International Journal of Organic Chemistry
Vol. 2 No. 4 (2012) , Article ID: 25892 , 10 pages DOI:10.4236/ijoc.2012.24052
13C NMR and Raman Studies of Fullerene-Based Poly (Acrylamides)
1Department of Physics, Maharana Pratap Engineering College, Kanpur, India
2Department of Chemistry, Christ Church Degree College, Kanpur, India
Email: sup4312@yahoo.com, ashu_dwi@yahoo.com, meetk_dwi@yahoo.co.in
Received July 27, 2012; revised September 3, 2012; accepted September 13, 2012
Keywords: Fullerene; Glass Transition Temperature; Poly (Acrylamide)
ABSTRACT
A new classification of the different types of fullerene-containing polymers is presented according to their different properties and applications they exhibit in a variety of fields. 13C NMR and Raman studies of a series of polymeric samples of fullerene-grafted poly (acrylamide), which were prepared by systematic variation of concentration of fullerene and acrylamide, are described. 13C NMR spectral analysis of the polymeric samples showed a peak for fullerene at 143 δppm and for poly (acrylamide) between 170 and 180 δppm and Raman spectral analysis of the polymeric samples gave the Raman band for fullerene between 1470 cm−1 and poly (acrylamide) at 2800 cm−1. The Tg value, obtained from DSC results, showed a high glass transition temperature at 100.94˚C revealing the presence of fullerene in the polymeric matrix. TGA analysis shows that polymer is thermally stable up to 340˚C.
1. Introduction
A polymer is a large molecule (macromolecule) composed of repeating structural units typically connected by covalent chemical bonds. While polymer in popular usage suggests plastic, the term actually refers to a large class of natural and synthetic materials with a wide variety of properties. The field of synthetic polymers or plastics is currently one of the fastest growing material industries. In addition to these synthetic materials, different types of carbon materials called fullerenes have recently been introduced [1]. Fullerene, in its most stable form C60, exhibits a variety of outstanding electronic, conducting, and magnetic properties [2] and acts as photo sensitizer and photorefractive material [3]. The science of fullerenes continues to be an exciting field, generating many articles with promising new applications every year. A search of the literature reveals that a lot of work has been carried out on synthesis, characterization of fullerenes, and doped fullerenes with alkali metals like potassium and rubidium ternary compounds, [4,5] poly fulleride ions in A C60 [6] (A is potassium, rubidium, or cesium), and fullerene doped with organic cations like morpholine and acridizine [7]. The literature survey also reveals that 13C NMR spectroscopic studies of fullerene and poly-ethylene oxide complexes, [8] magnetic resonance of solid fullerene and their compounds with alkali metals [9], superconductivity and nuclear magnetic resonance investigation of KTl1.5 doped C60 [10] have been carried out in past years. Synthesis and cationic photopolymerization of C60 derivative bearing a 2,4,6-tris(epoxynonyloxy)phenyl moiety (FB9ox) [11] has been carried out. In addition, the synthesis and determination of properties of poly (methyl methacrylate)/clay nanocomposites, prepared via in situ polymerization with nickel acetyl acetonate [Ni(acac)2] catalyst in combination with methyllalumoxane [12] have also been carried out. The literature also reveals Raman spectroscopic studies on pressure-induced polymerization of C60 at high temperature [13], rotation vibrational dynamics of C60 [14] and the vibrational Raman spectra of C60 [15]. High-temperature study of solid C60, pristine was performed by Raman spectroscopy up to 843 K [16]. These data could be used as a reference for in situ studies of C60 chemical transformation occurring at high temperature. Selective multi-addition of organocopper reagents to fullerenes [17] were also carried out. The literature survey shows sufficient study on radical polymerization of vinyl monomers in the form of homopolymer, copolymer, and terpolymer [18-20]. However, spectroscopic studies on fullerenegrafted vinyl monomers are still scarce. The present article therefore highlights 13C NMR and Raman spectroscopic studies of fullerene-grafted poly (acrylamide) polymers. Acrylamide as a monomer is used in a variety of synthetic processes to form polymers, copolymers. It is readily polymerized in the presence of free radicals, usually in aqueous solutions. Polyacrylamide plastics have numerous commercial applications, due in part to the tendency of polyacrylamide to adsorb many times its own mass in water. Feed ratios of C60, monomer & initiator are given in Table 1.
2. Experimental
Acrylamide was purified by standard methods and purified solvents were used as received. Benzoylperoxide (BPO) was recrystallized in chloroform. Fullerene (Alfa Aesar, 99.9% C60) was used as received and was dissolved in toluene as required.
2.1. Polymerization Procedure
The polymerization reactions were carried out under an inert atmosphere of nitrogen for 1.5 hr at 70˚C in toluene using BPO as an initiator. A series of samples of fullerene-grafted poly (acrylamide) were synthesized by systematic variation of fullerene and acrylamide as given in Table 2. The polymer was precipitated in acidified methanol and vaccum dried until a constant weight was obtained.
2.2. Spectral Analysis
1) 13C NMR Spectroscopy: 13C nuclear magnetic resonance (NMR) spectral analysis of fullerene-grafted poly (acrylamide) samples was carried in an ECX 500-JEOL NMR spectrometer using CDCl3 as a solvent.
2) Raman Spectroscopic Studies: Raman spectra of fullerene-based poly (acrylamide) were recorded by NSOM (near-field scanning optical microscope) using He-Ne laser having wavelength 514.7 nm.
3) FT-IR: The FTIR spectrum of formed polymer was recorded on vertex 70 (Bruker) instrument using KBr pellet.
2.3. Thermal Analysis
Differential scanning calorimetry was carried out on a V2.2 Dupont calorimeter, under nitrogen atmosphere at a heating rate of 10˚C/min. The sample weight was 3 - 5 mg.
2.4. Thermogravamatric Analysis
Thermogravimetric analysis was carried on TGA V5 1A DuPont 2100 at heating rate of 10˚C/min under nitrogen atmosphere.
3. Results and Discussion
13C NMR spectrum for pure fullerene (C60) shows a peak at 138 δppm (Figure 1) and that for poly (acrylamide)

Table 1. Feed ratios of C60, monomer & initiator.

Table 2. Variation of fullerene with poly (acrylamide).
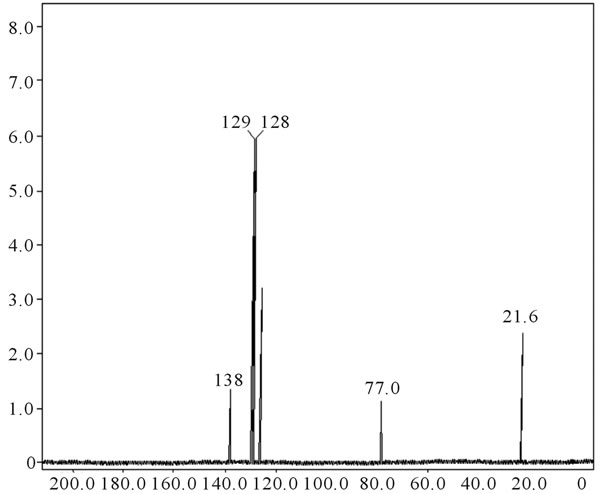
Figure 1. 13C NMR of sample pure fullerene (C60).
shows at 180 δppm (Figure 2) while 13C NMR spectrum for fullerene based poly (acrylamide) shows a peak of fullerene at 143 and 145 δppm, which is due to proton decoupling. Acrylamide group exists between 170 to 176 δppm. Peak values corresponding to CDCl3 were obtained between 76 and 77 δppm, equatorial positions of carbon atoms were obtained between 125 and 129 δppm. Peak values corresponding to CH2 were obtained between 36 and 38 δppm. Cα group (CH of acrylamide) exists between 40 to 46 δppm. Further polymeric samples were obtained by systematic variation of fullerene and poly (acrylamide). These samples were studied by 13C NMR spectroscopy, these spectra are given in Figures 1-8 and their comparison is given in Table 3. From the observed data, we find that there is a shift in peak values of poly (acrylamide) and fullerene, thereby showing incorporation of the polymer formed on the fullerene surface, which increases the solubility and processability of the polymer network.Raman spectroscopy yields important information about the structure of macromolecules. Raman spectrum for fullerene and polyacrylamide has been observed (Figures 9 and 10). We are able to determine the orientation and conformation of polymers and the density of vibrational states by using this technique. As observed in our Raman studies the polymer system of C60 shows Ag(1) mode above 500 cm−1 and Ag(2) mode between 1470 cm−1 and 1495 cm−1, where as the pure fullerene shows Ag(1) mode at 500 cm−1 sharp and Ag(2) mode at 1447 cm−1 sharp. Similarly all the Hg mode are not observed in the polymeric system (which are observed in the pure fullerene) A strong band of fullerene and poly (acrylamide) interaction is manifested as the pronounced shift of fullerene between 1400 - 1495 cm−1, which is usually obtained at about 1466 - 1470 cm−1. A strong band between 2900 and 3000 cm−1 shows the presence of CH2 stretching groups (Figures 11-15). There is no band at 1900 - 2400 cm−1, which indicates that the double bond of poly (acrylamide) is not present, i.e., poly (acrylamide) has been polymerized. Also, a band at 1740 - 1790 cm−1 shows the presence of carbonyl group of amide (Figures 11-15), a band between 1210 1295 cm−1 shows the presence of NH2 group. The near surface region of our samples, sampled by Raman spectroscopy seems to be even more inhomogeneous, differences were observed between different grains in some samples 21, comparative data of Raman spectra is given in Table 4. Differential scanning calorimetry (DSC) result shows a glass transition temperature (Tg) of polyacrylamide at 161˚C but glass transition temperature (Tg) of fullerene based poly (acrylamides) at 100.94˚C (Figures 16 and 17), The high value of Tg shows that the poly (acrylamide) has been incorporated on the C60 framework. The thermogravametric analysis of polyacrylamide and fullerene based poly (acrylamides) showed almost same results that the olymer is thermally
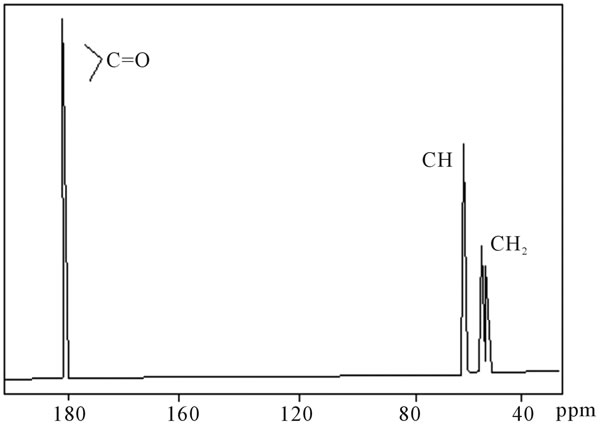
Figure 2. 13C NMR of sample polyacrylamide.
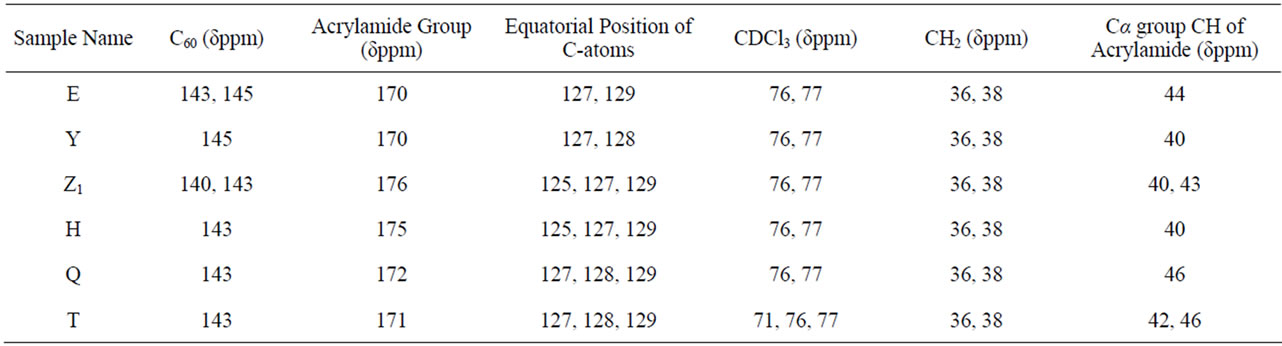
Table 3. 13C Nuclear magnetic resonance (NMR) spectral analysis of fullerene-grafted poly (acrylamide).
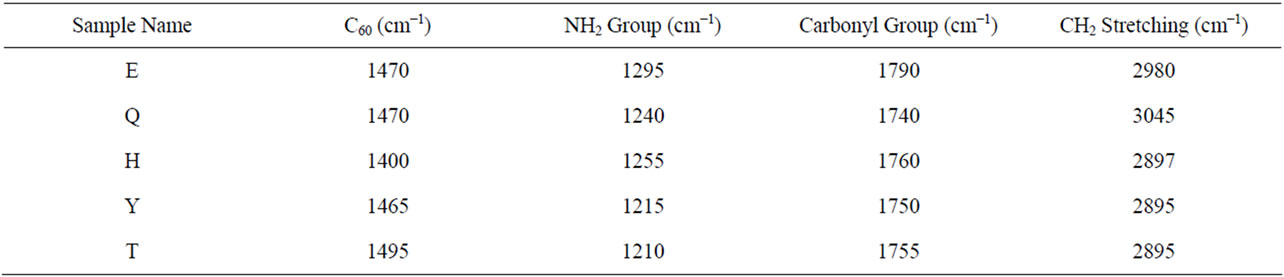
Table 4. Raman shifts of fullerene based poly (acrylamide).

Figure 3. 13C NMR of sample Z1.
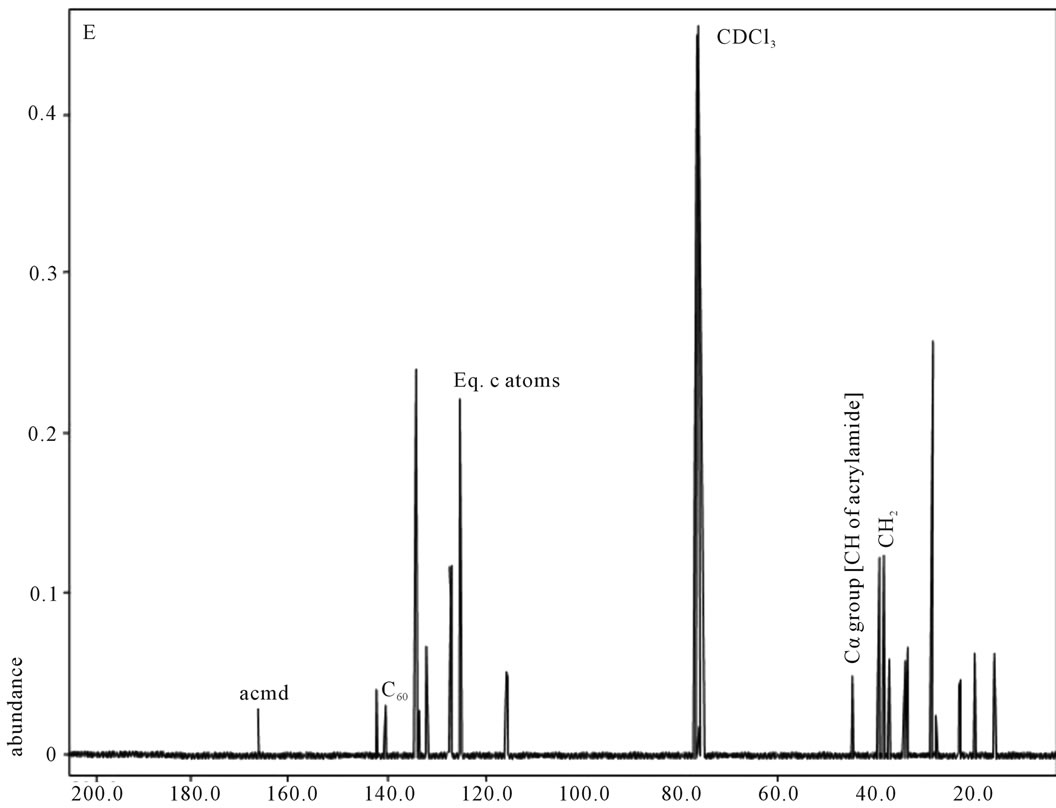
Figure 4. 13C NMR of sample E.
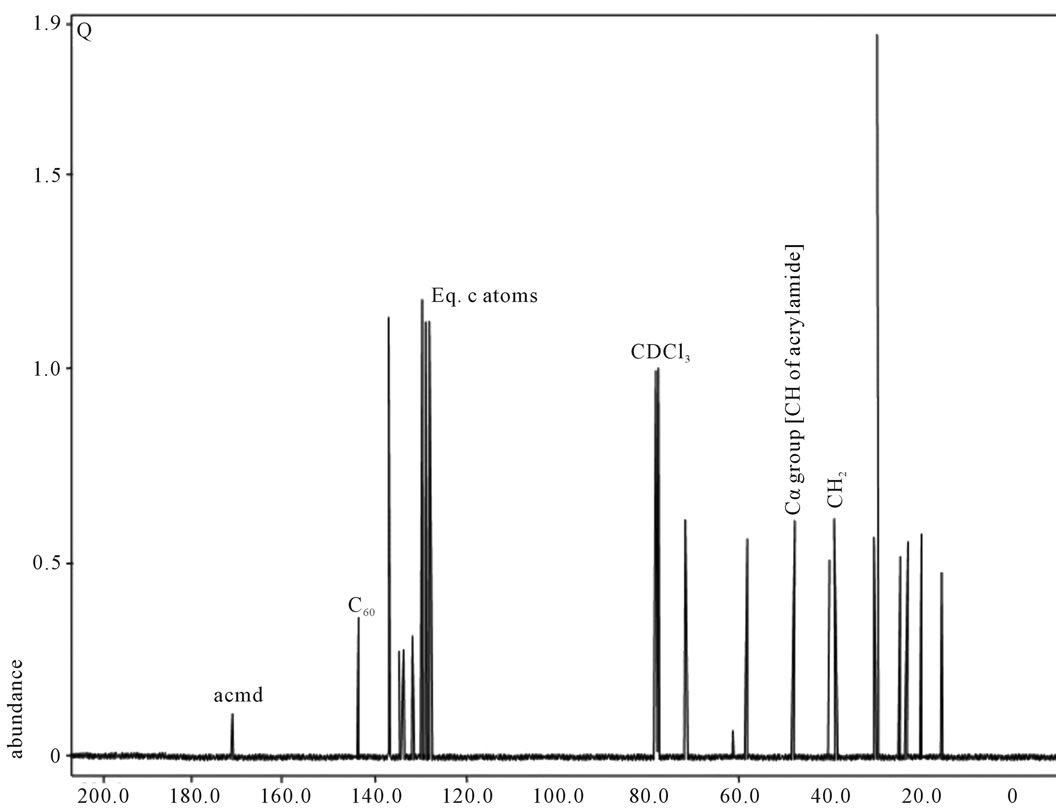
Figure 5. 13C NMR of sample Q.

Figure 6. 13C NMR of sample H.
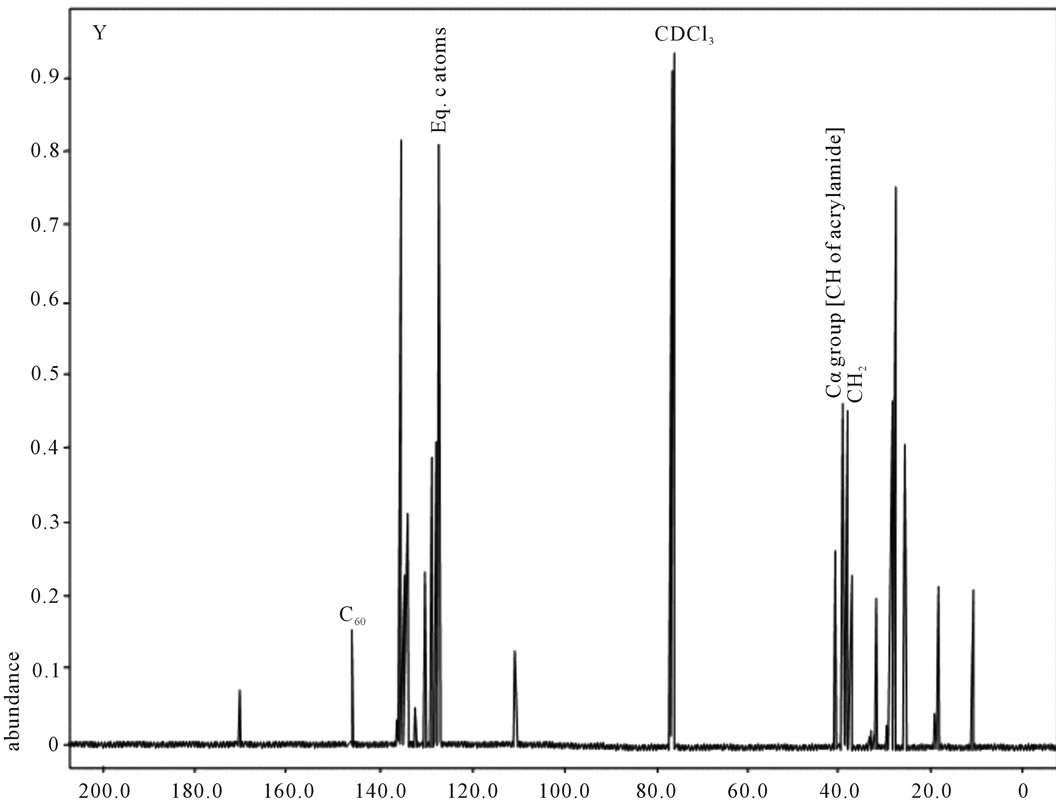
Figure 7. 13C NMR of sample Y.
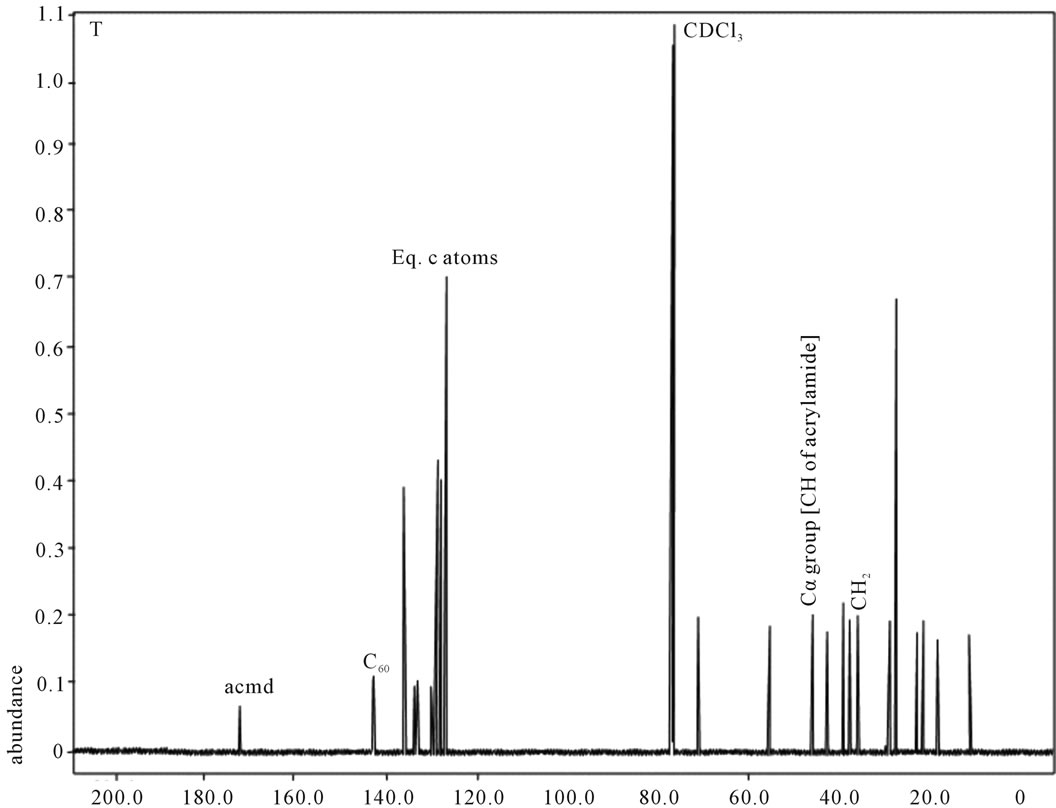
Figure 8. 13C NMR of sample T.
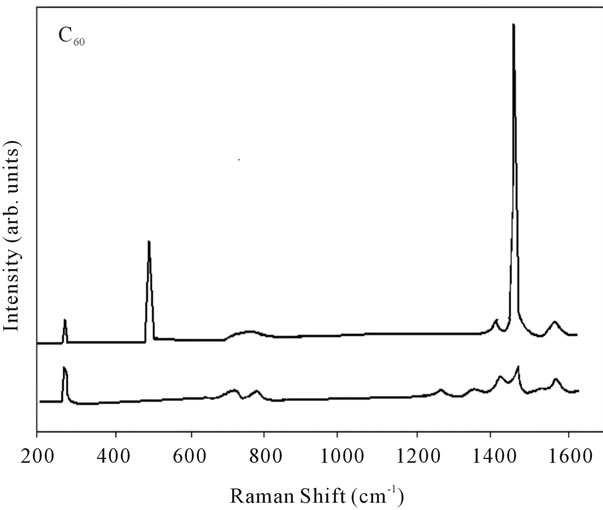
Figure 9. Raman spectra of pure fullerene (C60).

Figure 10. Raman spectra of polyacrylamide.
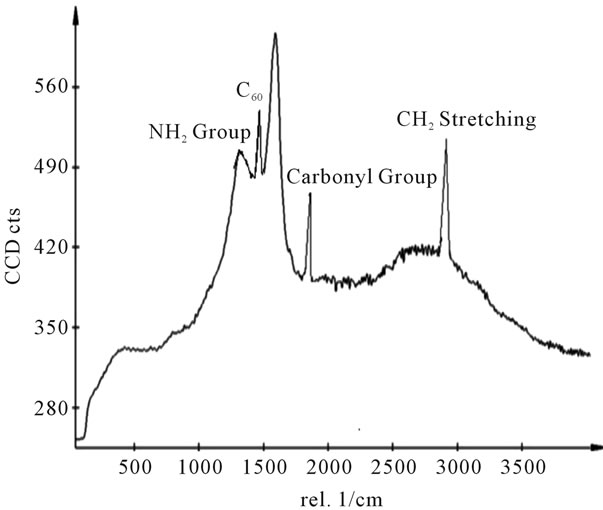
Figure 11. Raman spectra of sample E.

Figure 12. Raman spectra of sample Q.
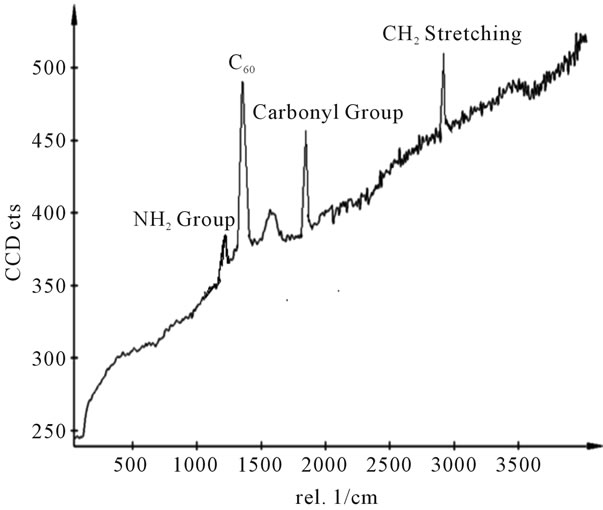
Figure 13. Raman spectra of sample H.
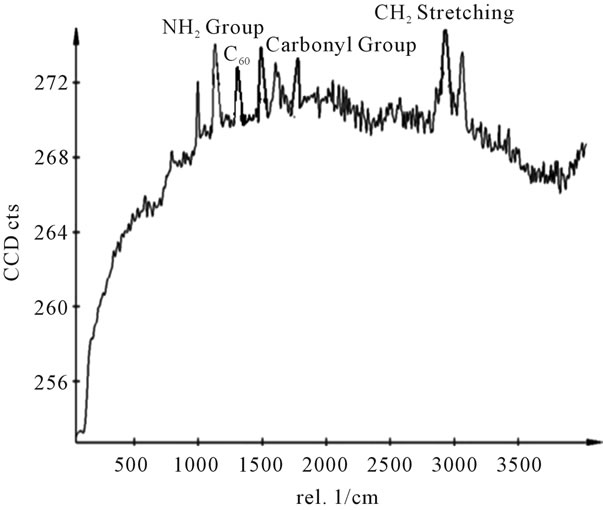
Figure 14. Raman spectra of sample Y.

Figure 15. Raman spectra of sample T.
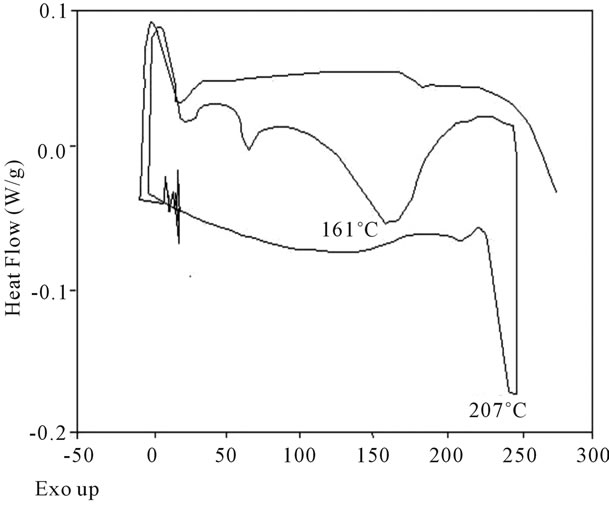
Figure 16. DSC Analysis of polyacrylamide.
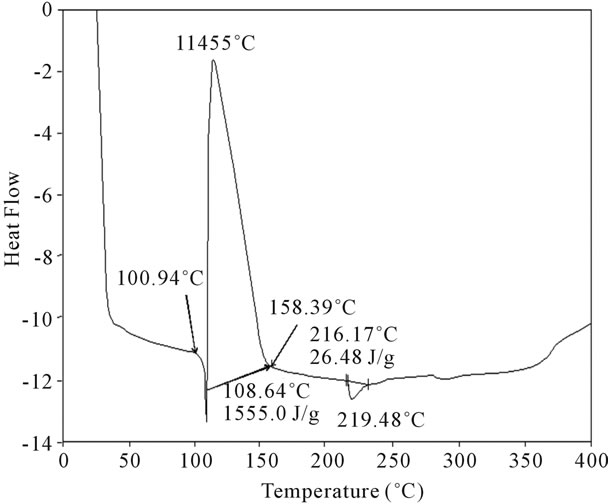
Figure 17. DSC Analysis of fullerene based poly (acrylamide).
stable upto 340˚C (Figures 18 and 19). FTIR spectrum of polyacrylamide (Figure 20), NH2 stretching vibrations of polyacrylamide and fullerene based poly (acrylamides) were observed at 3353 cm−1 and 3421.2 cm−1 respectively. The carbonyl group (C=O) stretching vibrations of poly (acrylamide) and fullerene based poly (acrylamides) were observed at 1658 cm−1 and 1784.78 cm−1 respectively (Figure 21). The whole reaction scheme for prepared fullerene based poly (acrylamides) shows in Figure 22.
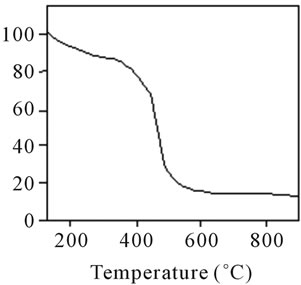
Figure 18. TG analysis of polyacrylamide.
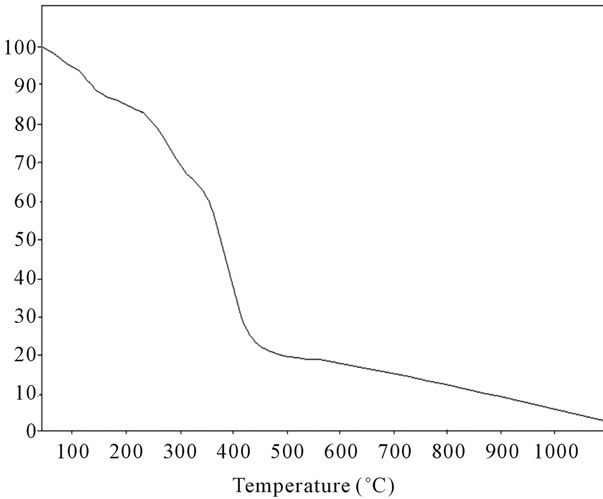
Figure 19. TG analysis of fullerene based poly (acrylamide).
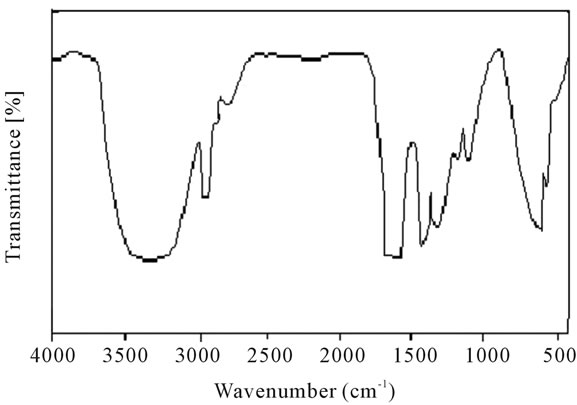
Figure 20. FT-IR spectrum of polyacrylamide.
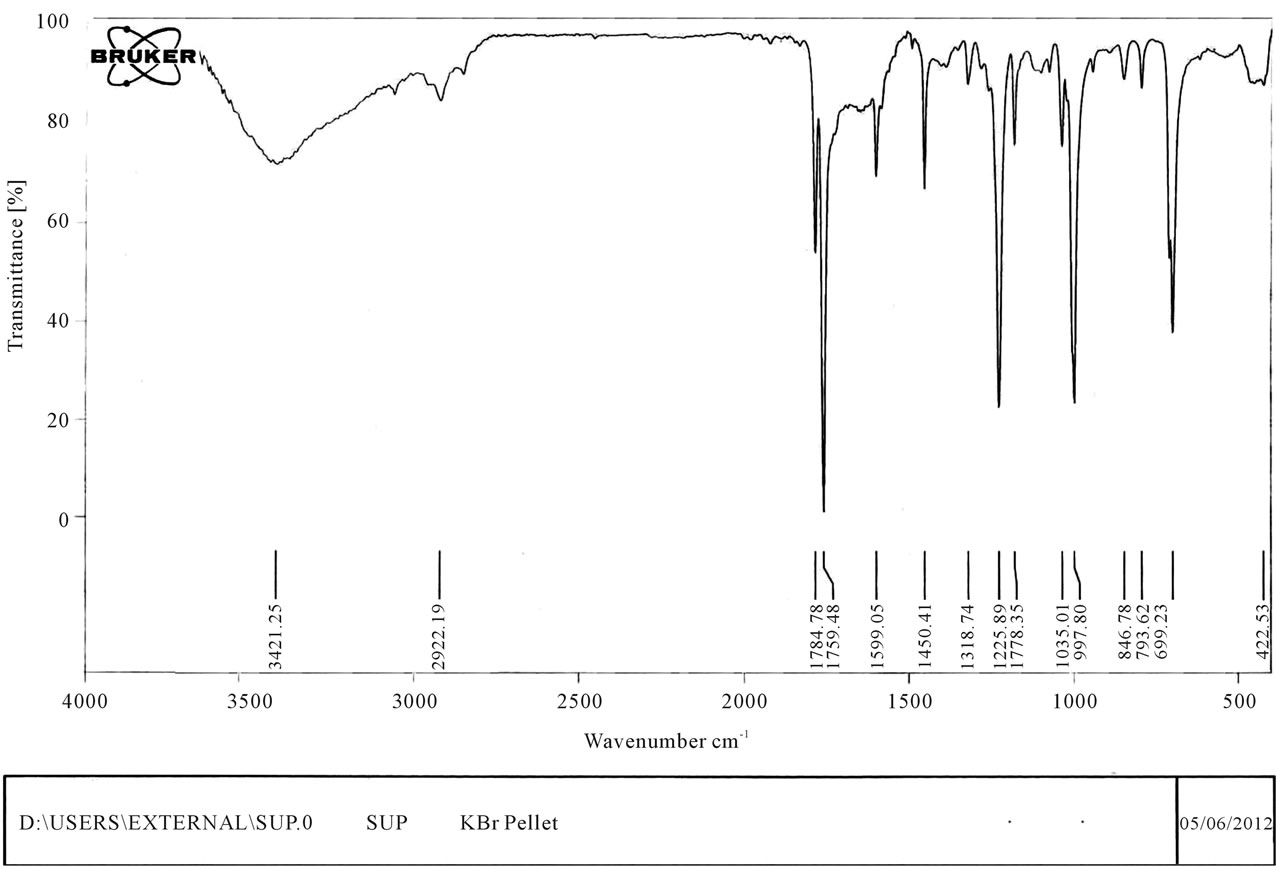
Figure 21. FT-IR spectrum of fullerene based poly (acrylamide).
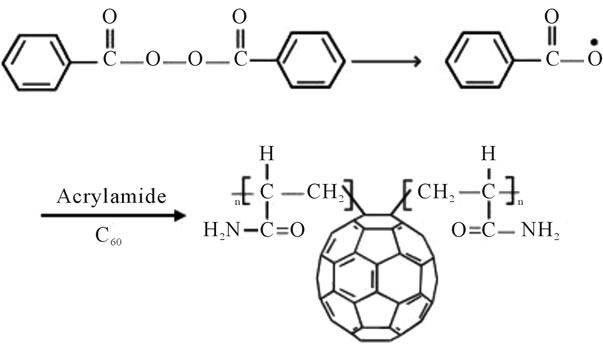
Figure 22. Reaction scheme for prepared fullerene based poly (acrylamides).
4. Conclusions
The present result obtained indicates that 13C NMR method employed in this work is suitable for investigating the interaction among pure C60, polyacrylamide and fullerene based poly (acrylamide) samples [22] which show there is a large shift occurs in the peak values of polymeric samples. In our study also the peak values obtained from 13C analysis, shows that there exist interactions between carbonyl group of polyacrylamide and the π-system of C60. This interaction may be due to the rapid isotropic rotation of C60. Raman study is governed by strict selection rule. The free isolated C60 molecule exhibit (two Ag + eight Hg) Raman allowed modes.
When C60 interacts with the acrylamide forming polymeric system, the symmetry of C60 is lowered signifycantly. It means the Raman study interpretates there is incorporation of acrylamide in fullerene thereby forming the polymer matrix. DSC and TGA analysis show that polymer is thermally stable. In all these studies definitely we observed the different peak values in prepared fullerene based poly (acrylamides) in comparison to pure fullerene and pure poly (acrylamide) which is due to the polymerization process.
REFERENCES
- H. Kroto, “Introduction to Special Issue on Fullerenes,” Carbon, Vol. 30, No. 8, 1992, pp. 1139-1141. doi:10.1016/0008-6223(92)90056-3
- L. A. Zheng, E. V. Barrera and R. D. Shull, “Formation and Stabilization of Nanosize Grains in Ferromagnetic Thin Films by Dispersed C60,” Journal of Applied Physics, Vol. 92, 2002, p. 523. doi:10.1063/1.1481204
- M L. artin-Gomis, J. Ortiz, F. Fernández-Lazaro, A. SastreSantos, B. Elliot and L. Echegoyen, “Synthesis and Characterization of New Trinitroflourene-Fullerene Dyads as Photosensitizers in Photorefractive Polymer Materials. Redox Behavior and Charge Transfer Properties,” Tetrahedron, Vol. 62, No. 9, 2006, pp. 2102-2109.
- H. Alloul, K. Holczer, Y. Yoshinari and O. Klein, “39K NMR Study of Phase Transitions and Electronic Properties in K3C60,” Physica C, Vol. 235, 1994, pp. 2509-2510. doi:10.1016/0921-4534(94)92475-9
- H. Alloul, K. Halczer, Y. Yoshinari and L. Forro, “NMR Study of RbC60,” Physica C, Vol. 235, 1994, pp. 2479-2480. doi:10.1016/0921-4534(94)92460-0
- S. Pekker, G. Oszlanyi and G. Faigel, “Structure and Stability of Covalently Bonded Polyfulleride Ions in AxC60 Salts,” Chemical Physics Letters, Vol. 282, 1998, pp. 435-441. doi:10.1016/S0009-2614(97)01248-7
- R. Zuzok, P. Wzietek, M. Fourmigue, P. Batail, P. Auban-Senzier, S. Roth and D. Jerome, “C60 Doped with Organic Cations: Magnetic Resonance Measurements,” Synthetic Metals, Vol. 56, No. 2-3, 1993, pp. 3235-3239. doi:10.1016/0379-6779(93)90108-9
- M. Li and Q. Chen, “Interactions between Fullerene (C60) and Poly(Ethylene Oxide) in Their Complexes as Revealed by High-Resolution Solidstate 13C NMR Spectroscopy,” Polymer, Vol. 44, No. 9, 2003, pp. 2793-2798. doi:10.1016/S0032-3861(03)00180-0
- K. Mizoguchi, “Magnetic Resonance of Fullerene Solids and Their Compounds,” Journal of Physics and Chemistry of Solids, Vol. 54, No. 12, 1993, pp. 1693-1698. doi:10.1016/0022-3697(93)90284-X
- M. Krous, J. Freyteg, S. Gartner, H. M. Vieth, W. Kratschmer and K. Luders, “Superconductivity and NMR Investigations of KTI1_5-Doped C60,” Zeitschrift fur Physik B: Condensed Matter, Vol. 85, 1991, p. 1. doi:10.1007/BF01387780
- K. Lintinen, A. Efimov, S. Hietala, S. Nagao, P. Jalkanen, N. Tkachenko and H. Lemmetyinen, “Cationic Photopolymerization of Liquid Fullerene Derivative under Visible Light,” Journal of Polymer Science Part A: Polymer Chemistry, Vol. 46, No. 15, 2008, p. 5194.
- L. Cui, N. H. Tarte and S. I. Woo, “Synthesis and Properties of Poly(Methylmethacrylate)/Clay Nanocomposites Prepared via in Situ Polymerization with Ni(acac)2 Catalyst,” Journal of Applied Polymer Science, Vol. 110, No. 2, 2008, pp. 784-790. doi:10.1002/app.28492
- A. V. Talyzin, L. S. Dubrovinsky, T. Le Bihan and U. Jansson, “Pressure-Induced Polymerization of C60 at High Temperatures: An in Situ Raman Study,” Physical Review B, Vol. 65, 2002, Article ID: 245413. doi:10.1103/PhysRevB.65.245413
- P. M. Rajailov, V. G. Hadjiev, A. R. Goni and C. Thomsen, “Rotation-Vibrational Dynamics of Solid C60: A Raman Study,” Physical Review B, Vol. 60, 1999, Article ID: 13351. doi:10.1103/PhysRevB.60.13351
- T. J. Dennis, J. P. Hare, H. W. Kroto, R. Taylor, D. R. M. Walton and P. J. Hendra, “The Vibrational Raman Spectra of C60 and C70,” Spectrochimica Acta, Vol. 47, 1991, pp. 1289-1292. doi:10.1016/0584-8539(91)80217-7
- A. V. Talyzin, A. Dzwilewski and T. Wagberg, “Temperature Dependence of C60 Raman Spectra up to 840 K,” Solid State Communication, Vol. 140, 2006, pp. 178-181. doi:10.1016/j.ssc.2006.07.043
- Y. Matsuo and E. Nakamura, “Selective Multiaddition of Organocopper Reagents to Fullerenes,” Chemical Review, Vol. 108, No. 8, 2008, pp. 3016-3028. doi:10.1021/cr0684218
- Y.-L. Liu, M.-C. Tseng and M.-H. Fangchiang, “Polymerization and Nanocomposites Properties of Multifunctional Methylmethacrylate POSS,” Journal of Polymer Science Part A: Polymer Chemistry, Vol. 46, No. 15, 2008, pp. 5157-5166.
- B. P. Agarwal and A. K. Srivastava, “Radical Terpolymerization by Zinc Acrylate, Acrylonitrile and Styrene Initiated by Styrene Arsenic Sulfide Complex,” Polymer Engineering & Science, Vol. 34, No. 6, 1994, pp. 528-531.
- U. Bhatnagar and A. K. Srivastava, “Metalylide Complex Initiated Radical Copolymerization of Methylmethacrylate and Styrene,” Journal of Macromolecular Science: Pure and Applied Chemistry, Vol. A29, No. 4-5, 1992, pp. 357-370. doi:10.1080/10101329208052166
- R. Murugan, S. Mohan and A. Bigotto, “FTIR and Polarized Raman Spectra of Acrylamide and Polyacrylamide,” Journal of the Korean Physical Society, Vol. 32, No. 4, 1998, pp. 505-512.
- S. Porwal, A. Dwivedi and M. Kamal, “13C NMR and Raman Studies of Fullerene-Based Poly(Methyl Methacrylate) Polymers,” International Journal of Polymer Analysis and Characterization, Vol. 14, 2009, pp. 551-562. doi:10.1080/10236660903086185

