Paper Menu >>
Journal Menu >>
 World Journal of Neuroscience, 2011, 1, 31-37 WJNS doi:10.4236/wjns.2011.13005 Published Online November 2011 (http://www.SciRP.org/journal/wjns/). Published Online November 2011 in SciRes. http://www.scirp.org/journal/WJNs Chronic effect of olive oil on some neurotransmitter contents in different brain regions and physiological, histological structure of liver and kidney of male albino rats A. E. Bawazir Department of Zoology, King Abdul-Aziz University Faculty of Science, Jeddah, Kingdom of Saudi Arabia. Email: maiibriaum@yahoo.com Received 30 April 2011; revised 7 June 2011; accepted 5 September 2011. ABSTRACT Olive oil is an important source of mono-unsaturated fat and a prime component of the Mediterranean diet. The beneficial health effects of olive oil are due to both its high content of mono-unsaturated fatty acids and its high content of anti-oxidative substances. The objective of this study was to investigate the basis for the epidemiological information relating to the health benefits associated with the consumption of ex- tra-virgin olive oil (EVOO). The effect of olive oil on norepinephrine (NE), dopamine (DA), serotonin (5-HT) and gamm-aminobutyric acid (GABA) con- tents in different brain regions and histological structure of liver and kindey of male albino rats was studied. The chronic administration of olive oil (7.5 mg/kg body wt.) caused a significant increase in norepinephrine (NE), dopamine (DA) , serotonin (5-HT) and gamm-aminobutyric acid (GABA) con- tent in different brain regions (Cerebellum, striatum, cerebral cortex, hypothalamus, brain steam and hip- pocampus) of male albino rats. The increase in NE, DA, 5-HT, and GABA content in the different CNS areas of male albino rat may be due to the inhibition of Ca2+/calmodulin binding which plays an important role in the release of these neurotransmitters. The results, also, revealed that urea and creatinne con- centrations in rats with oral administration with olive oil were decreased. Meanwhile, the activities of the enzymes AsT, AlT and ALP were elevated. The pre- sent results indicated that there is no change in tis- sues of kidney after treated with virgin olive oil. Olive oil may potentially be safe for use as a sedative drug. improvement also led to the reductions in risk of Alzheimer’s and Parkinson’s diseases. Keywords: Olive Oil; NE, DA, 5-HT; GABA; Brain Re- gions; Histological Structure of Liver and Kidney; Male Albino Rats; 1. INTRODUCTION Olive (Olea europaea L.) oil is a fundamental component of the Mediterranean diet (Serra-Majem et al., 2003). In the last few decades there has been a significant increase in the global consumption of olive oil, even in countries where it is not produced, such as the Canada and Japan [1]. This is due in large part to its nutritional and health- promoting effects [2], which have been related to the op- timal balance between saturated, monounsaturated (MUFA), and polyunsaturated fattyacids (PUFA), as well as tomi- nor components such as chlorophyll, polyphenols and to- copherols [3]. Vegetable oils have been historically present in many food stuffs and health care products. it play a leading role in human nutrition, and a source of many essential nutria- ents. Vegetable oil is generally obtained from the seeds of plants like soy, sunflower, rape, palm, peanut, corn, etc. Nevertheless, the importance of olive oil, obtained from a drupe fruit, is increasing due to the biological proper- ties of several of its components that preserve health and prevent many degenerative illnesses [4]. Effectively, olive oil has a beneficial effect on the cardiovascular system, lowering the plasma levels of cholesterol and polyun- saturated fatty acids, and decreasing in the same time the risk for low-density-lipoprotein oxidation, which leads to a healthy lipoprotein profile [5]. Furthermore, olive oil diets help in controlling blood pressure, glucose and lipids levels in diabetic patients [6] as well as in impro- ving the immune function by attenuating the inflamma- tory process [7]. Rojas-Molina et al., [8] and Awney, [9] were tested some vegetable oils in the Drosophila wing- spot test and found that extra-virgin olive oil (EVOO) was clearly not genotoxic, while soy oil showed a clear genotoxic activity. Many studies have been conducted to prove its potential through oil, whole fruit and leaf extract as cardiovascular disorders and anti-oxidant, gastroprotective effect, osteo- protective effect, endocrine effect, immunomodulatory  A. E. Bawazir / World Journal of Neuroscience 1 (2011) 31-37 Copyright © 2011 SciRes. WJNS 32 effect, anti-cancer, anti-viral and anti-microbial effects and cancers of the breast, skin, and colon [10-12]. Before 1400 years ago the Prophet of Islam, Muham- mad (SAW), use of oil is found in many religions and cultures. It has been used during special ceremonies and also as a general health measure. It is also known as the symbol for peace, wisdom and victory [13]. Since olive oil is a wild oil commonly available in Saudi Arabia and especially in the Mediterranean and its leaves are used in folk medicine for treatment, it is therefore deemed interesting to examine the effect of chronic ad- ministration of olive oil on norepinephrine (NE), dopa- mine (DA), serotonin (5-HT) and gamm-aminobutyric acid (GABA) contents in different brain regions and histo- logical structure of liver and kidney of male albino rats. 2. MATERIALS AND METHOD 2.1. Animals The experimental animals used in this study were male albino rats, Rattus rattus (90 g - 100 g ). They were sup- plied with food and water ad libitum under standard con- ditions of light, humidity and temperature (22˚C - 25˚C). 2.2. Olive Oil It is an oil obtained from the olive (O lea europaea; fam- ily Oleaceae), a traditional tree crop of the Mediterranean Basin. It is commonly used in cooking, cosmetics, phar- maceuticals, and soaps and as a fuel for traditional oil lamps. 2.3. Method 2.3.1. The Effect of Oli ve Oi l on Different Brain Regions of Male Albino Rats The animals were randomly divided into 2 groups. The 1st group (n = 6) was treated with saline vehicle and killed at the beginning of the experiment and served as a control. The 2nd group (n = 24) was normal rats orally administered with olive oil (7.5 ml/kg) through gastric tube for 4 week, [14]. The rat was killed by sudden decapitation at the designed times. The brain was rapidly and carefully ex- cised and then dissected on dry ice glass plate according to the method of Glowinski and Iversen [15] into the following regions: cerebellum, striatum, cerebral cortex, hypothalamus, brain steam and hippocampus. The brain tissues were wiped dry with a filter paper, weighed, wrap- ped in plastic films and then in aluminum foil and qui- ckly frozen in dry ice pending analysis. NE, DA and 5-HT were extracted and estimated ac- cording to the method of Chang [16] modified by Ciar- lone [17]. GABA was estimated according to the method of Sutton and Simmodes [18]. The fluorescence was mea- sured in Jenway 6200 fluorometer. 2.3.2. The effect of oli ve oi l on liver and k i d ney function of male albino rats Blood Sampling: The portion of blood samples were col- lected and allowed to coagulate at room temperature; EDTA (ethylene diamine tetracetic acid) was added to the other portion of blood and centrifuged at 3000 r.p.m. for 30 minutes. The clear, non-haemolysed supernatant sera and plasma were quickly removed divided into four portions for each animal, and stored at –20˚C for subse- quent analysis. For the measurement of AST, ALT and Alkaline phosphatase in liver and, Urea and creatinine in kinday [19]. 2.3.3. The E ffect of Olive Oil on Histol ogical St ructur es of Liver and Kinday of Male Albin o Ra ts After sacrifice of animals, part of the liver and kidany tissues from each animal from treated and control was removed and immersed in 10% buffered formalin solu- tion. Each part of liver and kidney tissues were kept in separate numbered small glass bottles and then embed- ded in paraffin, and sectioned. Four sections (5 microns in thickness) were taken from each liver and kinday tis- sues, each section being at a distance of at least 500 u from the proceeding one sections were stained with haematoxylin and eosin [20]. 2.3.4. Statistical Anal ysis The data are presented as mean + S.E. Statistical analy- ses between control and treated animals were performed using paired student ‘t’ [21]. 3. RESULTS As shown Tab le 1 the daily oral administration of olive oil (7.5 mg/kg b.wt.) caused a significant increase in dopamine content starting from the third and fourth week in cerebellum, striatum, cerebral cortex, hypo- thalamus, brain stem and hippocampus. The maximal increase (p < 0.001) in dopamine content was found in the cerebral cortex after 3 weeks (+103.68%). The results obtained from Table 2 showed that the daily oral administration of olive oil caused no significant in nor epinephrine content in all of tested areas after 1, 2, 3 and 4 week. Also, Table 3 showed that the daily oral ad- ministration of olive oil (7.5 mg /kg b.wt.) caused a sig- nificant increase in gamm-aminobutyric acid content start- ing from the third and four week in cerebellum, striatum, cerebral cortex, hypothalamus, brain stem and hippocam- pus. The maximal increase in gamm-aminobutyric acid content was found in the cerebral cortex after 4 weeks (+117.17%), while the daily oral administration of olive oil caused a significant(p < 0.001) increase in serotonin content starting from two week in brain stem and from the third and four week in all brain area till the end of the experiment. The maximal increase (p < 0.001) in serotonin 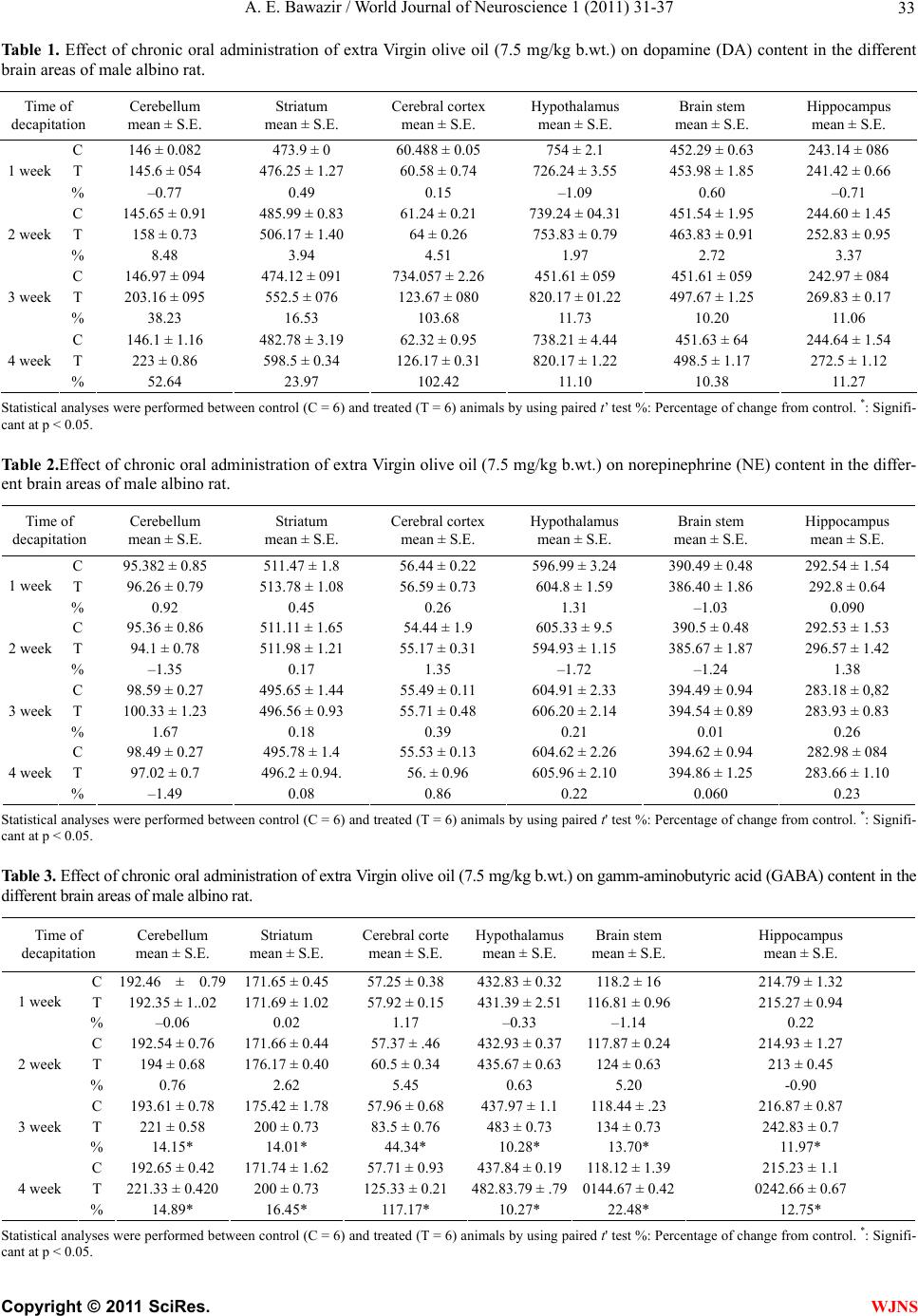 A. E. Bawazir / World Journal of Neuroscience 1 (2011) 31-37 Copyright © 2011 SciRes. WJNS 33 Ta bl e 1 . Effect of chronic oral administration of extra Virgin olive oil (7.5 mg/kg b.wt.) on dopamine (DA) content in the different brain areas of male albino rat. Time of decapitation Cerebellum mean ± S.E. Striatum mean ± S.E. Cerebral cortex mean ± S.E. Hypothalamus mean ± S.E. Brain stem mean ± S.E. Hippocampus mean ± S.E. C 146 ± 0.082 473.9 ± 0 60.488 ± 0.05 754 ± 2.1 452.29 ± 0.63 243.14 ± 086 T 145.6 ± 054 476.25 ± 1.27 60.58 ± 0.74 726.24 ± 3.55 453.98 ± 1.85 241.42 ± 0.66 1 week % –0.77 0.49 0.15 –1.09 0.60 –0.71 C 145.65 ± 0.91 485.99 ± 0.83 61.24 ± 0.21 739.24 ± 04.31 451.54 ± 1.95 244.60 ± 1.45 T 158 ± 0.73 506.17 ± 1.40 64 ± 0.26 753.83 ± 0.79 463.83 ± 0.91 252.83 ± 0.95 2 week % 8.48 3.94 4.51 1.97 2.72 3.37 C 146.97 ± 094 474.12 ± 091 734.057 ± 2.26 451.61 ± 059 451.61 ± 059 242.97 ± 084 T 203.16 ± 095 552.5 ± 076 123.67 ± 080 820.17 ± 01.22 497.67 ± 1.25 269.83 ± 0.17 3 week % 38.23 16.53 103.68 11.73 10.20 11.06 C 146.1 ± 1.16 482.78 ± 3.19 62.32 ± 0.95 738.21 ± 4.44 451.63 ± 64 244.64 ± 1.54 T 223 ± 0.86 598.5 ± 0.34 126.17 ± 0.31 820.17 ± 1.22 498.5 ± 1.17 272.5 ± 1.12 4 week % 52.64 23.97 102.42 11.10 10.38 11.27 Statistical analyses were performed between control (C = 6) and treated (T = 6) animals by using paired t’ test %: Percentage of change from control. *: Signifi- cant at p < 0.05. Table 2.Effect of chronic oral administration of extra Virgin olive oil (7.5 mg/kg b.wt.) on norepinephrine (NE) content in the differ- ent brain areas of male albino rat. Time of decapitation Cerebellum mean ± S.E. Striatum mean ± S.E. Cerebral cortex mean ± S.E. Hypothalamus mean ± S.E. Brain stem mean ± S.E. Hippocampus mean ± S.E. C 95.382 ± 0.85 511.47 ± 1.8 56.44 ± 0.22 596.99 ± 3.24 390.49 ± 0.48 292.54 ± 1.54 T 96.26 ± 0.79 513.78 ± 1.08 56.59 ± 0.73 604.8 ± 1.59 386.40 ± 1.86 292.8 ± 0.64 1 week % 0.92 0.45 0.26 1.31 –1.03 0.090 C 95.36 ± 0.86 511.11 ± 1.65 54.44 ± 1.9 605.33 ± 9.5 390.5 ± 0.48 292.53 ± 1.53 T 94.1 ± 0.78 511.98 ± 1.21 55.17 ± 0.31 594.93 ± 1.15 385.67 ± 1.87 296.57 ± 1.42 2 week % –1.35 0.17 1.35 –1.72 –1.24 1.38 C 98.59 ± 0.27 495.65 ± 1.44 55.49 ± 0.11 604.91 ± 2.33 394.49 ± 0.94 283.18 ± 0,82 T 100.33 ± 1.23 496.56 ± 0.93 55.71 ± 0.48 606.20 ± 2.14 394.54 ± 0.89 283.93 ± 0.83 3 week % 1.67 0.18 0.39 0.21 0.01 0.26 C 98.49 ± 0.27 495.78 ± 1.4 55.53 ± 0.13 604.62 ± 2.26 394.62 ± 0.94 282.98 ± 084 T 97.02 ± 0.7 496.2 ± 0.94. 56. ± 0.96 605.96 ± 2.10 394.86 ± 1.25 283.66 ± 1.10 4 week % –1.49 0.08 0.86 0.22 0.060 0.23 Statistical analyses were performed between control (C = 6) and treated (T = 6) animals by using paired t' test %: Percentage of change from control. *: Signifi- cant at p < 0.05. Table 3. Effect of chronic oral administration of extra Virgin olive oil (7.5 mg/kg b.wt.) on gamm-aminobutyric acid (GABA) content in the different brain areas of male albino rat. Time of decapitation Cerebellum mean ± S.E. Striatum mean ± S.E. Cerebral corte mean ± S.E. Hypothalamus mean ± S.E. Brain stem mean ± S.E. Hippocampus mean ± S.E. C 192.46 ± 0.79171.65 ± 0.45 57.25 ± 0.38 432.83 ± 0.32118.2 ± 16 214.79 ± 1.32 T 192.35 ± 1..02 171.69 ± 1.02 57.92 ± 0.15 431.39 ± 2.51116.81 ± 0.96215.27 ± 0.94 1 week % –0.06 0.02 1.17 –0.33 –1.14 0.22 C 192.54 ± 0.76 171.66 ± 0.44 57.37 ± .46 432.93 ± 0.37117.87 ± 0.24214.93 ± 1.27 T 194 ± 0.68 176.17 ± 0.40 60.5 ± 0.34 435.67 ± 0.63124 ± 0.63 213 ± 0.45 2 week % 0.76 2.62 5.45 0.63 5.20 -0.90 C 193.61 ± 0.78 175.42 ± 1.78 57.96 ± 0.68 437.97 ± 1.1118.44 ± .23 216.87 ± 0.87 T 221 ± 0.58 200 ± 0.73 83.5 ± 0.76 483 ± 0.73 134 ± 0.73 242.83 ± 0.7 3 week % 14.15* 14.01* 44.34* 10.28* 13.70* 11.97* C 192.65 ± 0.42 171.74 ± 1.62 57.71 ± 0.93 437.84 ± 0.19118.12 ± 1.39215.23 ± 1.1 T 221.33 ± 0.420 200 ± 0.73 125.33 ± 0.21 482.83.79 ± .790144.67 ± 0.420242.66 ± 0.67 4 week % 14.89* 16.45* 117.17* 10.27* 22.48* 12.75* Statistical analyses were performed between control (C = 6) and treated (T = 6) animals by using paired t' test %: Percentage of change from control. *: Signifi- cant at p < 0.05. 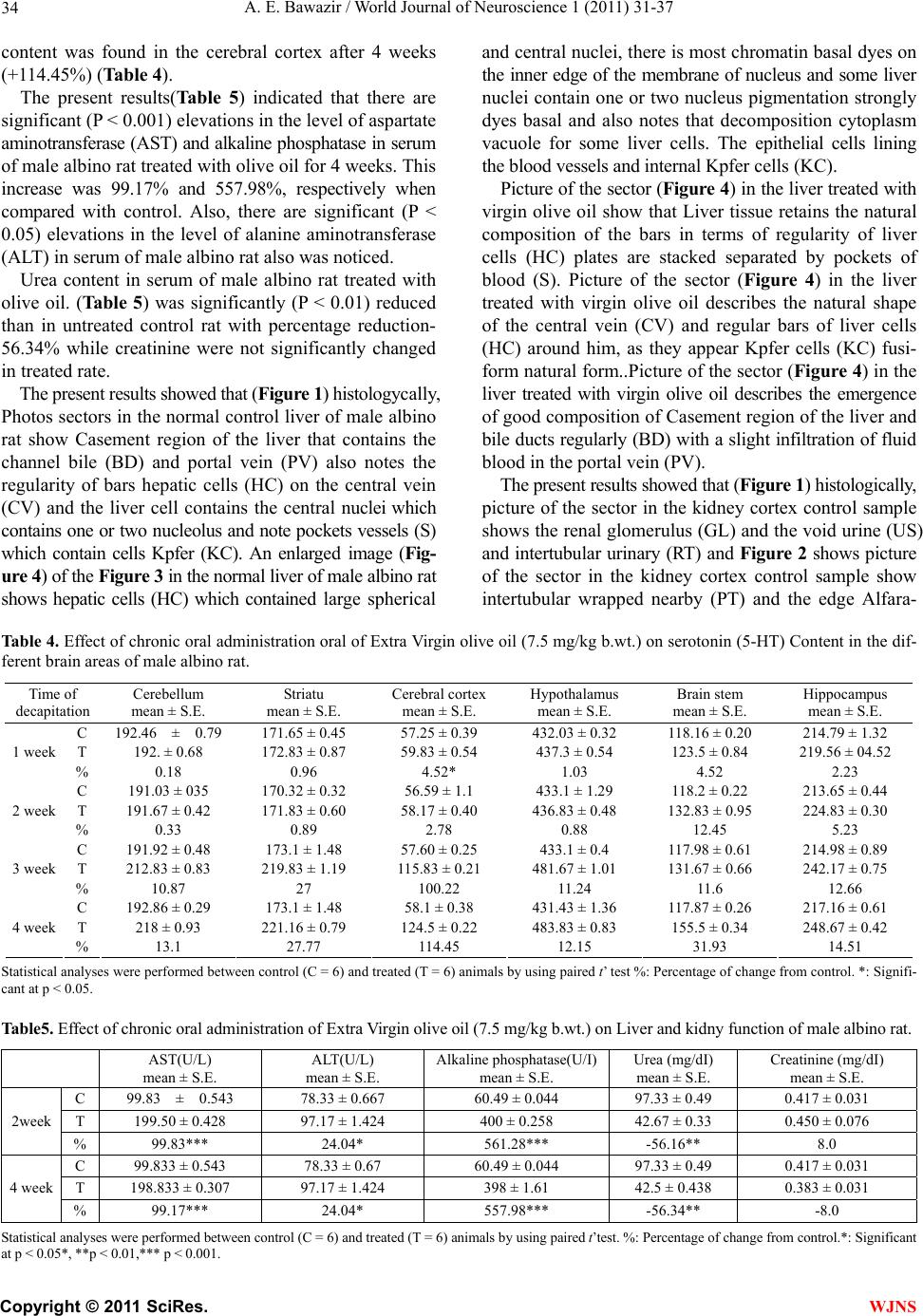 A. E. Bawazir / World Journal of Neuroscience 1 (2011) 31-37 Copyright © 2011 SciRes. WJNS 34 content was found in the cerebral cortex after 4 weeks (+114.45%) (Table 4). The present results(Table 5) indicated that there are significant (P < 0.001) elevations in the level of aspartate aminotransferase (AST) and alkaline phosphatase in serum of male albino rat treated with olive oil for 4 weeks. This increase was 99.17% and 557.98%, respectively when compared with control. Also, there are significant (P < 0.05) elevations in the level of alanine aminotransferase (ALT) in serum of male albino rat also was noticed. Urea content in serum of male albino rat treated with olive oil. (Tabl e 5) was significantly (P < 0.01) reduced than in untreated control rat with percentage reduction- 56.34% while creatinine were not significantly changed in treated rate. The present results showed that (Figure 1) histologycally, Photos sectors in the normal control liver of male albino rat show Casement region of the liver that contains the channel bile (BD) and portal vein (PV) also notes the regularity of bars hepatic cells (HC) on the central vein (CV) and the liver cell contains the central nuclei which contains one or two nucleolus and note pockets vessels (S) which contain cells Kpfer (KC). An enlarged image (Fig- ure 4 ) of the Figure 3 in the normal liver of male albino rat shows hepatic cells (HC) which contained large spherical and central nuclei, there is most chromatin basal dyes on the inner edge of the membrane of nucleus and some liver nuclei contain one or two nucleus pigmentation strongly dyes basal and also notes that decomposition cytoplasm vacuole for some liver cells. The epithelial cells lining the blood vessels and internal Kpfer cells (KC). Picture of the sector (Figure 4) in the liver treated with virgin olive oil show that Liver tissue retains the natural composition of the bars in terms of regularity of liver cells (HC) plates are stacked separated by pockets of blood (S). Picture of the sector (Figure 4) in the liver treated with virgin olive oil describes the natural shape of the central vein (CV) and regular bars of liver cells (HC) around him, as they appear Kpfer cells (KC) fusi- form natural form..Picture of the sector (Figure 4) in the liver treated with virgin olive oil describes the emergence of good composition of Casement region of the liver and bile ducts regularly (BD) with a slight infiltration of fluid blood in the portal vein (PV). The present results showed that (Figure 1) histologically, picture of the sector in the kidney cortex control sample shows the renal glomerulus (GL) and the void urine (US) and intertubular urinary (RT) and Figure 2 shows picture of the sector in the kidney cortex control sample show intertubular wrapped nearby (PT) and the edge Alfara- Table 4. Effect of chronic oral administration oral of Extra Virgin olive oil (7.5 mg/kg b.wt.) on serotonin (5-HT) Content in the dif- ferent brain areas of male albino rat. Time of decapitation Cerebellum mean ± S.E. Striatu mean ± S.E. Cerebral cortex mean ± S.E. Hypothalamus mean ± S.E. Brain stem mean ± S.E. Hippocampus mean ± S.E. C 192.46 ± 0.79 171.65 ± 0.45 57.25 ± 0.39 432.03 ± 0.32 118.16 ± 0.20 214.79 ± 1.32 T 192. ± 0.68 172.83 ± 0.87 59.83 ± 0.54 437.3 ± 0.54 123.5 ± 0.84 219.56 ± 04.52 1 week % 0.18 0.96 4.52* 1.03 4.52 2.23 C 191.03 ± 035 170.32 ± 0.32 56.59 ± 1.1 433.1 ± 1.29 118.2 ± 0.22 213.65 ± 0.44 T 191.67 ± 0.42 171.83 ± 0.60 58.17 ± 0.40 436.83 ± 0.48 132.83 ± 0.95 224.83 ± 0.30 2 week % 0.33 0.89 2.78 0.88 12.45 5.23 C 191.92 ± 0.48 173.1 ± 1.48 57.60 ± 0.25 433.1 ± 0.4 117.98 ± 0.61 214.98 ± 0.89 T 212.83 ± 0.83 219.83 ± 1.19 115.83 ± 0.21 481.67 ± 1.01 131.67 ± 0.66 242.17 ± 0.75 3 week % 10.87 27 100.22 11.24 11.6 12.66 C 192.86 ± 0.29 173.1 ± 1.48 58.1 ± 0.38 431.43 ± 1.36 117.87 ± 0.26 217.16 ± 0.61 T 218 ± 0.93 221.16 ± 0.79 124.5 ± 0.22 483.83 ± 0.83 155.5 ± 0.34 248.67 ± 0.42 4 week % 13.1 27.77 114.45 12.15 31.93 14.51 Statistical analyses were performed between control (C = 6) and treated (T = 6) animals by using paired t’ test %: Percentage of change from control. *: Signifi- cant at p < 0.05. Table5. Effect of chronic oral administration of Extra Virgin olive oil (7.5 mg/kg b.wt.) on Liver and kidny function of male albino rat. AST(U/L) mean ± S.E. ALT(U/L) mean ± S.E. Alkaline phosphatase(U/I) mean ± S.E. Urea (mg/dI) mean ± S.E. Creatinine (mg/dI) mean ± S.E. C 99.83 ± 0.543 78.33 ± 0.667 60.49 ± 0.044 97.33 ± 0.49 0.417 ± 0.031 T 199.50 ± 0.428 97.17 ± 1.424 400 ± 0.258 42.67 ± 0.33 0.450 ± 0.076 2week % 99.83*** 24.04* 561.28*** -56.16** 8.0 C 99.833 ± 0.543 78.33 ± 0.67 60.49 ± 0.044 97.33 ± 0.49 0.417 ± 0.031 T 198.833 ± 0.307 97.17 ± 1.424 398 ± 1.61 42.5 ± 0.438 0.383 ± 0.031 4 week % 99.17*** 24.04* 557.98*** -56.34** -8.0 Statistical analyses were performed between control (C = 6) and treated (T = 6) animals by using paired t’test. %: Percentage of change from control.*: Significant at p < 0.05*, **p < 0.01,*** p < 0.001. 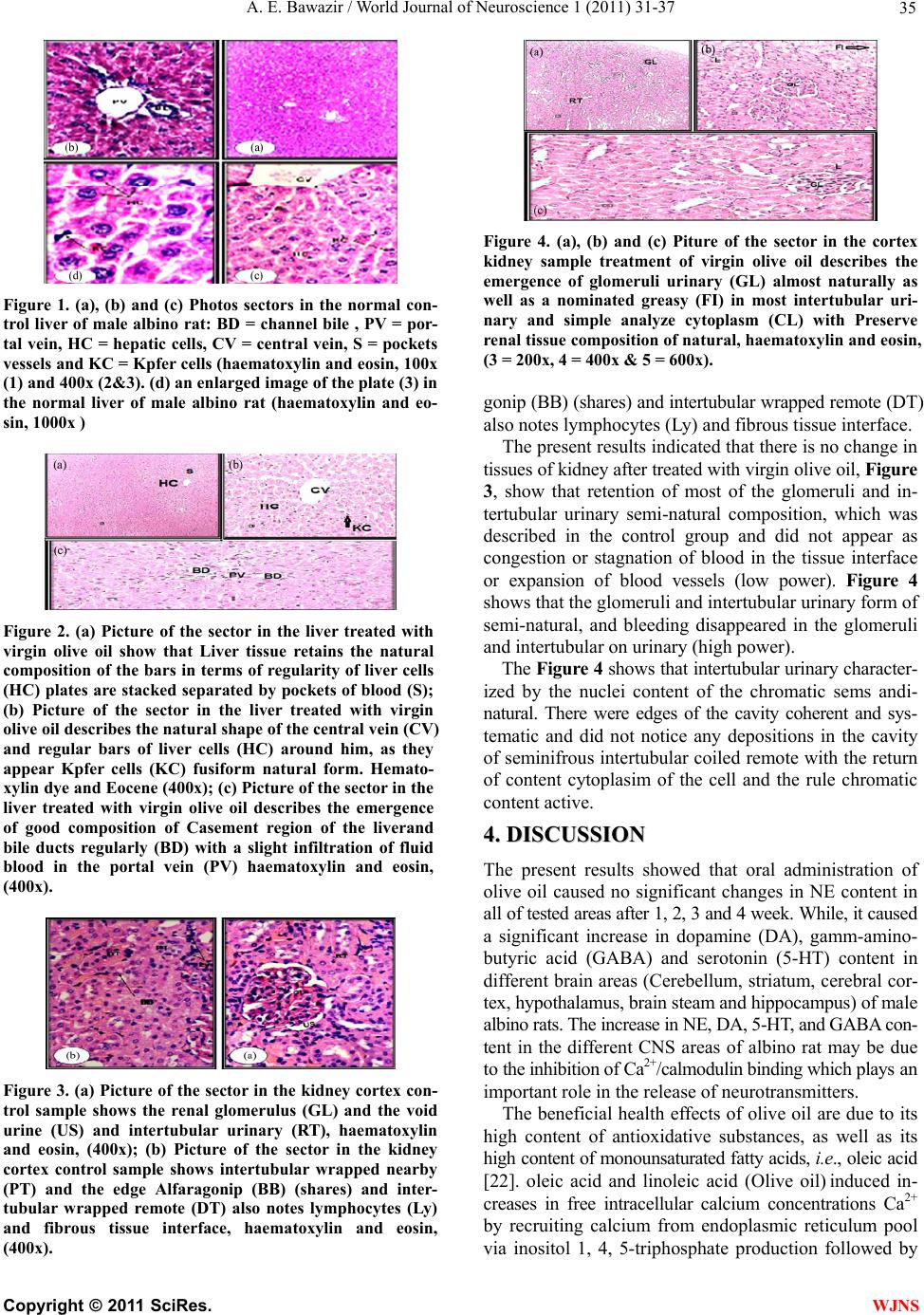 A. E. Bawazir / World Journal of Neuroscience 1 (2011) 31-37 Copyright © 2011 SciRes. WJNS 35 Figure 1. (a), (b) and (c) Photos sectors in the normal con- trol liver of male albino rat: BD = channel bile , PV = por- tal vein, HC = hepatic cells, CV = central vein, S = pockets vessels and KC = Kpfer cells (haematoxylin and eosin, 100x (1) and 400x (2&3). (d) an enlarged image of the plate (3) in the normal liver of male albino rat (haematoxylin and eo- sin, 1000x ) Figure 2. (a) Picture of the sector in the liver treated with virgin olive oil show that Liver tissue retains the natural composition of the bars in terms of regularity of liver cells (HC) plates are stacked separated by pockets of blood (S); (b) Picture of the sector in the liver treated with virgin olive oil describes the natural shape of the central vein (CV) and regular bars of liver cells (HC) around him, as they appear Kpfer cells (KC) fusiform natural form. Hemato- xylin dye and Eocene (400x); (c) Picture of the sector in the liver treated with virgin olive oil describes the emergence of good composition of Casement region of the liverand bile ducts regularly (BD) with a slight infiltration of fluid blood in the portal vein (PV) haematoxylin and eosin, (400x). Figure 3. (a) Picture of the sector in the kidney cortex con- trol sample shows the renal glomerulus (GL) and the void urine (US) and intertubular urinary (RT), haematoxylin and eosin, (400x); (b) Picture of the sector in the kidney cortex control sample shows intertubular wrapped nearby (PT) and the edge Alfaragonip (BB) (shares) and inter- tubular wrapped remote (DT) also notes lymphocytes (Ly) and fibrous tissue interface, haematoxylin and eosin, (400x). Figure 4. (a), (b) and (c) Piture of the sector in the cortex kidney sample treatment of virgin olive oil describes the emergence of glomeruli urinary (GL) almost naturally as well as a nominated greasy (FI) in most intertubular uri- nary and simple analyze cytoplasm (CL) with Preserve renal tissue composition of natural, haematoxylin and eosin, (3 = 200x, 4 = 400x & 5 = 600x). gonip (BB) (shares) and intertubular wrapped remote (DT) also notes lymphocytes (Ly) and fibrous tissue interface. The present results indicated that there is no change in tissues of kidney after treated with virgin olive oil, Figure 3, show that retention of most of the glomeruli and in- tertubular urinary semi-natural composition, which was described in the control group and did not appear as congestion or stagnation of blood in the tissue interface or expansion of blood vessels (low power). Figure 4 shows that the glomeruli and intertubular urinary form of semi-natural, and bleeding disappeared in the glomeruli and intertubular on urinary (high power). The Figure 4 shows that intertubular urinary character- ized by the nuclei content of the chromatic sems andi- natural. There were edges of the cavity coherent and sys- tematic and did not notice any depositions in the cavity of seminifrous intertubular coiled remote with the return of content cytoplasim of the cell and the rule chromatic content active. 4 4. . D DI IS SC CU US SS SI IO ON N The present results showed that oral administration of olive oil caused no significant changes in NE content in all of tested areas after 1, 2, 3 and 4 week. While, it caused a significant increase in dopamine (DA), gamm-amino- butyric acid (GABA) and serotonin (5-HT) content in different brain areas (Cerebellum, striatum, cerebral cor- tex, hypothalamus, brain steam and hippocampus) of male albino rats. The increase in NE, DA, 5-HT, and GABA con- tent in the different CNS areas of albino rat may be due to the inhibition of Ca2+/calmodulin binding which plays an important role in the release of neurotransmitters. The beneficial health effects of olive oil are due to its high content of antioxidative substances, as well as its high content of monounsaturated fatty acids, i.e., oleic acid [22]. oleic acid and linoleic acid (Olive oil) induced in- creases in free intracellular calcium concentrations Ca2+ by recruiting calcium from endoplasmic reticulum pool via inositol 1, 4, 5-triphosphate production followed by  A. E. Bawazir / World Journal of Neuroscience 1 (2011) 31-37 Copyright © 2011 SciRes. WJNS 36 calcium influx via opening of store-operated calcium chan- nels. Oleic acid and linoleic acid also induced phospho- rylation of Src-protein-tyrosine kinases, particularly of Fyn59 and Yes62. linoleic acid -evoked phosphorylation of Fyn59 and Yes62 was implicated in the activation of SOC channels. Reverse transcription-quantitative PCR revealed that the CD36-positive gustatory cells possessed mRNA of enzymes like tryptophan hydroxylase-1, L-aromatic amino acid decarboxylase, tyrosine hydroxylase, and do- pamine β-hydroxylase, involved in the synthesis of mono- ami ne neurotransmitters. Interestingly, the addition of li- noleic acid to these cells induced the release of 5-hydro- xytryptamine and noradrenalin (norepinephrine, nepine- phrine) to the extracellular environment. The linoleic acid induced release of these neurotrans- mitters was curtailed by SOC channel blockers and Src- PTK inhibitors. These results altogether demonstrate that LA binds to mouse CD36-positive gustatory cells, It is clear from the literature that DHA is involved in a variety of processes in neural cells and that its role is far more complex than simply influencing cell membrane pro- perties [26]. Unsaturated fatty acids are important consti- tuents of neuronal cell membranes and have neuropro- tective, antioxidant, and anti-inflammatory properties [27]. Previous studies had verified their effects on regulat- ing cholesterol levels and hypertension, two risk factors commonly associated with heart disease and Alzheimer’s disease.the Polyphenols could prevent damage caused by oxidation or neural death in cell cultures [28]. These results demonstrate that diet contain olive oil is capable of inducing the generation of new cells in the adult brain, and of strengthening the neural networks which become affected with age and in neurogenerative proc- esses such as Alzheimer's disease, as well as protecting neurons from oxidative and neural damage, two pheno- mena which occur at the origin of many diseases affect- ing the central nervous system [29]. A study from Ger- many supports that suspicion, and offers hope that diets high in extra virgin olive oil may help reduce brain da- mage under the effect of free radicals [30]. The aminotransaminases [aspartate aminotransferase (AST) and alanine aminotransferase (ALT)] represent an important link between carbohydrate and amino acid me- tabolic pathways [19]. Also, these enzymes are consid- ered good sensitive tools for detection of any variations in the physiological process of living organisms [31,32]. The concentration of these enzymes (AST&ALT) in liver of treated rats were significantly increased than that in control rat. The elevation in the activities of these en- zymes could be due to a variety of conditions including muscle damage, intestinal and hepatic injury and toxic hepatitis [33]. As well, the increase in serum alkaline phosphatase (ALP) activity in patients could be attrib- uted to hepatocytes injury and interruptions in their na- tural activities [33]. Urea content in serum of male al- bino rat treated with olive oil was depleted reduced than in untreated rat while creatinine was not significantly changed in treated rate. Elevated liver enzymes are the cause of the destruct- tion of cells and the occurrence of damage to tissue where attributed scientists Tdmillo liver tissue as a result of increased free radicals and LPO and high enzymes GOT, GPT destruction Bernchimip liver and the accumulation of triglycerides and release enzymes into the bloodstream, which have a clear indication of the emergence of necro- sis cell of liver tissue Where scientists attributed the de- struction of liver tissue as a result of increased free radicals and LPO and high enzymes GOT, GPT and the accumu- lation of triglycerides and release enzymes into the blood- stream, which have a clear indication of the emergence of cellular necrosis of the liver tissue [34]. 5. CONCLUSIONS Olive oil may potentially be safe for use as a sedative drug improved the central nervous system also led to the reductions in risk of Alzheimer’s and Parkinson’s dis- eases. The olive oil could be used as a protector against Alzheimer’s and Albarkson a result of the different char- acteristics. REFERENCES [1] Mili, S. (2006) Olive oil marketing innon-traditionalmar- kets: Prospects and strategies. NewMedit, 5, 27-37. [2] Monteleone, E., Caporale, G., Carlucci, A. and Pagliarini, E. (1998) Optimisation of extra virgin olive oil quality. Journal of the Science of Food and Agriculture, 77, 31- 37. doi:10.1002/(SICI)1097-0010(199805)77:1<31::AID-JSF A998>3.0.CO;2-F [3] Lazzez, A., Perri, E., Caravita, M.A., Khlif, M. and Cossentini, M. (2008) Influence of olive maturity stage and geographical origin on some minor components in virgin olive oil of the Chemlali Variety. Journal of Agri- cultural and Food Chemistry, 56, 982-988. doi:10.1021/jf0722147 [4] Mackenbach, J.P. (2007) The Mediterranean diet story illustrates that “why” questions are as important as “how” questions in disease explanation. Journal of Clinical Epi- demiology, 60, 105-109. doi:10.1016/j.jclinepi.2006.05.001 [5] Halliwell, B. and Gutteridge, J.M.C. (1999) Free radicals, other reactive species and disease. In: Free Radicals in Biology and Medicine, Third Edition, Oxford University Press, Oxford, 617-783. [6] Toobert, D.J., Glasgow, R.E., Strycker, L.A., Barrera, M.Jr., Radcliffe, J.L., Wander, R.C. and Bagdade, J.D. (2003) Biologic and quality-of-life outcomes from the Mediter- ranean lifestyle program: A randomized clinical trial,  A. E. Bawazir / World Journal of Neuroscience 1 (2011) 31-37 Copyright © 2011 SciRes. WJNS 37 Diabetes Care, 26, 2288-2293. doi:10.2337/diacare.26.8.2288 [7] Wahle, K.W.J., Caruso, D., Ochoa, J.J. and Quiles, J.L. (2004) Olive oil and modulation of cell signaling in dis- ease prevention. Lipids, 39, 1223-1231. doi:10.1007/s11745-004-1351-y [8] Rojas-Molina, M., Campos-Sلnchez, J., Analla, M., Mu˜noz- Serrano, A. and Alonso-Moraga, A. (2005) Genotoxicity of vegetable cooking oils in the drosophila wing spot test. Environmental and Molecular Mutagenesis, 45, 90-95. doi:10.1002/em.20078 [9] Awney, H. (2010) The effect of green tea and olive oil on the mutagenic activity of heterocyclic amines extracted from common food consumed in Saudi Arabia. Interna- tional Journal of Food Sciences and Nutrition, [Epub ahead of print]. [10] Samova, L.I., Shode, F.O., Ramnanan, P. and Nadar, A. (2003) Antihypertensive, antiatherosclerotic and antioxi- dantactivity of triterpenoids isolated from Oleaeuropaea, subspecies Africana leaves. Journal of Ethnopharmacology, 84, 299-305. doi:10.1016/S0378-8741(02)00332-X [11] Owen, R.W., Haubner, R. and Wurtele, G. (2004) Olives and olive oil in cancer prevention. European Journal of Cancer Prevention, 13, 319-326. doi:10.1097/01.cej.0000130221.19480.7e [12] Omar, S.H. (2008) Pharmacy department, sebai institute of health. Pharmacognosy reviews, 2, 96-100. [13] Omar, S.H. (2010) Oleuropein in olive and its pharma- cological effects. Scientific Pharmaceuticals, 78, 133- 154. doi:10.3797/scipharm.0912-18 [14] Luciane, A., Faine, H., Rodrigues, G., Cristiano Galhardi, M., Geovana, M. and Ebaid, X. (2006) Effects of olive oil and its minor constituents on serum lipids, oxidative st. Canadian Journal of Physiology and Pharmacology, 84, 239. doi:10.1139/y05-124 [15] Glowinski, J. and Iversen, L.L. (1966) Regional studies catecholamines in the rat brain I. The disposition of [3H] norepinephrine, [3H] dopamine and [3H] dopa in various regions of the brain. Journal of Neurochemistry, 13, 655-669. doi: 10 .1111/j .1471-4159.1966.tb09873.x [16] Chang, C.C. (1964) Asensitive method for spectrophoto metric assay of catecholamines. International Journal of Neuropharmacology, 3, 643-649. doi:10.1016/0028-3908(64)90089-9 [17] Ciarlone, A.E. (1978) Further modification of a fluoro- metric method for analyzing brain amines. Microchemi- cal Journal, 23, 9-12. doi:10.1016/0026-265X(78)90034-6 [18] Sutton, I. and Simmonds, M.A. (1973) Effects of acute and chronic ethanol on the gama-aminobutyric acid sys- tem in rat brain. Biochemical Pharmacology, 22, 1685- 1692. doi:10.1016/0006-2952(73)90381-X [19] Christic, J.H. and Michelson, E.H. (1978) Transaminase levels in the digestive gland-gonad of Schistosoma man- soni infected Biomplalaria alexandrina. Comparative Physiology and Biochemistry, 50, 233-236. [20] Harris, H.F. (1900) On the rapid conversion of haema- toxylin into haematin staining reactions. Journal of Ap- plied Microscopy and Laboratory Methods, 3, 777. [21] Hill, H.B. (1971) Principles of medical statistics. 9th Edition, Oxford University Press. [22] Pérez-Jiménez, F., Ruano, J., Perez-Martinez, P., Lo- pez-Segura, F. and Lopez-Miranda, J. (2007) The influ- ence of olive oil on human health: Not a question of fat alone. Molecular Nutrition and Food Research, 51, 1199-1208. [23] Abdelghani, E.-Y., Aziz, H., Philippe, B. and Khan, N.A. (2008) Linoleic acid induces calcium signaling, src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. Journal of Biological Ch- emistry, 283, 12949-12959. doi:10.1074/jbc.M707478200 [24] Visoli, F., Poli, A. and Gall, C. (2002) Antioxidant and other biological activities of phenols from olives and olive oil. Medicinal Research Reviews, 22, 65-75. doi:10.1002/med.1028 [25] Ruiz-Gutiérrez, V., Vázquez, C.M. and Santa-Maria, C. (2001) Liver lipid composition and antioxidant enzyme activities of spontaneously hypertensive rats after inges- tion of dietary fats (fish, olive and high-oleic sunflower oils). Bioscience Reports, 21, 271-285. doi:10.1023/A:1013277914213 [26] Sinclair, A.J., Begg, D., Mathai, M. and Weisinger, R.S. (2007) Omega 3 fatty acids and the brain: Review of studies in depression. Asia Pacific Journal of Clin ical Nu- trition, 16, 391-397. [27] De Lau, L.M.L., Bornebroek, M., Witteman, J.C.M., Hofman, A., Koudstaal, P.J. and Breteler, M.M.B. (2005) Dietary fatty acids and the risk of Parkinson disease. Neurology, 64, 2040-2045. doi:10.1212/01.WNL.0000166038.67153.9F [28] Zaslaver, M., Offer, S., Kerem, Z., Stark, A.H., Weller, J.I., Eliraz, A. and Madar, Z. (2005) Natural compounds derived from foods modulate nitric oxide production and oxidative status in epithelial lung cells. Journal of Agri- cultural and Food Chemistry, 53, 9934-9939. doi:10.1021/jf052000u [29] Tony, V., Juan, H., Irene, Bolea., Bartolomé, Ramirez., Neus, A., Jordi, R., José, R.M., Cristina, G., Mercè, B. and Mercedes, U. (2009) A diet enriched in polyphenols and polyunsaturated fatty acids, LMN diet, induces neu- rogenesis in the subventricular zone and hippocampus of adult mouse brain. Journal of Alzheimer’s Disease, 18. [30] Schaffer, S., Podstawa, M., Visioli, F., Bogani, P., Müller, W.E. and Eckert, G.P. (2007) Hydroxytyrosol-rich olive mill wastewater extract protects brain cells in vitro and ex vivo. Journal of Agricultural and Food Chemistry, 55, 5043-5049. doi:10.1021/jf0703710 [31] Nevo, E., Shimony, T. and Libin, M. (1978) Pollution selection of allozyme polymorphisms in barnacles. Ex- perentia, 36, 1562-1564. doi:10.1007/BF02034674 [32] Tolba, M.R., Mohammed, B. and Mohammed, A. (1997) Effect of some heavy metals on respiration, mean en- zyme activity and total protein of the pulmonate snails Biomphalaria alexandrina and Bulinus truncates. Journal of Egyptian German Society of Zoology, 24, 17-35. [33] Farkaset, J., Farkas, P. and Hyde, D. (2004) Liver and gastroenterology tests, ini basic skills in interpreting la- boratory data. Mary Lee 3rd Edition, American Society of Health System Pharmacists, Bethesda, Maryland, USA, 330-336. [34] Alhazza, I.M. (2007) Antioxidant and hypolipidemic effects of olive oil in normal and diabetic male rats. Saudi Journal of Biological Sciences, 14, 69-74. |

