995) Plantas Medicinais. 48th Edition.
- Castillo, L., González-Coloma, A., González, A., Díaz, M., Santos, E., Alonso-Paz, E., Bassagoda, M.J. and Rossini, C. (2009) Screening of Uruguayan Plants for Deterrent Activity against Insects. Industrial Crops and Products, 29, 235- 240. http://dx.doi.org/10.1016/j.indcrop.2008.05.004
- Aichholz, R., Spitzer, V. and Lorbeer, E. (1997) Analysis of Cyanolipids and Triacylglycerols from Sapindaceae Seed Oils with High-Temperature Gas Chromatography and High-Temperature Gas Chromatography-Chemical Ionization Mass Spectrometry. Journal of Chromatography A, 787, 181-194. http://dx.doi.org/10.1016/S0021-9673(97)00650-X
- Braekman, J.C., Daloze, D. and Pasteels, J.M. (1982) Cyanogenic and Other Glucosides in a Neo-Guinean Bug Leptocoris isolata. Possible Precursors in Its Host Plant. Biochemical Systematics and Ecology, 10, 355-3664. http://dx.doi.org/10.1016/0305-1978(82)90010-2
- Díaz, M., González, A., Castro-Gamboa, I., Gonzáles, D. and Rossini, C. (2008) First Record of L-Quebrachitol in Allophylus edulis (Sapindaceae). Carbohydrate Research, 343, 2699-2700. http://dx.doi.org/10.1016/j.carres.2008.07.014
- Wagner, H. and Bladt, S. (1996) Plant Drug Analysis. A Thin Layer Chromatography Atlas.
- Gutierrez, C., Fereres, A., Reina, M., Cabrera, R. and Gonzalez-Coloma, A. (1997) Behavioral and Sublethal Effects of Structurally Related Lower Terpenes on Myzus persicae. Journal of Chemical Ecology, 23, 1641-1650. http://dx.doi.org/10.1023/B:JOEC.0000006428.00568.c5
- Bellomo, A., Camarano, S., Rossini, C. and Gonzalez, D. (2009) Enantiospecific Synthesis and Insect Feeding Activity of Sulfur-Containing Cyclitols. Carbohydrate Research, 344, 44-51. http://dx.doi.org/10.1016/j.carres.2008.09.026
- Lowry, R. (2011) VassarStats Web Site for Statistical Computation. http://faculty.vassar.edu/lowry/VassarStats.html
- Reina, M., Nold, M., Santana, O., Orihuela, J.C. and Gonzalez-Coloma, A. (2002) C-5-Substituted Antifeedant Silphinene Sesquiterpenes from Senecio palmensis. Journal of Natural Products, 65, 448-453. http://dx.doi.org/10.1021/np0105533
- Ragasa, C.Y., Ganzon, J., Hofilena, J., Tamboong, B. and Rideout, J.A. (2003) A New Furanoid Diterpene from Caesalpinia pulcherrima. Chemical & Pharmaceutical Bulletin, 51, 1208-1210. http://dx.doi.org/10.1248/cpb.51.1208
- Aguiar, J.C.D., Santiago, G.M.P., Lavor, P.L., Veras, H.N.H., Ferreira, Y.S., Lima, M.A.A., Arriaga, A.M.C., Lemos, T.L.G., Lima, J.Q., de Jesus, H.C.R., Alves, P.B. and Braz-Filho, R. (2010) Chemical Constituents and Larvicidal Activity of Hymenaea courbaril Fruit Peel. Natural Product Communications, 5, 1977-1980.
- da Silva Gois, R.W., de Sousa, L.M., Lemos, T.L.G., Arriaga, A.M.C., Andrade-Neto, M., Santiago, G.M.P., Ferreira, Y.S., Alves, P.B. and de Jesusc, H.C.R. (2011) Chemical Composition and Larvicidal Effects of Essential Oil from Bauhinia acuruana (Moric) against Aedes aegypti. Journal of Essential Oil Research, 23, 59-62. http://dx.doi.org/10.1080/10412905.2011.9700484
- Ben Jemaa, M.J., Tersim, N., Toudert, K.T. and Khouja, M.L. (2012) Insecticidal Activities of Essential Oils from Leaves of Laurus nobilis L. from Tunisia, Algeria and Morocco, and Comparative Chemical Composition. Journal of Stored Products Research, 48, 97-104. http://dx.doi.org/10.1016/j.jspr.2011.10.003
- Li, W.Q., Jiang, C.H., Chu, S.S., Zuo, M.X. and Liu, Z.L. (2010) Chemical Composition and Toxicity against Sitophilus zeamais and Tribolium castaneum of the Essential Oil of Murraya exotica Aerial Parts. Molecules, 15, 5831-5839. http://dx.doi.org/10.3390/molecules15085831
- Yang, K., Zhou, Y.X., Wang, C.F., Du, S.S., Deng, Z.W., Liu, Q.Z. and Liu, Z.L. (2011) Toxicity of Rhododendron anthopogonoides Essential Oil and Its Constituent Compounds towards Sitophilus zeamais. Molecules, 16, 7320-7330. http://dx.doi.org/10.3390/molecules16097320
- Cantrell, C.L., Klun, J.A., Bryson, C.T., Kobaisy, M. and Duke, S.O. (2005) Isolation and Identification of Mosquito Bite Deterrent Terpenoids from Leaves of American (Callicarpa americana) and Japanese (Callicarpa japonica) Beautyberry. Journal of Agricultural and Food Chemistry, 53, 5948-5953. http://dx.doi.org/10.1021/jf0509308
- Aciole, S.D.G., Piccoli, C.F., Duque, J.E., Costa, E.V., Navarro-Silva, M.A., Marques, F.A., Maia, B., Pinheiro, M.L.B. and Rebelo, M.T. (2011) Insecticidal Activity of Three Species of Guatteria (Annonaceae) against Aedes aegypti (Diptera: Culicidae). Revista Colombiana De Entomologia, 37, 262-268.
- de Moraes, S.M., Facundo, V.A., Bertini, L.M., Cavalcanti, E.S.B., dos Anjos, J.F., Ferreira, S.A., de Brito, E.S. and de Souza Neto, M.A. (2007) Chemical Composition and Larvicidal Activity of Essential Oils from Piper Species. Biochemical Systematics and Ecology, 35, 670-675. http://dx.doi.org/10.1016/j.bse.2007.05.002
- Cetin, H., Yanikoglu, A. and Cilek, J.E. (2011) Larvicidal Activity of Selected Plant Hydrodistillate Extracts against the House Mosquito, Culex pipiens, a West Nile Virus Vector. Parasitology Research, 108, 943-948. http://dx.doi.org/10.1007/s00436-010-2136-z
- Dua, V.K., Alam, M.F., Pandey, A.C., Rai, S., Chopra, A.K., Kaul, V.K. and Dash, A.P. (2008) Insecticidal Activity of Valeriana jatamansi (Valerianaceae) against Mosquitoes. Journal of the American Mosquito Control Association, 24, 315-318. http://dx.doi.org/10.2987/5642.1
- Dua, V.K., Pandey, A.C. and Dash, A.P. (2010) Adulticidal Activity of Essential Oil of Lantana camara Leaves against Mosquitoes. Indian Journal of Medical Research, 131, 434-439.
- Zhu, L. and Tian, Y.J. (2011) Chemical Composition and Larvicidal Activity of Blumea densiflora Essential Oils against Anopheles anthropophagus: A Malarial Vector Mosquito. Parasitology Research, 109, 1417-1422. http://dx.doi.org/10.1007/s00436-011-2388-2
- Bachrouch, O., Mediouni-Ben Jemaa, J., Wissem, A.W., Talou, T., Marzouk, B. and Abderraba, M. (2010) Composition and Insecticidal Activity of Essential Oil from Pistacia lentiscus L. against Ectomyelois ceratoniae Zeller and Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Journal of Stored Products Research, 46, 242-247. http://dx.doi.org/10.1016/j.jspr.2010.07.001
- Alva, M., Popich, S., Borkosky, S., Cartagena, E. and Bardon, A. (2012) Bioactivity of the Essential Oil of an Argentine Collection of Acanthospermum hispidum (Asteraceae). Natural Product Communications, 7, 245-248.
- Chu, S.S., Liu, Q.R., Jiang, G.H. and Liu, Z.L. (2012) Chemical Composition and Insecticidal Activity of the Essential Oil of Amethystea caerulea L. Natural Product Research, 26, 1207-1212. http://dx.doi.org/10.1080/14786419.2010.547195
- Francois, T., Michel, J.D.P., Lambert, S.M., Ndifor, F., Vyry, W.N.A., Henri, A.Z.P. and Chantal, M. (2009) Comparative Essential Oils Composition and Insecticidal Effect of Different Tissues of Piper capense L., Piper guineense Schum. et Thonn., Piper nigrum L. and Piper umbellatum L. Grown in Cameroon. African Journal of Biotechnology, 8, 424-431.
- Zoubiri, S. and Baaliouamer, A. (2012) Chemical Composition and Insecticidal Properties of Lantana camara L. Leaf Essential Oils from Algeria. Journal of Essential Oil Research, 24, 377-383. http://dx.doi.org/10.1080/10412905.2012.692910
- Prachayasittikul, S., Suphapong, S., Worachartcheewan, A., Lawung, R., Ruchirawat, S. and Prachayasittikul, V. (2009) Bioactive Metabolites from Spilanthes acmella Murr. Molecules, 14, 850-867. http://dx.doi.org/10.3390/molecules14020850
- Burns, D., Reynolds, W.F., Buchanan, G., Reese, P.B. and Enriquez, R.G. (2000) Assignment of H-1 and C-13 Spectra and Investigation of Hindered Side-Chain Rotation in Lupeol Derivatives. Magnetic Resonance in Chemistry, 38, 488- 493. http://dx.doi.org/10.1002/1097-458X(200007)38:7<488::AID-MRC704>3.0.CO;2-G
- Gonzalez-Coloma, A., Lopez Balboa, C., Santana, O., Reina, M. and Fraga, B.M. (2011) Triterpene-Based Plant Defenses. Phytochemistry Reviews, 10, 245-260. http://dx.doi.org/10.1007/s11101-010-9187-8
- Brimson, J.M., Brimson, S.J., Brimson, C.A., Rakkhitawatthana, V. and Tencomnao, T. (2012) Rhinacanthus nasutus Extracts Prevent Glutamate and Amyloid-Beta Neurotoxicity in HT-22 Mouse Hippocampal Cells: Possible Active Compounds Include Lupeol, Stigmasterol and Beta-Sitosterol. International Journal of Molecular Sciences, 13, 5074- 5097. http://dx.doi.org/10.3390/ijms13045074
- de Oliveira, P.V., Lemos, R.P.L. and Conserva, L.M. (2012) Chemical Constituents of Rourea doniana. Revista Brasileira De Farmacognosia—Brazilian Journal of Pharmacognosy, 22, 451-454. http://dx.doi.org/10.1590/S0102-695X2011005000223
- Dong, L., Zhang, Y., Cheng, B., Wu, Y., Li, Y., Yao, H. and Li, Y. (2012) Chemical Constituents from Ampelopsis sinica var. Hancei Prevent Liver Damage. Latin American Journal of Pharmacy, 31, 195-199.
- dos Santos, E.O., Meira, M., do Vale, A.E., David, J.M., de Queiroz, L.P. and David, J.P. (2012) Isolation and Characterization of New Ceramides from Aerial Parts of Lepidaploa cotoneaster. Natural Product Communications, 7, 781-783.
- Fayek, N.M., Monem, A.R.A., Mossa, M.Y., Meselhy, M.R. and Shazly, A.H. (2012) Chemical and Biological Study of Manilkara zapota (L.) Van Royen Leaves (Sapotaceae) Cultivated in Egypt. Pharmacognosy Research, 4, 85-91. http://dx.doi.org/10.4103/0974-8490.94723
- Keawsa-ard, S., Natakankitkul, S., Liawruangrath, S., Teerawutgulrag, A., Trisuwan, K., Charoenying, P., Pyne, S.G. and Liawruangrath, B. (2012) Anticancer and Antibacterial Activities of the Isolated Compounds from Solanum spirale Roxb. Leaves. Chiang Mai Journal of Science, 39, 445-454.
- Kumari, A. and Kakkar, P. (2012) Lupeol Prevents Acetaminophen-Induced in Vivo Hepatotoxicity by Altering the Bax/Bcl-2 and Oxidative Stress-Mediated Mitochondrial Signaling Cascade. Life Sciences, 90, 561-570. http://dx.doi.org/10.1016/j.lfs.2012.01.012
- Mishra, P.M., Sree, A. and Panigrahi, M. (2012) Isolation of a Lupane Triterpene Fatty Acid Ester with Antibacterial Activity from the Leaves of Finlaysonia obovata. Chemistry of Natural Compounds, 48, 161-163. http://dx.doi.org/10.1007/s10600-012-0191-7
- Sakong, B.M., Ahmed, A.S., McGaw, L.J. and Eloff, J.N. (2012) Isolation and Characterization of Compounds from Calodendrum capense and Lydenburgia cassinoides with Antimicrobial Potential against Opportunistic Pathogens. South African Journal of Botany, 79, 210-210.
- Thoison, O., Sevenet, T., Niemeyer, H.M. and Russell, G.B. (2004) Insect Antifeedant Compounds from Nothofagus dombeyi and N. pumilio. Phytochemistry, 65, 2173-2176. http://dx.doi.org/10.1016/j.phytochem.2004.04.002
- Kojima, H., Sato, N., Hatano, A. and Ogura, H. (1990) Constituents of the Labiatae Plants. 5. Sterol Glucosides from Prunella vulgaris. Phytochemistry, 29, 2351-2355. http://dx.doi.org/10.1016/0031-9422(90)83073-A
- Kolak, U., Topcu, G., Birteksoz, S., Otuk, G. and Ulubelen, A. (2005) Terpenoids and Steroids from the Roots of Salvia blepharochlaena. Turkish Journal of Chemistry, 29, 177-186.
- Santana, O., Reina, M., Fraga, B.M., Sanz, J. and Gonzalez-Coloma, A. (2012) Antifeedant Activity of Fatty Acid Esters and Phytosterols from Echium wildpretii. Chemistry & Biodiversity, 9, 567-576. http://dx.doi.org/10.1002/cbdv.201100083
- Behmer, S.T. and Elias, D.O. (1999) The Nutritional Significance of Sterol Metabolic Constraints in the Generalist Grasshopper Schistocerca americana. Journal of Insect Physiology, 45, 339-348. http://dx.doi.org/10.1016/S0022-1910(98)00131-0
- Hamrouni, I., Chraief, I., Hammami, M. and Marzouk, B. (2005) Sterol Composition and Accumulation in Maturing Borage (Borago officinalis L.) Seeds. Rivista Italiana delle Sostanze Grasse, 82, 83-86.
- Schmidt, J., Spengler, B., Adam, G. and Budzikiewicz, H. (1993) Sterol Constituents in Seeds of Ornithopus sativus. Phytochemistry, 33, 506-507. http://dx.doi.org/10.1016/0031-9422(93)85550-B
- Sivakumar, G., Bati, C.B., Perri, E. and Uccella, N. (2006) Gas Chromatography Screening of Bioactive Phytosterols from Mono-Cultivar Olive Oils. Food Chemistry, 95, 525-528. http://dx.doi.org/10.1016/j.foodchem.2005.04.003
- Tori, M., Katto, A. and Sono, M. (1999) Nine New Clerodane Diterpenoids from Rhizomes of Solidago altissima. Phytochemistry, 52, 487-493. http://dx.doi.org/10.1016/S0031-9422(99)00273-3
- Fazio, C., Passannanti, S., Paternostro, M.P. and Piozzi, F. (1992) Neo-Clerodane Diterpenoids from Stachys rosea. Phytochemistry, 31, 3147-3149. http://dx.doi.org/10.1016/0031-9422(92)83463-9
- Niu, H.M., Zeng, D.Q., Long, C.L., Peng, Y.H., Wang, Y.H., Luo, J.F., Wang, H.S., Shi, Y.N., Tang, G.H. and Zhao, F.W. (2010) Clerodane Diterpenoids and Prenylated Flavonoids from Dodonaea viscosa. Journal of Asian Natural Products Research, 12, 7-14. http://dx.doi.org/10.1080/10286020903407379
- Omosa, L.K., Midiwo, J.O., Derese, S., Yenesew, A., Peter, M.G. and Heydenreich, M. (2010) Neo-Clerodane Diterpenoids from the Leaf Exudate of Dodonaea angustifolia. Phytochemistry Letters, 3, 217-220. http://dx.doi.org/10.1016/j.phytol.2010.08.001
- Vaccarini, C.E., Palacios, S.M., Meragelman, K.M. and Sosa, V.E. (2002) Antifeedant Activity of Metabolites from Viguiera tucumanensis. Natural Product Letters, 16, 323-327. http://dx.doi.org/10.1080/10575630290030711
- Banerjee, H.N. (1936) Clerodin from Clerodendron infortunatum. Science and Culture, 2, 163.
- Klein Gebbinck, E.A., Jansen, B.J.M. and de Groot, A. (2002) Insect Antifeedant Activity of Clerodane Diterpenes and Related Model Compounds. Phytochemistry, 61, 737-770. http://dx.doi.org/10.1016/S0031-9422(02)00174-7
- Sosa, M.E. and Tonn, C.E. (2008) Plant Secondary Metabolites from Argentinean Semiarid Lands: Bioactivity against Insects. Phytochemistry Reviews, 7, 3-24. http://dx.doi.org/10.1007/s11101-006-9056-7
NOTES

*Corresponding author.


