 American Journal of Plant Sciences, 2011, 2, 702-715 doi:10.4236/ajps.2011.25085 Published Online November 2011 (http://www.SciRP.org/journal/ajps) Copyright © 2011 SciRes. AJPS Influence of Planting Date on Seed Protein, Oil, Sugars, Minerals, and Nitrogen Metabolism in Soybean under Irrigated and Non-Irrigated Environments* Nacer Bellaloui1, Krishna N. Reddy2, Anne M. Gillen1, Daniel K. Fisher2, Alemu Mengistu3 1Crop Genetics Research Unit, USDA-ARS, Stoneville, MS, USA; 2Crop Production Systems Research Unit, USDA-ARS, Stone- ville, USA; 3 Crop Genetics Research Unit, USDA-ARS, Jackson, TN, USA. Email: nacer.bellaloui@ars.usda.gov Received August 17th, 2011; revised September 21st, 2011; accepted October 15th, 2011. ABSTRACT Information on the effect of planting date and irrigation on soybean [Glycine max (L.) Merr.] seed composition in the Early Soybean Production System (ESPS) is deficient, and what is available is inconclusive. The objective of this re- search was to investigate the effects of planting date on seed protein, oil, fatty acids, sugars, and minerals in soybean grown under irrigated (I) and non-irrigated (NI) co ndition s. A 2-yr field experiment was conducted in Stoneville, MS in 2007 and 2008. Soybean was planted during second week of April (early planting) and second week of May (late plant- ing) each year. Results showed that under irrigated condition, early planting increased seed oil (up to 16% increase) and oleic acid (up to 22.8% increase), but decreased protein (up to 6.6% decrease), linoleic (up to 10.9% decrease) and linolenic acids (up to 27.7% decrease) compared to late planting. Under I conditions, late planting resulted in higher sucrose and raffinose and lower stachyose compared with early planting. Under NI conditions, seed of early planting had higher protein (up to 4% increase) and oleic acid (up to 25% increase) and lower oil (up to10.8% de- crease) and linolenic acids (up to 13% decrease) than those of late planting. Under NI, stachyose concentration was higher than sucrose or raffinos e, especially in early planting. Under I, early planting resulted in lower lea f and seed B, Fe, and P concentrations compared with those of late planting. Under NI, however, early planting resulted in higher accumulation of leaf B and P, but lower seed B and P compared with those of late planting. This research demonstrated that both irrigation and planting date have a significant influence on seed protein, oil, unsaturated fatty acids, and sugars. Our results suggest that seed of late planting accumulate more B, P, and Fe than those of early planting, and this could be a beneficial gain. Limited translocation of nutrients from leaves to seed under NI is undesirable. Soybean producers may u se this information to maintain yield and seed quality , and soybean breeders to select for seed quality traits and mineral translocation efficiency in stress environments. Keywords: Mineral Nutrition, Oligosaccharides, Raffinose, Stachyose, Seed Composition, Sucrose 1. Introduction Soybean is a major crop in the word and a source of pro- tein, oil, carbohydrates, and other nutrients for humans and animals [1]. Seed contains about 40% protein, 20% oil, and 33% carbohydrates [2]. Soybean seed soluble carbohydrates, including disaccharides (sucrose) and oli- gosaccharides (raffinose and stachyose), contribute to seed quality [3]. The average soybean seed contains 9% to 12% total soluble carbohydrates, of which 4% to 5% are sucrose (C12H22O11), 1 to 2% are raffinose (C18H32O16), and 3.5% to 4.5% are stachyose (C24H42O21) [4]. Raffinose and stachyose are undesirable seed quality traits because they have detrimental effects on food and feed quality, causing flatulence or diarrhea in nonruminants [3], and soybean with low raffinose and stachyose is desirable because of increased feed energy efficiency, mineral uptake, and reduced flatulence for nonruminant animals. Soybean seed with high sucrose is desirable because it *Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does no imply recommendation or endorsement by the U.S. Department o A riculture.  Influence of Planting Date on Seed Protein, Oil, Sugars, Minerals, and Nitrogen Metabolism in Soybean under 703 Irrigated and Non-Irrigated Environments improves taste and flavor in tofu, soymilk, and nato [1]. Although the Early Soybean Production System (ESPS) showed yield benefit under irrigated and non-irrigated conditions [5], lower seed quality and substandard ger- mination of seed [6,7] and variability of seed composi- tion constituents [8-10] are still a challenge. Planting date was used as a management strategy to optimize yield and seed quality. This is because changing of the planting date would lead to a change in environmental factors, including temperature and rainfall. Although it is well established that a late planting date results in higher seed quality (seed germination), in ESPS for maturity group IV and V, effects of planting date on seed composition constituents (protein, oil, fatty acids, and sugars) have not been well investigated. Previous research showed that oil concentration in- creased with early planting, but this increase pattern was not consistent across locations [11-13]. On the other hand, it was found that protein concentration increased and oil concentration decreased with late planting [12,13]. This variability in seed composition constituents across envi- ronment and location is still a challenge for normal and novel modified seed constituent lines. For example, it was reported that the instability of high oleate germplasm lines across environments was due to mainly to the effect of temperature on the enzymes controlling biosynthesis of soybean seed fatty acids, especially at a seed-fill (R5-R6) stage [14-16]. It was suggested that palmitic and lino- lenic acids may decrease with later planting date, but stearic acid may increase, and this may be due to tem- perature changes during seed maturation at later planting [15]. On the other hand, oleic acid levels increased and linoleic and linolenic acid levels decreased when soy- beans were grown in warmer environments [17]. Higher oleic acid and lower linoleic and linolenic acids in soy- bean seed oil are desirable because of their contribution to the stability of the oil. The effect of temperature on oleic and linolenic acid was explained previously in that temperature may affect oleate and linoleate desaturases [18], decrease oleyl and linoleyl desaturase activities at 35˚C [19], decrease ω-6 desaturase enzyme, encoded by the FAD2-1A gene, and desaturases degraded at high growth temperatures of 30˚C [20]. Based on the above literature, effects of planting date on seed composition are still inconclusive. Therefore, the objective of this research was to further investigate effects of planting date and irrigation on seed protein, oil, fatty acids, sugars, and mineral nutrition. Since nitrogen is an essential nutrient for seed protein, and nitrogen metabo- lism in legumes is a result of both N2 fixation and as- similation [21,22], the effect of planting date and irriga- tion on nitrogen fixation and assimilation were also in- vestigated. 2. Materials and Methods 2.1. Field and Growth Conditions A 2-yr field study was conducted during 2007 and 2008 at the USDA-ARS Crop Production Systems Research farm, Stoneville, MS (33˚26'N latitude), The soil was a Dundee silt loam (fine-silty, mixed, active, thermic Typic Endoqualf) with pH 6.7, 1.1% organic matter, a cation exchange capacity of 15 cmol/kg, and soil textural fra- ctions of 26% sand, 55% silt, and 19% clay. The experi- mental area was disked, subsoiled, disked, and bedded in the fall of the previous year. Prior to planting, the raised beds were smoothed as needed. Soybean was planted in 102-cm wide rows using a MaxEmerge 2 planter (Deere and Co., Moline, IL) at 285,000 seeds/ha. Soybean cul- tivar “AG4604RR/S” was planted April 9 and May 10 in 2007 and April 8 and May 12 in 2008. Pendimethalin at 1.12 kg·ai/ha plus paraquat at 1.12 kg·ai/ha were applied to the entire experimental area immediately after each planting. Paraquat was applied to kill existing weeds at planting, Pendimethalin was used to provide early-season weed control. Glyphosate at 0.84 kg·ae/ha was applied at 4 - 5 weeks after planting soybean to the entire experi- mental area for postemergence weed control. Herbicides were applied with a tractor-mounted sprayer with TeeJet 8004 standard flat spray nozzles (TeeJet Spraying Sy- stems Co., Wheaton, IL), delivering 187 L/ha water at 179 kPa. All plots were hand weeded periodically through- out the season to keep weed-free. No fertilizer nitrogen was applied and the crop was irrigated on an as-needed basis each year. Each treatment plot consisted of twelve rows spaced 102-cm apart and 15.2 m long. At harvest about 200 soybean pods were randomly sampled from the middle four rows for seed. Soybean from middle eight rows in each plot were harvested using a combine, and grain yield was adjusted to 13% moisture. 2.2. Seed Analysis for Protein, Oil, and Fatty Acids Mature seed collected at harvest were analyzed for pro- tein, oil, and fatty acids. Approximately 25 g of seed from each plot were ground using a Laboratory Mill 3600 (Perten, Springfield, IL). Analyses were conducted by near infrared reflectance [23] using a diode array feed analyzer AD 7200 (Perten, Springfield, IL). Calibrations were developed by the University of Minnesota, using Perten’s Thermo Galactic Grams PLS IQ software. The calibration curve has been regularly updated for unique samples according to AOAC methods [24,25]. Analyses of protein and oil were performed based on a seed dry matter basis [23,26]. Copyright © 2011 SciRes. AJPS  Influence of Planting Date on Seed Protein, Oil, Sugars, Minerals, and Nitrogen Metabolism in Soybean under 704 Irrigated and Non-Irrigated Environments 2.3. Seed Analysis for Sucrose, Raffinose, and Stachyose Matured seed collected at harvest from each planting date were analyzed for sucrose, raffinose, and stachyose concentrations [10]. About 25 g of seed from each plot were ground using a Laboratory Mill 3600 (Perten, Spring- field, IL). Analyses were conducted by near infrared reflectance [9,23] using an AD 7200 array feed analyzer (Perten, Springfield, IL). Calibrations were developed by the Department of Agronomy and Plant Genetics, Uni- versity of Minnesota St Paul, MN, using Thermo Galac- tic Grams PLS IQ software, developed by Perten com- pany (Perten, Springfield, IL). Analyses of sugars were performed based on a seed dry matter basis [23,26]. 2.4. Seed N, S, and Mineral Composition Mature seed collected at harvest were analyzed for N, S, Ca, Mg, and Zn concentrations at The University of Georgia’s Soil, Plant, and Water Laboratory, Athens, GA. Seed Ca, Mg, and Zn concentrations were analyzed by digesting 0.5 g of dried ground seed in HNO3 in a mi- crowave digestion system. Values were then determined using inductively coupled plasma spectrometry. Nitrogen and S were measured in a 0.25-g sample using an ele- mental analyzer (LECO CNS-2000, LECO Corporation, MI). For seed B, Fe, and P, concentrations were deter- mined as indicated in the following sections. 2.5. Nitrate Reductase Activity Nitrate reductase activity (NRA) was measured in the fully expanded leaves at R1-R2 from each plot. The mea- surement of NRA was made according to the method of [27] and was described for soybean in detail by others [28]. To determine potential NRA (PNRA) where nitrate availability is not limited, nitrate at a concentration of 10 mM as KNO3, was added to the incubation solution. Ni- trate reductase activity was expressed as µmol 2 NO ·g fwt–1·hour–1). 2.6. Acetylene Reduction Assay Ten soybean plants were randomly sampled from each plot at R1-R2 for nitrogenase activity (nitrogen fixation activity, NFA) measurement. Plants were excavated with roots and shoot and transported to the laboratory for NA. Nitrogenase activity was assayed within 30 min of colle- ction using the acetylene reduction assay to measure NA [29,30]. Roots with nodules intact were excised and in- cubated in 1 L Mason jars (two jars per plot). Six roots were placed in the Mason jars and sealed, and a 10% volume of acetylene was added. After 1 h of incubation at room temperature, gas samples were removed and analyzed by gas chromatography using a flame ioniza- tion detector (FID) and a thermal conductivity detector (TCD) for determination of ethylene. 2.7. Boron Measurement Boron concentration was measured in seed from each plot using the Azomethine-H method [31]. Calcium car- bonate powder was added to 1.0 g seed samples before ashing at 500˚C for 8 hours to prevent losses of volatile B compounds. Ashed samples, then, were extracted with 20 ml of 2 M HCl at 90˚C for 10 min, filtered and trans- ferred to plastic vials. A 2 ml sample of the solution was added to 4 ml of buffer solution (containing 25% ammo- nium acetate, 1.5% EDTA, and 12.5% acetic acid) and 4 ml of freshly prepared azomethine-H solution (0.45% azomethine-H and 1% of ascorbic acid) [32]. Samples were left at room temperature for at least 45 min for color development, and B concentration was determined using a Beckman Coulter DU 800 spectrophotometer (Fullerton, CA, USA) at 420 nm. 2.8. Iron Measurement Seed iron from each plot was measured after acid wet digestion, extraction, and reaction of the reduced ferrous Fe with 1, 10-phenanthroline [10,33,34]. A 2 g sample of dried ground seed was digested in nitric acid (70% m/m HNO3). After the acids were removed by volatilization, the soluble constituents were dissolved in 2 M HCl. Standard solutions of iron were prepared in 0.4 M HCl and ranged from 0.0 to 4 μg·ml−1 Fe. Phenanthroline so- lution of 0.25% m/v was prepared in 25% v/v ethanol. A fresh quinol solution (1% m/v) reagent was prepared on the day of use. An aliquot of approximately 4 ml was pipetted into a 25 ml volumetric flask. A concentration of 0.4 M HCl solution was used to dilute the aliquot to 5 ml. A volume of quinol solution was added and mixed, and then 3 ml of phenanthroline solution and 5 ml of tri- sodium citrate solution (8% m/v) were added. The mix- ture solution containing the aliquot, HCl, phenanthroline, tri-sodium citrate, was diluted to 25 ml. The mixture stood for 4 h and the absorbance of the samples was read at 510 nm using a Beckman Coulter DU 800 spectro- photometer. 2.9. Phosphorus Measurement Seed phosphorus was measured spectrophotometrically as the yellow phospho-vanado-molybdate complex [35, 36]. A dry seed sample of 2 g was ashed, then 10 ml of 6 M HCl was added. Samples were placed in a water bath at 70˚C to evaporate the solution. After drying, the sam- ples were kept under heat, and 2 ml of 36% m/m HCl was added, and gently boiled. Then, 10 ml of water was Copyright © 2011 SciRes. AJPS 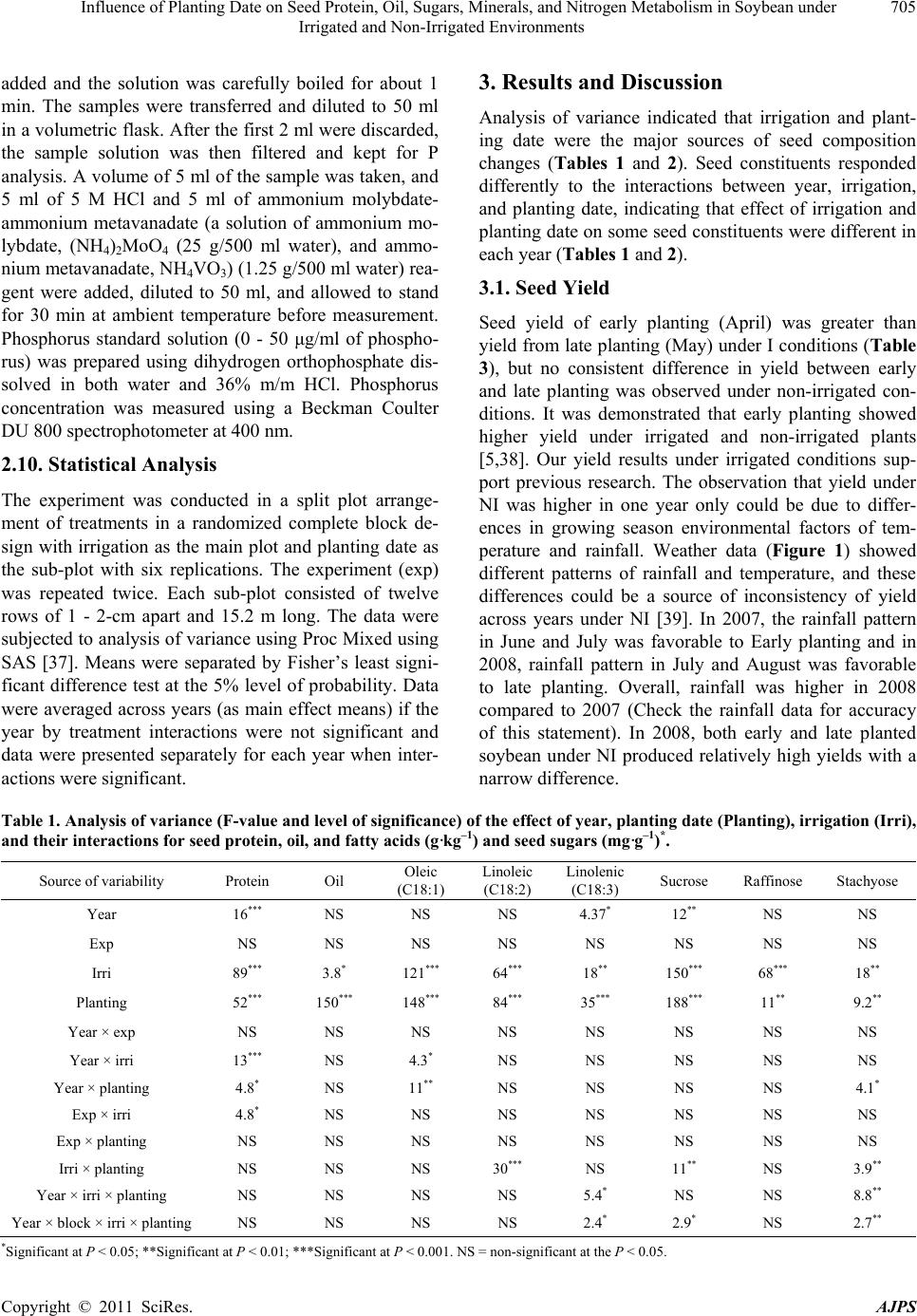 Influence of Planting Date on Seed Protein, Oil, Sugars, Minerals, and Nitrogen Metabolism in Soybean under Irrigated and Non-Irrigated Environments Copyright © 2011 SciRes. AJPS 705 added and the solution was carefully boiled for about 1 min. The samples were transferred and diluted to 50 ml in a volumetric flask. After the first 2 ml were discarded, the sample solution was then filtered and kept for P analysis. A volume of 5 ml of the sample was taken, and 5 ml of 5 M HCl and 5 ml of ammonium molybdate- ammonium metavanadate (a solution of ammonium mo- lybdate, (NH4)2MoO4 (25 g/500 ml water), and ammo- nium metavanadate, NH4VO3) (1.25 g/500 ml water) rea- gent were added, diluted to 50 ml, and allowed to stand for 30 min at ambient temperature before measurement. Phosphorus standard solution (0 - 50 μg/ml of phospho- rus) was prepared using dihydrogen orthophosphate dis- solved in both water and 36% m/m HCl. Phosphorus concentration was measured using a Beckman Coulter DU 800 spectrophotometer at 400 nm. 2.10. Statistical Analysis The experiment was conducted in a split plot arrange- ment of treatments in a randomized complete block de- sign with irrigation as the main plot and planting date as the sub-plot with six replications. The experiment (exp) was repeated twice. Each sub-plot consisted of twelve rows of 1 - 2-cm apart and 15.2 m long. The data were subjected to analysis of variance using Proc Mixed using SAS [37]. Means were separated by Fisher’s least signi- ficant difference test at the 5% level of probability. Data were averaged across years (as main effect means) if the year by treatment interactions were not significant and data were presented separately for each year when inter- actions were significant. 3. Results and Discussion Analysis of variance indicated that irrigation and plant- ing date were the major sources of seed composition changes (Tables 1 and 2). Seed constituents responded differently to the interactions between year, irrigation, and planting date, indicating that effect of irrigation and planting date on some seed constituents were different in each year (Tables 1 and 2). 3.1. Seed Yield Seed yield of early planting (April) was greater than yield from late planting (May) under I conditions (Table 3), but no consistent difference in yield between early and late planting was observed under non-irrigated con- ditions. It was demonstrated that early planting showed higher yield under irrigated and non-irrigated plants [5,38]. Our yield results under irrigated conditions sup- port previous research. The observation that yield under NI was higher in one year only could be due to differ- ences in growing season environmental factors of tem- perature and rainfall. Weather data (Figure 1) showed different patterns of rainfall and temperature, and these differences could be a source of inconsistency of yield across years under NI [39]. In 2007, the rainfall pattern in June and July was favorable to Early planting and in 2008, rainfall pattern in July and August was favorable to late planting. Overall, rainfall was higher in 2008 compared to 2007 (Check the rainfall data for accuracy of this statement). In 2008, both early and late planted soybean under NI produced relatively high yields with a narrow difference. Table 1. Analysis of variance (F-value and level of significance) of the effect of year, planting date (Planting), irrigation (Irri), and their interactions for seed protein, oil, and fatty acids (g·kg–1) and seed sugars (mg·g–1)*. Source of variability Protein Oil Oleic (C18:1) Linoleic (C18:2) Linolenic (C18:3) Sucrose Raffinose Stachyose Year 16*** NS NS NS 4.37* 12** NS NS Exp NS NS NS NS NS NS NS NS Irri 89*** 3.8* 121*** 64*** 18** 150*** 68*** 18** Planting 52*** 150*** 148*** 84*** 35*** 188*** 11** 9.2** Year × exp NS NS NS NS NS NS NS NS Year × irri 13*** NS 4.3* NS NS NS NS NS Year × planting 4.8* NS 11** NS NS NS NS 4.1* Exp × irri 4.8* NS NS NS NS NS NS NS Exp × planting NS NS NS NS NS NS NS NS Irri × planting NS NS NS 30*** NS 11** NS 3.9** Year × irri × planting NS NS NS NS 5.4* NS NS 8.8** Year × block × irri × planting NS NS NS NS 2.4* 2.9* NS 2.7** *Significant at P < 0.05; **Significant at P < 0.01; ***Significant at P < 0.001. NS = non-significant at the P < 0.05. 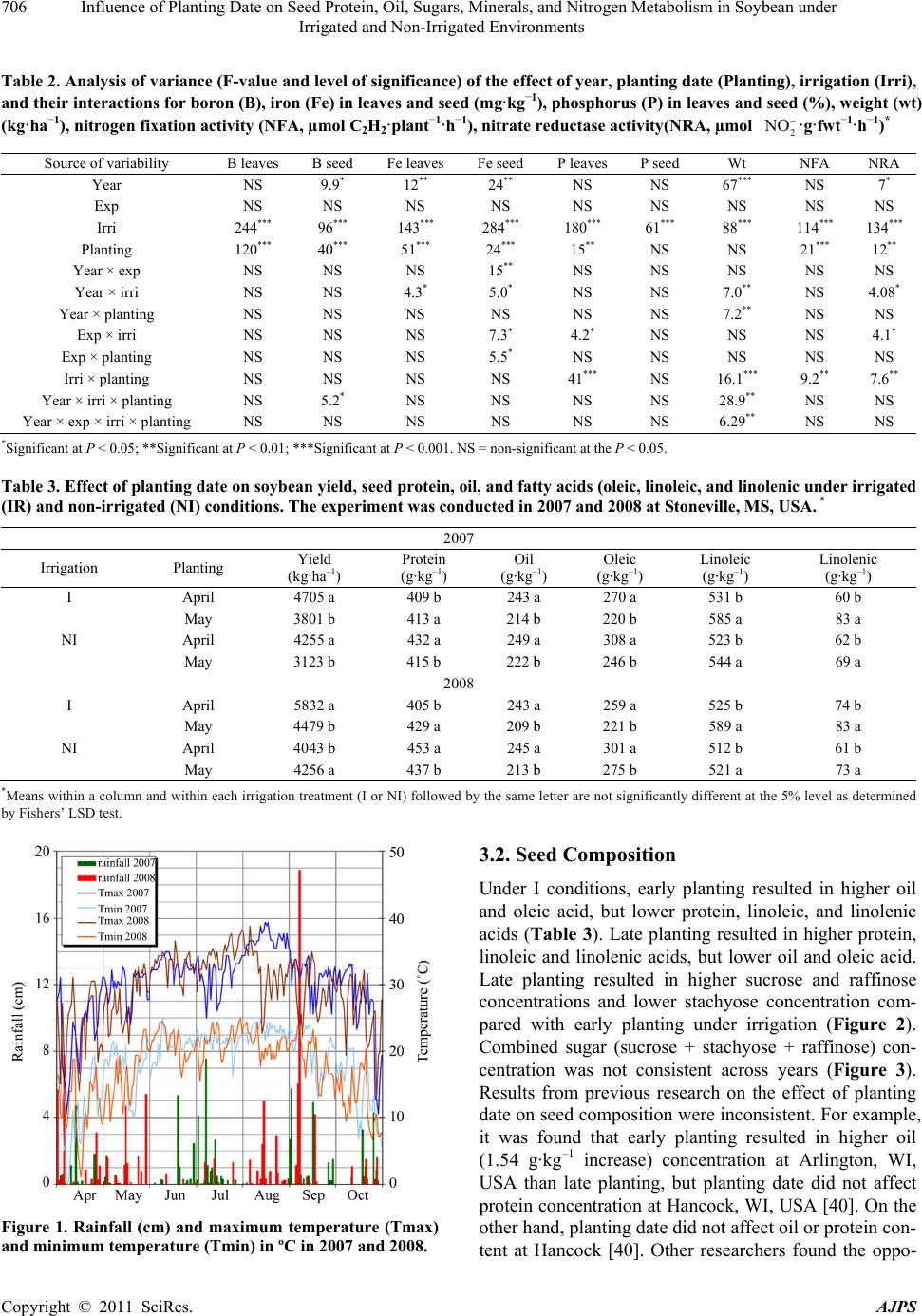 Influence of Planting Date on Seed Protein, Oil, Sugars, Minerals, and Nitrogen Metabolism in Soybean under 706 Irrigated and Non-Irrigated Environments Table 2. Analysis of variance (F-value and level of significance) of the effect of year, planting date (Planting), irrigation (Irri), and their interactions for boron (B), iron (Fe) in leaves and seed (mg·kg–1), phosphorus (P) in leaves and seed (%), weight (wt) (kg·ha–1), nitrogen fixation activity (NFA, µmol C2H2·plant–1·h–1), nitrate reductase activity(NRA, µmol ·g·fwt–1·h–1)* 2 NO Source of variability B leaves B seed Fe leaves Fe seed P leaves P seed Wt NFA NRA Year NS 9.9* 12** 24** NS NS 67*** NS 7* Exp NS NS NS NS NS NS NS NS NS Irri 244*** 96*** 143*** 284*** 180*** 61*** 88*** 114*** 134*** Planting 120*** 40*** 51*** 24*** 15** NS NS 21*** 12** Year × exp NS NS NS 15** NS NS NS NS NS Year × irri NS NS 4.3* 5.0* NS NS 7.0** NS 4.08* Year × planting NS NS NS NS NS NS 7.2** NS NS Exp × irri NS NS NS 7.3* 4.2* NS NS NS 4.1* Exp × planting NS NS NS 5.5* NS NS NS NS NS Irri × planting NS NS NS NS 41*** NS 16.1*** 9.2** 7.6** Year × irri × planting NS 5.2* NS NS NS NS 28.9** NS NS Year × exp × irri × planting NS NS NS NS NS NS 6.29** NS NS *Significant at P < 0.05; **Significant at P < 0.01; ***Significant at P < 0.001. NS = non-significant at the P < 0.05. Table 3. Effect of planting date on soybean yield, seed protei n, oil, and fatty acids (oleic, linoleic, and linolenic under irrigated (IR) and non-irrigated (NI) conditions. The experiment was conducted in 2007 and 2008 at Stoneville, MS, USA. * 2007 Irrigation Planting Yield (kg·ha–1) Protein (g·kg–1) Oil (g·kg–1) Oleic (g·kg–1) Linoleic (g·kg–1) Linolenic (g·kg–1) I April 4705 a 409 b 243 a 270 a 531 b 60 b May 3801 b 413 a 214 b 220 b 585 a 83 a NI April 4255 a 432 a 249 a 308 a 523 b 62 b May 3123 b 415 b 222 b 246 b 544 a 69 a 2008 I April 5832 a 405 b 243 a 259 a 525 b 74 b May 4479 b 429 a 209 b 221 b 589 a 83 a NI April 4043 b 453 a 245 a 301 a 512 b 61 b May 4256 a 437 b 213 b 275 b 521 a 73 a *Means within a column and within each irrigation treatment (I or NI) followed by the same letter are not significantly different at the 5% level as determined by Fishers’ LSD test. Figure 1. Rainfall (cm) and maximum temperature (Tmax) and minimum temperature (Tmin) in ºC in 2007 and 2008. 3.2. Seed Composition Under I conditions, early planting resulted in higher oil and oleic acid, but lower protein, linoleic, and linolenic acids (Table 3). Late planting resulted in higher protein, linoleic and linolenic acids, but lower oil and oleic acid. Late planting resulted in higher sucrose and raffinose concentrations and lower stachyose concentration com- pared with early planting under irrigation (Figure 2). Combined sugar (sucrose + stachyose + raffinose) con- centration was not consistent across years (Figure 3). Results from previous research on the effect of planting date on seed composition were inconsistent. For example, it was found that early planting resulted in higher oil (1.54 g·kg–1 increase) concentration at Arlington, WI, USA than late planting, but planting date did not affect protein concentration at Hancock, WI, USA [40]. On the other hand, planting date did not affect oil or protein con- tent at Hancock [40]. Other researchers found the oppo- Copyright © 2011 SciRes. AJPS 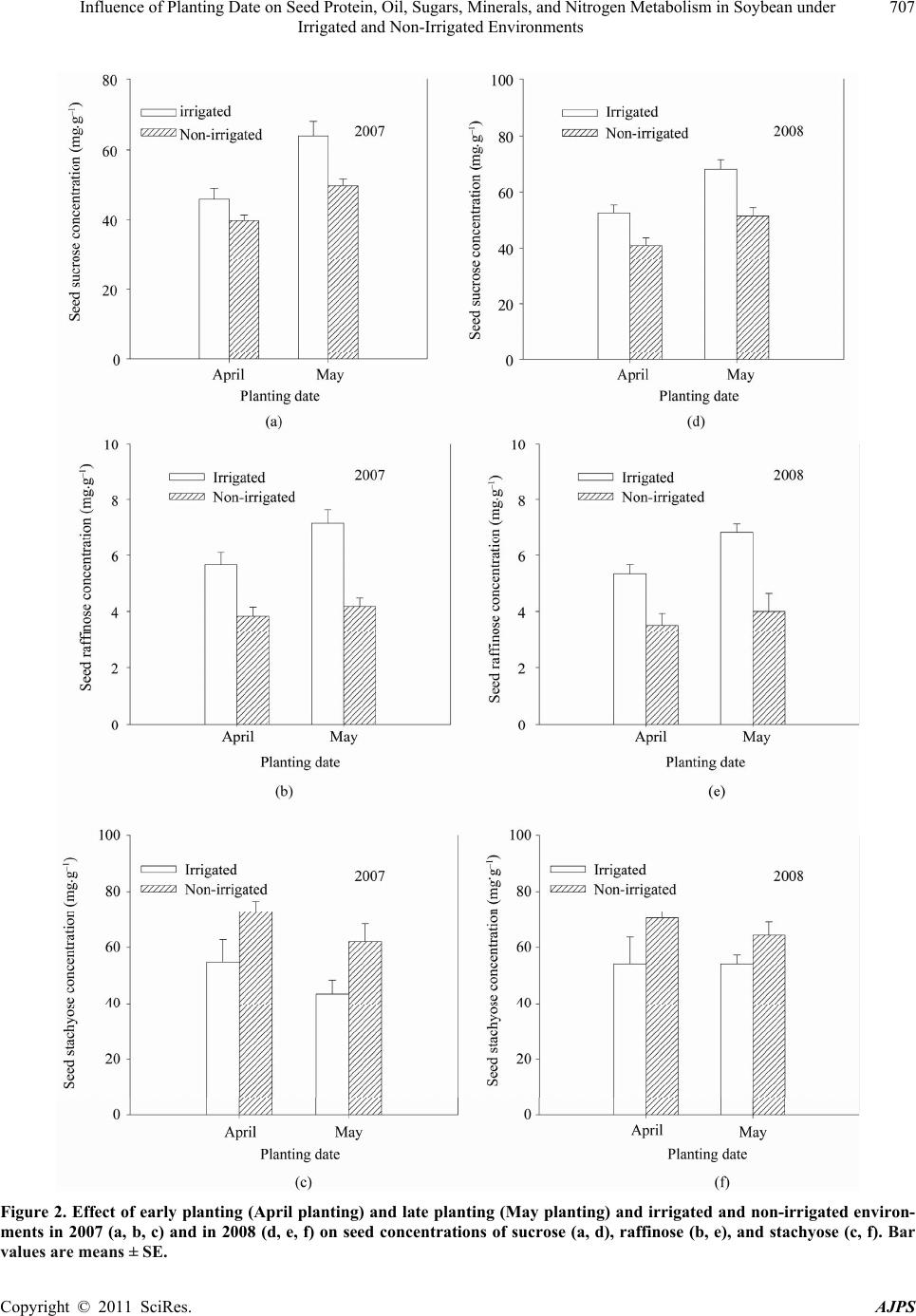 Influence of Planting Date on Seed Protein, Oil, Sugars, Minerals, and Nitrogen Metabolism in Soybean under 707 Irrigated and Non-Irrigated Environments Figure 2. Effect of early planting (April planting) and late planting (May planting) and irrigated and non-irrigated environ- ments in 2007 (a, b, c) and in 2008 (d, e, f) on seed concentrations of sucrose (a, d), raffinose (b, e), and stachyose (c, f). Bar values are means ± SE. Copyright © 2011 SciRes. AJPS 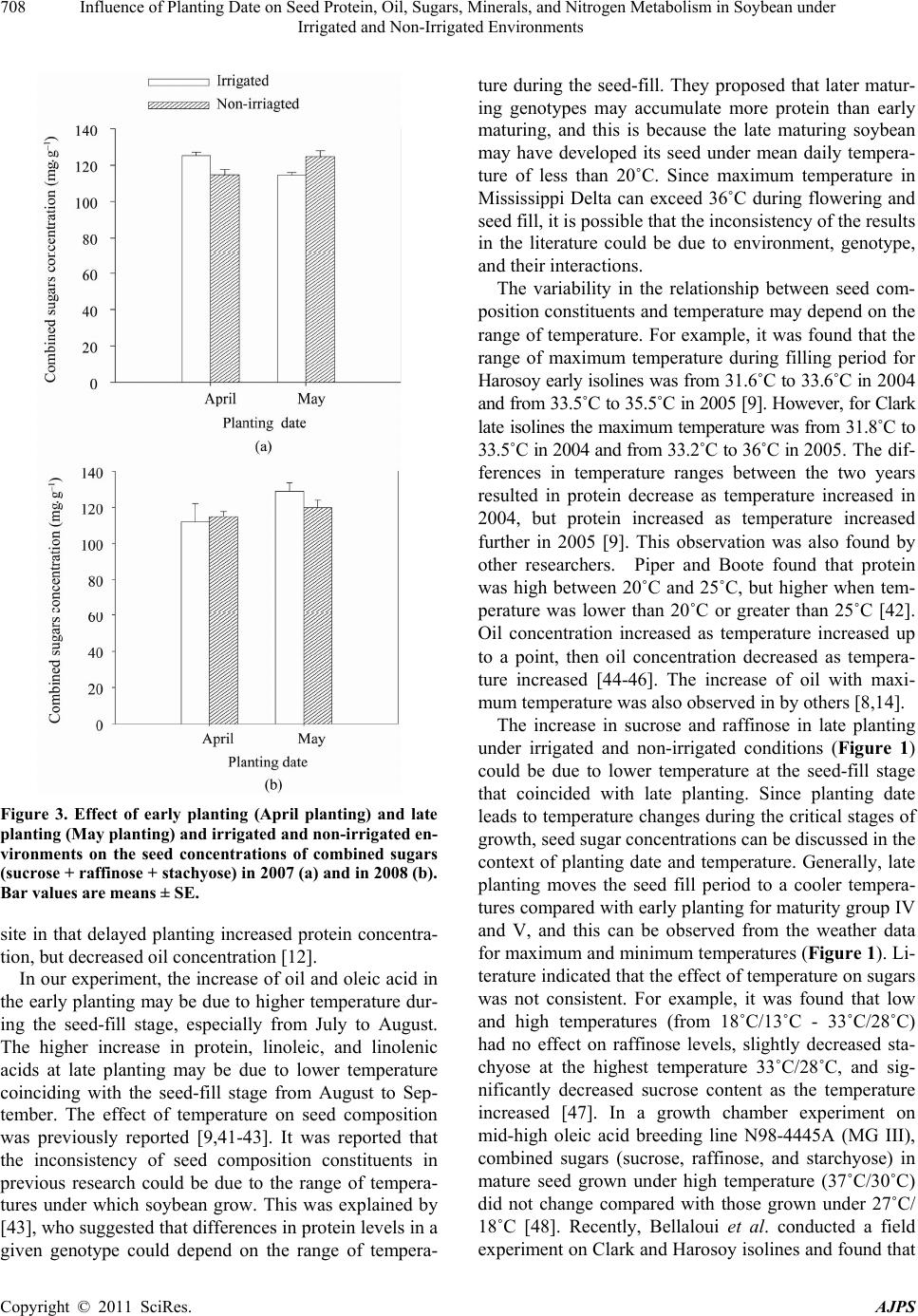 Influence of Planting Date on Seed Protein, Oil, Sugars, Minerals, and Nitrogen Metabolism in Soybean under 708 Irrigated and Non-Irrigated Environments Figure 3. Effect of early planting (April planting) and late planting (May planting) and irrigated and non-irrigated en- vironments on the seed concentrations of combined sugars (sucrose + raffinose + stachyose) in 2007 (a) and in 2008 (b). Bar values are means ± SE. site in that delayed planting increased protein concentra- tion, but decreased oil concentration [12]. In our experiment, the increase of oil and oleic acid in the early planting may be due to higher temperature dur- ing the seed-fill stage, especially from July to August. The higher increase in protein, linoleic, and linolenic acids at late planting may be due to lower temperature coinciding with the seed-fill stage from August to Sep- tember. The effect of temperature on seed composition was previously reported [9,41-43]. It was reported that the inconsistency of seed composition constituents in previous research could be due to the range of tempera- tures under which soybean grow. This was explained by [43], who suggested that differences in protein levels in a given genotype could depend on the range of tempera- ture during the seed-fill. They proposed that later matur- ing genotypes may accumulate more protein than early maturing, and this is because the late maturing soybean may have developed its seed under mean daily tempera- ture of less than 20˚C. Since maximum temperature in Mississippi Delta can exceed 36˚C during flowering and seed fill, it is possible that the inconsistency of the results in the literature could be due to environment, genotype, and their interactions. The variability in the relationship between seed com- position constituents and temperature may depend on the range of temperature. For example, it was found that the range of maximum temperature during filling period for Harosoy early isolines was from 31.6˚C to 33.6˚C in 2004 and from 33.5˚C to 35.5˚C in 2005 [9]. However, for Clark late isolines the maximum temperature was from 31.8˚C to 33.5˚C in 2004 and from 33.2˚C to 36˚C in 2005. The dif- ferences in temperature ranges between the two years resulted in protein decrease as temperature increased in 2004, but protein increased as temperature increased further in 2005 [9]. This observation was also found by other researchers. Piper and Boote found that protein was high between 20˚C and 25˚C, but higher when tem- perature was lower than 20˚C or greater than 25˚C [42]. Oil concentration increased as temperature increased up to a point, then oil concentration decreased as tempera- ture increased [44-46]. The increase of oil with maxi- mum temperature was also observed in by others [8,14]. The increase in sucrose and raffinose in late planting under irrigated and non-irrigated conditions (Figure 1) could be due to lower temperature at the seed-fill stage that coincided with late planting. Since planting date leads to temperature changes during the critical stages of growth, seed sugar concentrations can be discussed in the context of planting date and temperature. Generally, late planting moves the seed fill period to a cooler tempera- tures compared with early planting for maturity group IV and V, and this can be observed from the weather data for maximum and minimum temperatures (Figure 1). Li- terature indicated that the effect of temperature on sugars was not consistent. For example, it was found that low and high temperatures (from 18˚C/13˚C - 33˚C/28˚C) had no effect on raffinose levels, slightly decreased sta- chyose at the highest temperature 33˚C/28˚C, and sig- nificantly decreased sucrose content as the temperature increased [47]. In a growth chamber experiment on mid-high oleic acid breeding line N98-4445A (MG III), combined sugars (sucrose, raffinose, and starchyose) in mature seed grown under high temperature (37˚C/30˚C) did not change compared with those grown under 27˚C/ 18˚C [48]. Recently, Bellaloui et al. conducted a field experiment on Clark and Harosoy isolines and found that Copyright © 2011 SciRes. AJPS 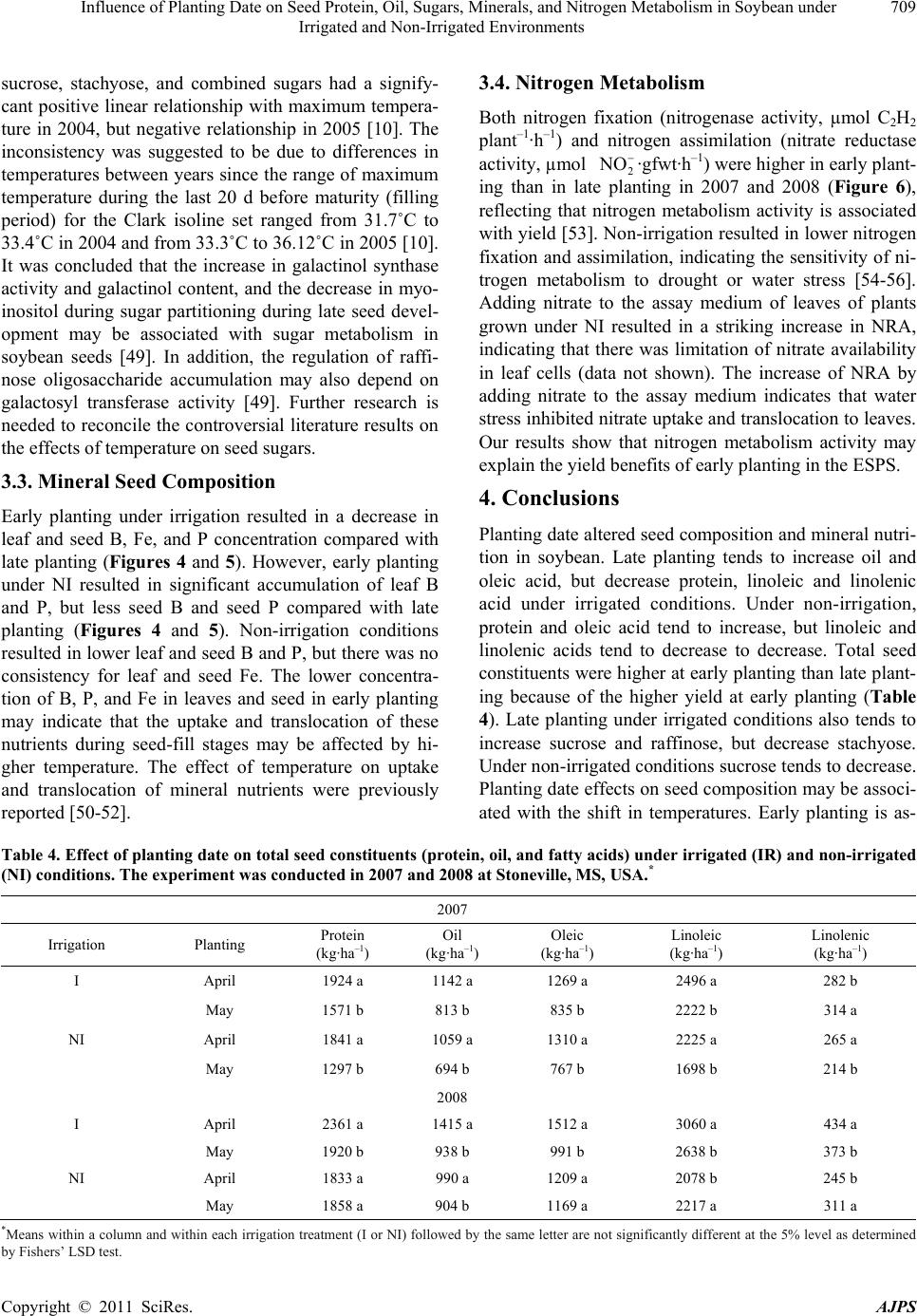 Influence of Planting Date on Seed Protein, Oil, Sugars, Minerals, and Nitrogen Metabolism in Soybean under 709 Irrigated and Non-Irrigated Environments sucrose, stachyose, and combined sugars had a signify- cant positive linear relationship with maximum tempera- ture in 2004, but negative relationship in 2005 [10]. The inconsistency was suggested to be due to differences in temperatures between years since the range of maximum temperature during the last 20 d before maturity (filling period) for the Clark isoline set ranged from 31.7˚C to 33.4˚C in 2004 and from 33.3˚C to 36.12˚C in 2005 [10]. It was concluded that the increase in galactinol synthase activity and galactinol content, and the decrease in myo- inositol during sugar partitioning during late seed devel- opment may be associated with sugar metabolism in soybean seeds [49]. In addition, the regulation of raffi- nose oligosaccharide accumulation may also depend on galactosyl transferase activity [49]. Further research is needed to reconcile the controversial literature results on the effects of temperature on seed sugars. 3.3. Mineral Seed Composition Early planting under irrigation resulted in a decrease in leaf and seed B, Fe, and P concentration compared with late planting (Figures 4 and 5). However, early planting under NI resulted in significant accumulation of leaf B and P, but less seed B and seed P compared with late planting (Figures 4 and 5). Non-irrigation conditions resulted in lower leaf and seed B and P, but there was no consistency for leaf and seed Fe. The lower concentra- tion of B, P, and Fe in leaves and seed in early planting may indicate that the uptake and translocation of these nutrients during seed-fill stages may be affected by hi- gher temperature. The effect of temperature on uptake and translocation of mineral nutrients were previously reported [50-52]. 3.4. Nitrogen Metabolism Both nitrogen fixation (nitrogenase activity, µmol C2H2 plant–1·h–1) and nitrogen assimilation (nitrate reductase activity, µmol 2 NO ·gfwt·h–1) were higher in early plant- ing than in late planting in 2007 and 2008 (Figure 6), reflecting that nitrogen metabolism activity is associated with yield [53]. Non-irrigation resulted in lower nitrogen fixation and assimilation, indicating the sensitivity of ni- trogen metabolism to drought or water stress [54-56]. Adding nitrate to the assay medium of leaves of plants grown under NI resulted in a striking increase in NRA, indicating that there was limitation of nitrate availability in leaf cells (data not shown). The increase of NRA by adding nitrate to the assay medium indicates that water stress inhibited nitrate uptake and translocation to leaves. Our results show that nitrogen metabolism activity may explain the yield benefits of early planting in the ESPS. 4. Conclusions Planting date altered seed composition and mineral nutri- tion in soybean. Late planting tends to increase oil and oleic acid, but decrease protein, linoleic and linolenic acid under irrigated conditions. Under non-irrigation, protein and oleic acid tend to increase, but linoleic and linolenic acids tend to decrease to decrease. Total seed constituents were higher at early planting than late plant- ing because of the higher yield at early planting (Table 4). Late planting under irrigated conditions also tends to increase sucrose and raffinose, but decrease stachyose. Under non-irrigated conditions sucrose tends to decrease. Planting date effects on seed composition may be associ- ated with the shift in temperatures. Early planting is as- Table 4. Effect of planting date on total seed constituents (protein, oil, and fatty acids) under irrigated (IR) and non-irrigated (NI) conditions. The experiment was conducted in 2007 and 2008 at Stoneville, MS, USA.* 2007 Irrigation Planting Protein (kg·ha–1) Oil (kg·ha–1) Oleic (kg·ha–1) Linoleic (kg·ha–1) Linolenic (kg·ha–1) I April 1924 a 1142 a 1269 a 2496 a 282 b May 1571 b 813 b 835 b 2222 b 314 a NI April 1841 a 1059 a 1310 a 2225 a 265 a May 1297 b 694 b 767 b 1698 b 214 b 2008 I April 2361 a 1415 a 1512 a 3060 a 434 a May 1920 b 938 b 991 b 2638 b 373 b NI April 1833 a 990 a 1209 a 2078 b 245 b May 1858 a 904 b 1169 a 2217 a 311 a *Means within a column and within each irrigation treatment (I or NI) followed by the same letter are not significantly different at the 5% level as determined by Fishers’ LSD test. Copyright © 2011 SciRes. AJPS 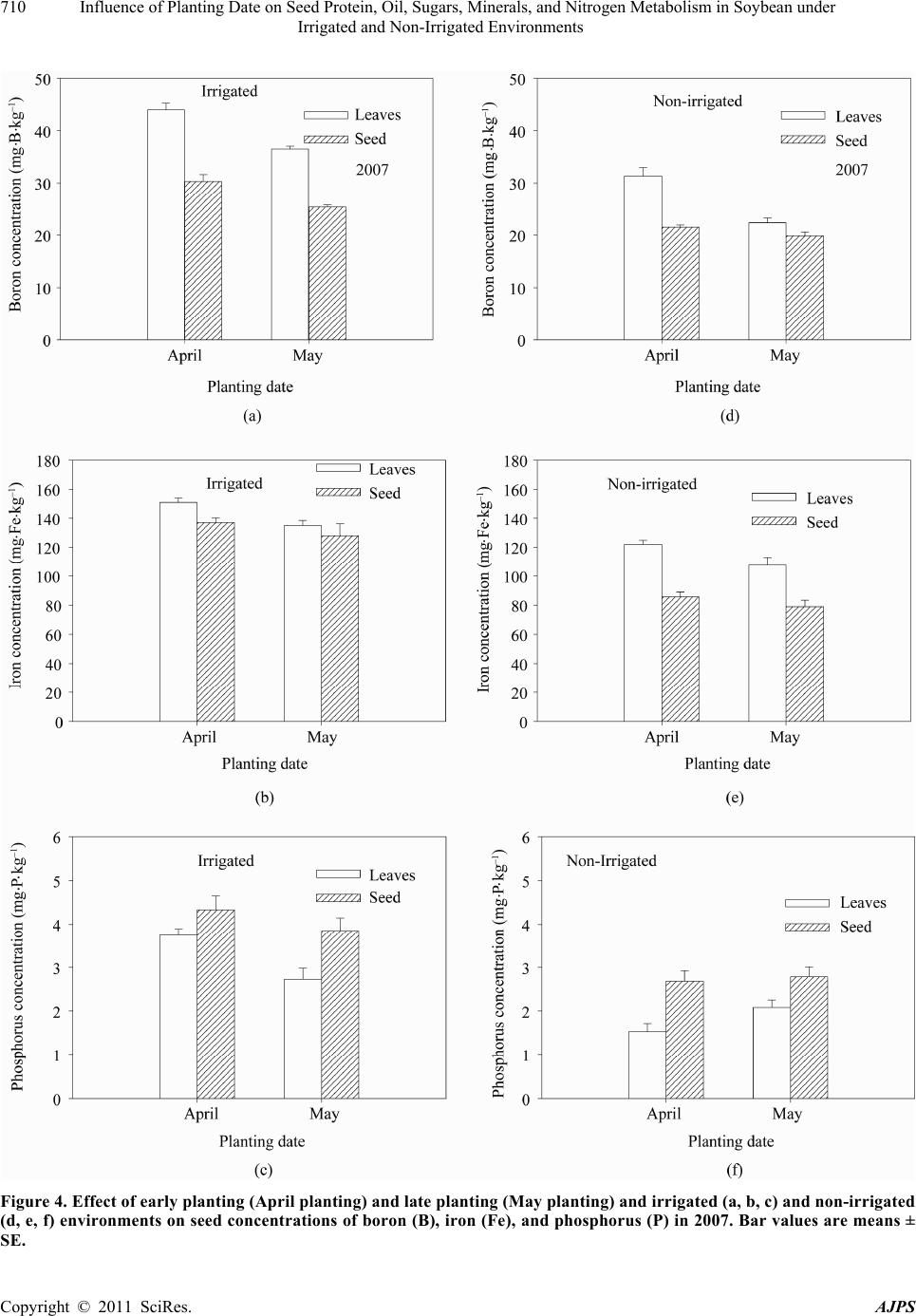 Influence of Planting Date on Seed Protein, Oil, Sugars, Minerals, and Nitrogen Metabolism in Soybean under 710 Irrigated and Non-Irrigated Environments Figure 4. Effect of early planting (April planting) and late planting (May planting) and irrigated (a, b, c) and non-irrigated (d, e, f) environments on seed concentrations of boron (B), iron (Fe), and phosphorus (P) in 2007. Bar values are means ± SE. Copyright © 2011 SciRes. AJPS 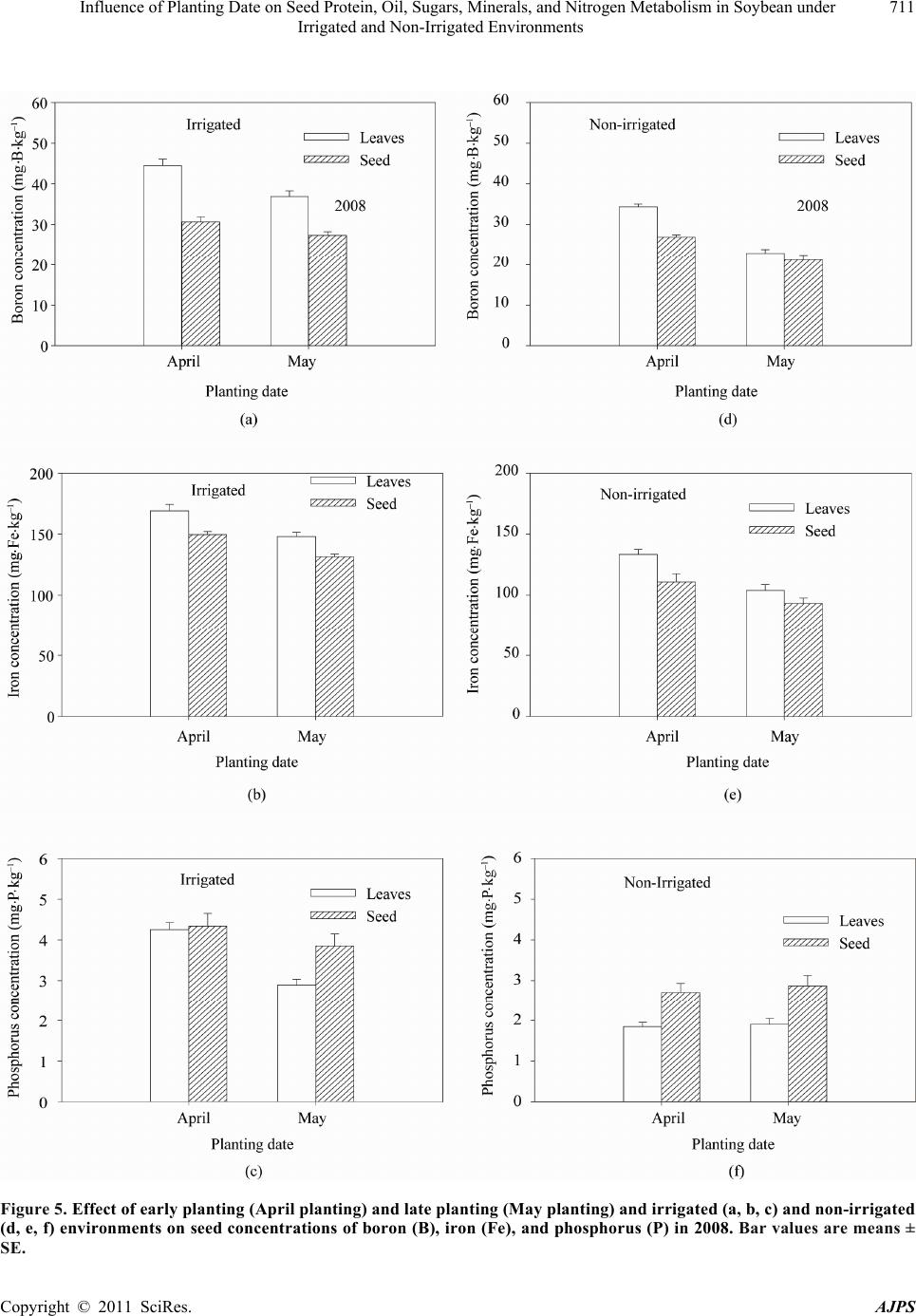 Influence of Planting Date on Seed Protein, Oil, Sugars, Minerals, and Nitrogen Metabolism in Soybean under 711 Irrigated and Non-Irrigated Environments Figure 5. Effect of early planting (April planting) and late planting (May planting) and irrigated (a, b, c) and non-irrigated (d, e, f) environments on seed concentrations of boron (B), iron (Fe), and phosphorus (P) in 2008. Bar values are means ± SE. Copyright © 2011 SciRes. AJPS 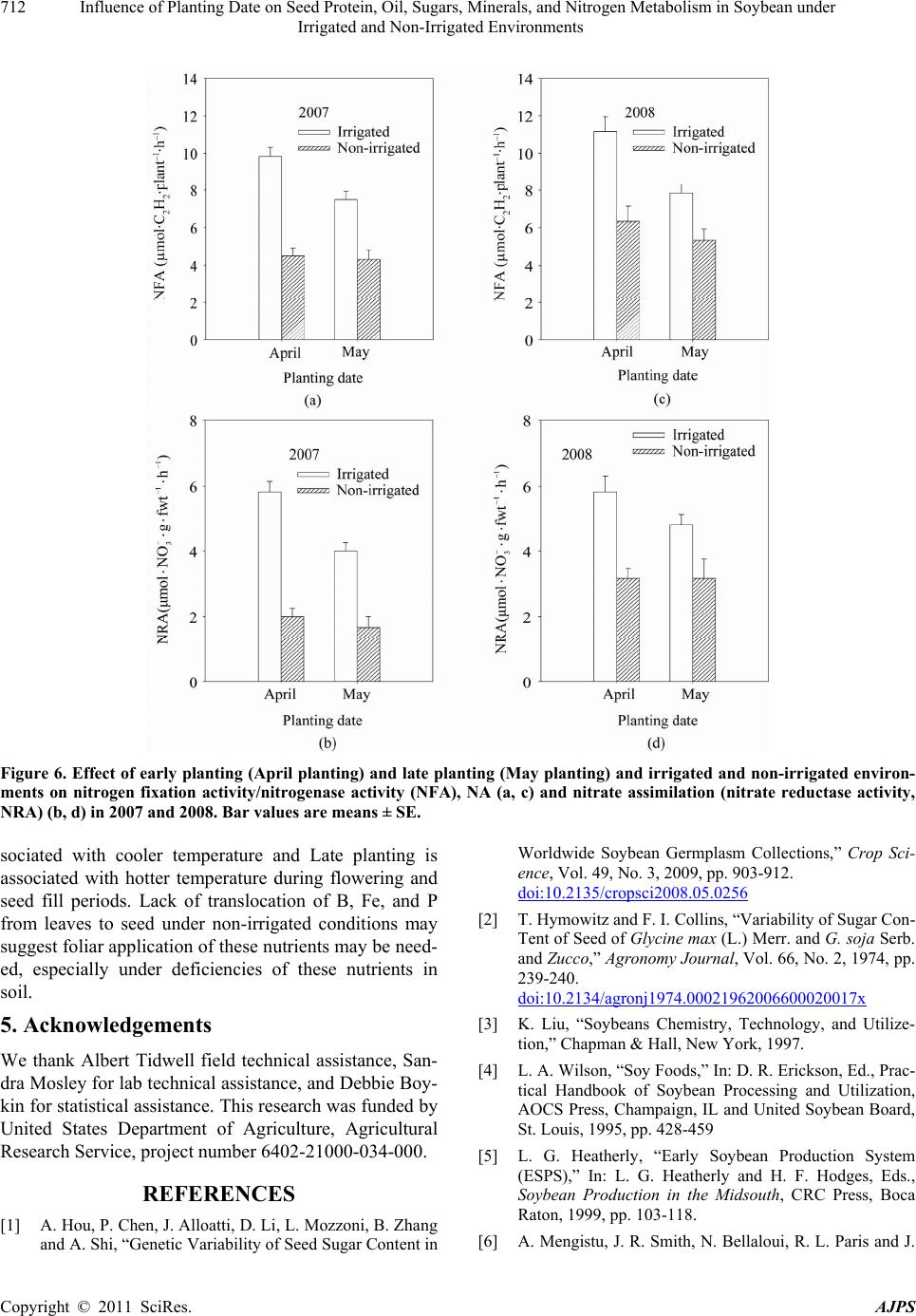 Influence of Planting Date on Seed Protein, Oil, Sugars, Minerals, and Nitrogen Metabolism in Soybean under Irrigated and Non-Irrigated Environments Copyright © 2011 SciRes. AJPS 712 Figure 6. Effect of early planting (April planting) and late planting (May planting) and irrigated and non-irrigated environ- ments on nitrogen fixation activity/nitrogenase activity (NFA), NA (a, c) and nitrate assimilation (nitrate reductase activity, NRA) (b, d) in 2007 and 2008. Bar values are means ± SE. sociated with cooler temperature and Late planting is associated with hotter temperature during flowering and seed fill periods. Lack of translocation of B, Fe, and P from leaves to seed under non-irrigated conditions may suggest foliar application of these nutrients may be need- ed, especially under deficiencies of these nutrients in soil. 5. Acknowledgements We thank Albert Tidwell field technical assistance, San- dra Mosley for lab technical assistance, and Debbie Boy- kin for statistical assistance. This research was funded by United States Department of Agriculture, Agricultural Research Service, project number 6402-21000-034-000. REFERENCES [1] A. Hou, P. Chen, J. Alloatti, D. Li, L. Mozzoni, B. Zhang and A. Shi, “Genetic Variability of Seed Sugar Content in Worldwide Soybean Germplasm Collections,” Crop Sci- ence, Vol. 49, No. 3, 2009, pp. 903-912. doi:10.2135/cropsci2008.05.0256 [2] T. Hymowitz and F. I. Collins, “Variability of Sugar Con- Tent of Seed of Glycine max (L.) Merr. and G. soja Serb. and Zucco,” Agronomy Journal, Vol. 66, No. 2, 1974, pp. 239-240. doi:10.2134/agronj1974.00021962006600020017x [3] K. Liu, “Soybeans Chemistry, Technology, and Utilize- tion,” Chapman & Hall, New York, 1997. [4] L. A. Wilson, “Soy Foods,” In: D. R. Erickson, Ed., Prac- tical Handbook of Soybean Processing and Utilization, AOCS Press, Champaign, IL and United Soybean Board, St. Louis, 1995, pp. 428-459 [5] L. G. Heatherly, “Early Soybean Production System (ESPS),” In: L. G. Heatherly and H. F. Hodges, Eds., Soybean Production in the Midsouth, CRC Press, Boca Raton, 1999, pp. 103-118. [6] A. Mengistu, J. R. Smith, N. Bellaloui, R. L. Paris and J.  Influence of Planting Date on Seed Protein, Oil, Sugars, Minerals, and Nitrogen Metabolism in Soybean under 713 Irrigated and Non-Irrigated Environments A. Wrather, “Irrigation and Time of Harvest Effects on Evaluation of Selected Soybean Accessions against Pho- mopsis longicolla,” Crop Science, 2010, Vol. 50, No. 5, pp. 2055-2064. doi:10.2135/cropsci2009.11.0657 [7] J. R. Smith, A. Mengistu, R. L. Nelson and R. L. Paris, “Identification of Soybean Accessions with High Ger- minability in High-Temperature Environments,” Crop Science, Vol. 48, No. 6, 2008, pp. 2279-2288. doi:10.2135/cropsci2008.01.0026 [8] N. Bellaloui, A. Mengistu and R. L. Paris, “Soybean Seed Composition in Cultivars Differing in Resistance to Char- coal Rot (Macrophomina phaseolina),” Journal of Agri- cultural Science, Vol. 146, 2008, pp. 667-675. doi:10.1017/S0021859608007971 [9] N. Bellaloui, J. R. Smith, J. D. Ray and A. M. Gillen, “Effect of Maturity on Seed Composition in the Early Soybean Production System as Measured on Near-Isog- enic Soybean Lines,” Crop Sc ience , Vol. 49, No. 2, 2009, pp. 608-620. doi:10.2135/cropsci2008.04.0192 [10] N. Bellaloui, K. N. Reddy, A. M Gillen and C. A. Abel, “Nitrogen Metabolism and Seed Composition as Influ- Enced by Foliar Boron Application in Soybean,” Plant and Soil, Vol. 336, No. 1-2, 2010, pp.143-155. doi:10.1007/s11104-010-0455-6 [11] P. Pedersen and J. G. Lauer, “Response of Soybean Yield Components to Management System and Planting Date,” Agronomy Journal, Vol. 96, No.5, 2004, pp. 1372-1381. doi:10.2134/agronj2004.1372 [12] M. V. Kane, C. C. Steele, L. J. Grabau, C. T. MacKown and D. F. Hildebrand, “Early-Maturing Soybean Crop- ping System: III. Protein and Oil Contents and Oil Com- position,” Agronomy Journal, Vol. 89, No. 3, 1997, pp. 464-469. doi:10.2134/agronj1997.00021962008900030016x [13] T. C. Helms, C. R. Jr. Hurburgh, , R. L. Lussenden and D. A. Whited, “Economic Analysis of Increased Protein and Decreased Yield Due to Delayed Planting of Soybean,” Journal of Production Agriculture, 1990, Vol. 3, pp. 367- 371. [14] R. W. Howell and F. I. Collins, “Factors Affecting Lino- Lenic and Linoleic Acid Content of Soybean Oil,” Agronomy Journal, Vol. 49, No. 11, 1957, pp. 593-597. doi:10.2134/agronj1957.00021962004900110007x [15] J. R. Wilcox and J. F. Cavins, “Normal and Low Lino- lenic Acid Soybean Strains: Response to Planting Date,” Crop Science, Vol. 32, No. 5, 1992, pp.1248-1251. doi:10.2135/cropsci1992.0011183X003200050037x [16] E. Bachlava and A. J. Cardinal, “Correlation between Temperature and Oleic Acid Seed Content in Three Seg- regating Soybean Populations,” Crop Science, Vol. 49, No. 4, 2009, pp. 1328-1335. doi:10.2135/cropsci2008.11.0660 [17] B. F. Carver, J. W. Burton, T. E. Jr. Carter and R. F. Wil- son, “Response to Environmental Variation of Soybean Lines Selected for Altered Unsaturated Fatty Acid Com- position,” Crop Science, Vol. 26, No. 6, 1986, pp. 1176-1180. doi:10.2135/cropsci1986.0011183X002600060021x [18] J. W. Burton, “Recent Developments in Breeding Soy- Beans for Improved Oil Quality,” Fat Science Technol- ogy, Vol. 93, 1991, pp. 121-128. [19] T. M. Cheesbrough, “Changes in the Enzymes for Fatty Acid Synthesis and Desaturation during Acclimation of Developing Soybean Seeds to Altered Growth Tempera- ture,” Plant Physiology, Vol. 90, 1989, pp. 760-764. doi:10.1104/pp.90.2.760 [20] G. O. Tang, W. P. Novitzky, H. C. Griffin, S. C. Huber and R. E. Dewey, “Oleate Desaturase Enzymes of Soy- bean: Evidence of Regulation through Differential Stabil- ity and Phosphorylation,” Plant Journal, Vol. 44, No. 3, 2005, pp. 433-446. doi:10.1111/j.1365-313X.2005.02535.x [21] J. M. Caba, C. Lluch and F. Ligero,“ Distribution of Ni- trate Reductase Activity in Vicia Faba: Effect of Nitrate and Plant Genotype,” Physiolgia Plantarum, Vol. 93, No. 4, 1995, pp. 667-672. doi:10.1111/j.1399-3054.1995.tb05115.x [22] Y. Kanayama, K. Kimura, Y. Nakamura and T. Ike, “Pu- rification and Characterization of Nitrate Reductase from Nodule Cytosol of Soybean Plants,” Physiologia Planta- rum, Vol. 105, No. 3, 1999, pp. 396-401. doi:10.1034/j.1399-3054.1999.105302.x [23] J. R. Wilcox and R. M. Shibles, “Interrelationships among Seed Quality Attributes in Soybean,” Crop Sci- ence, Vol. 41, No. 1, 2001, pp. 11-14. doi:10.2135/cropsci2001.41111x [24] AOAC, “Method 988.05,” In: K. Helrich, Ed., Official Methods of Analysis, 15th Edition, The Association of Official Analytical Chemists, Inc., Arlington, 1990a. [25] AOAC, “Method 920.39,” In: K. Helrich, Ed., Official Methods of Analysis, 15th Edition, The Association of Official Analytical Chemists, Inc., Arlington, 1990b. [26] E. Boydak, M. Alpaslan, M. Hayta, S. Gercek and M. Simsek, “Seed Composition of Soybeans Grown in the Harran Region of Turkey as Affected by Row Spacing and Irrigation,” Journal of Agricultural and Food Chem- istry, Vol. 50, No. 16, 2002, pp. 4718-4720. doi:10.1021/jf0255331 [27] L. Klepper and R. H. Hageman, “The Occurrence of Ni- trate Reductase in Apple Leaves,” Plant Physiology, Vol. 44, No. 1, 1969, pp. 110-114. doi:10.1104/pp.44.1.110 [28] N. Bellaloui, K. N. Reddy, R. M. Zablotowicz and A. Mengistu, “Simulated Glyphosate Drift Influences Nitrate Assimilation and Nitrogen Fixation in Non-Glyphosate- Resistant Soybean,” Journal of Agriculture and Food Chemistry, Vol. 54, 2006, pp. 3357-3364. [29] R. W. F. Hardy, D. Holsten, E. K. Jackson and R. C. Burns, “The Acetylene-Ethylene Assay for Nitrogen Fix- ation: Laboratory and Field Evaluation,” Plant Physiol- ogy, Vol. 43, No. 8, 1968, pp. 1185-1207. doi:10.1104/pp.43.8.1185 [30] R. M. Zablotowicz, D. D. Focht and G. H. Cannell, Copyright © 2011 SciRes. AJPS  Influence of Planting Date on Seed Protein, Oil, Sugars, Minerals, and Nitrogen Metabolism in Soybean under 714 Irrigated and Non-Irrigated Environments “Nodulation and N, Fixation of Field Grown California Cowpeas as Influenced by Irrigated and Droughted Con- ditions” Agronomy Journal, Vol. 73, No. 1, 1981, pp. 9-12. doi:10.2134/agronj1981.00021962007300010003x [31] G. Lohse, “Microanalytical Azomethine-H Method for Boron Determination in Plant Tissue,” Communications in Soil Science and Plant Analysis, Vol. 13, No. 2, 1982, pp. 127-134. doi:10.1080/00103628209367251 [32] M. K. John, H. H. Chuah and J. H. Neufeld, “Application of Improved Azomethine-H Method to the Determination of Boron in Soils and Plants,” Analytical Letters, Vol. 8, No. 8, 1975, pp. 559-568. doi:10.1080/00032717508058240 [33] S. L Bandemer and P. J. Schaible, “Determination of Iron. a Study of the O-Phenanthrolinemethod,” Industrial and Engineering Chemistry Analytical Edition, Vol. 16, No. 5, 1944, pp. 317-319. doi:10.1021/i560129a013 [34] R. L. Leoppert and W. P. Inskeep, “Colorimetric Deter- mination of Ferrous Iron and Ferric Iron by the 1,10-Phenanthroline Method,” In: J. M. Bigham, Ed., Methods of Soil Analysis: Part 3, Chemical Methods, Soil Science Society of America, Madison, 1996, pp. 659-661. [35] A. J. Cavell, “The Colorimetric Determination of Phos- phorus in Plant Materials,” Journal of the Science of Food and Agriculture, Vol. 6, No. 8, 1955, pp. 479-480. doi:10.1002/jsfa.2740060814 [36] Analytical Methods Committee Analyst, London, 1959, pp. 214. [37] SAS, “SAS 9.1 TS LeVel 1M3, Windows Version 5.1. 2600,” SAS Institute, Cary, 2001 [38] N. Bellaloui and A. Mengistu, “Seed Composition Is In- fluenced by Irrigation Regimes and Cultivar Differences in Soybean,” Irrigation Science, Vol. 26, No. 3, 2008, pp. 261-268. doi:10.1007/s00271-007-0091-y [39] A. Mengistu, L. Castlebury, R. Smith, J. Ray and N. Bel- laloui, “Seasonal Progress of Phomopsis Longicolla In- fection on Soybean Plant Parts and Its Relationship to Seed Quality,” Plant Disease, 2009, Vol. 93, No. 10, pp. 1009-1018. doi:10.1094/PDIS-93-10-1009 [40] J. Gao, X. Hao, K. D. Thelen and G. P. Robertson, “Ag- ronomic Management System and Precipitation Effects on Soybean Oil and Fatty Acid Profiles,” Crop Science, Vol. 149, No. 3, 2009, pp. 1049-1057. doi:10.2135/cropsci2008.08.0497 [41] D. M. Maestri, D. O. Labuckas, J. M. Meriles, A. L. La- marques, J. A. Zygadlo and C. A. Guzman, “Seed Com- Position of Soybean Cultivars Evaluated in Different En- vironmental Regions,” Journal of the Science of Food and Agriculture, Vol. 77, No. 4, 1998, pp. 494-498. doi:10.1002/(SICI)1097-0010(199808)77:4<494::AID-JS FA69>3.0.CO;2-B [42] E. L. Piper and K. J. Boote, “Temperature and Cultivar Effects on Soybean Seed Oil and Protein Concentra- tions,” Journal of American Oil Chemists’ Society, Vol. 76, 1999, pp. 1233-1242. [43] J. L. Dardanelli, M. Balzarini, M. J. Martinez, M. Cuni- berti, S. Resnik, S. F. Ramunda, R. Herrero and H. Bai- gorri, “Soybean Maturity Groups, Environments, and Their Interaction Define Mega-Environments for Seed Composition in Argentina,” Crop Science, Vol. 46, No. 5, 2006, pp. 1939-1947. doi:10.2135/cropsci2005.12-0480 [44] D. L. Dornbos and R. E. Mullen, “Soybean Seed Protein and Oil Contents and Fatty-Acid Composition Adjust- ments by Drought and Temperature,” Journal of Ameri- can Oil Chemists’ Society, Vol. 69, 1992, pp. 228-231. [45] L. R. Gibson and R. E. Mullen, “Soybean Seed Quality Reductions by High Day and Night Temperature,” Crop Science, Vol. 36, 1996, pp. 1615-1619. doi:10.2135/cropsci1996.0011183X003600060034x [46] J. M. G. Thomas, K. J. Boote, L. H. Jr. Allen, M. Gallo-Meagher and J. M. Davis, “Seed Physiology and Metabolism: Elevated Temperature and Carbon Dioxide Effects on Soybean Seed Composition and Transcript Abundance,” Crop Science, Vol. 43, No. 4, 2003, pp. 1548-1557. doi:10.2135/cropsci2003.1548 [47] R. B. Wolf, J. F. Cavins, R. Kleiman and L. T. Black, “Effect of Temperature on Soybean Seed Constituents: Oil, Protein, Moisture, Fatty Acids, Amino Acid, and Sugars,” Journal of American Oil Chemists’ Society, Vol. 59, 1982, pp. 230-232. [48] C. Ren, K. D. Bilyeu and P. R. Beuselinck, “Composition, Vigor, and Proteome of Mature Soybean Seeds Devel- oped under High Temperature,” Crop Science, Vol. 49, No. 3, 2009, pp.1010-1022. doi:10.2135/cropsci2008.05.0247 [49] C. A. Lowell and T. M. Ku, “Oligosaccharide Metabo- lism and Accumulation in Developing Soybean Seeds,” Crop Science, Vol. 29, No. 2, 1989, pp. 459-465. doi:10.2135/cropsci1989.0011183X002900020044x [50] P. J. Kramer, “Effect of Temperature on Water and Ion Transport in Soybean and Broccoli Systems,” Plant Physiology, Vol. 64, No. 1, 1979, pp. 83-87. doi:10.1104/pp.64.1.83 [51] C. Engels and H. Marschner, “Effect of Suboptimal Root Zone Temperatures at Varied Nutrient Supply and Shoot Meristem Temperature on Growth and Nutrient Concen- trations in Maize Seedlings (Zea mays L.),” Plant and Soil, Vol. 126, No. 2, 1990, pp. 215-25. doi:10.1007/BF00012825 [52] C. Engels, L. Munkle and H. Marschner, “Effect of Root Zone Temperature and Shoot Demand on Uptake and Xylem Transport of Macronutrients in Maize (Zea mays L.) Journal of Experimental Botany, Vol. 43, No. 4, 1992, pp. 537-547. doi:10.1093/jxb/43.4.537 [53] O. Uchikawa, K. Tanaka, M. Miyazaki and Y. Matsue, “Effects of Planting Pattern on Growth, Yield and Nitro- gen Fixation Activity of Soybean Cropped with Late Planting and Non-Intertillage Cultivation Method in Northern Kyusyu,” Japanese Journal of Crop Science, Vol. 78, No. 2, 2009, pp. 163-169. doi:10.1626/jcs.78.163 [54] R. Serraj, “Effects of Drought on Nitrogen Fixation in Soybean Root Nodules,” Indian Journal of Experimental Copyright © 2011 SciRes. AJPS  Influence of Planting Date on Seed Protein, Oil, Sugars, Minerals, and Nitrogen Metabolism in Soybean under Irrigated and Non-Irrigated Environments Copyright © 2011 SciRes. AJPS 715 Biology, Vol. 41, 2003, pp.1136-41. [55] J. G. Streeter, “Effects of Drought Stress on Legume Symbiotic Nitrogen Fixation: Physiological Mecha- nisms,” Plant, Cell & Environment, Vol. 26, No. 8, 2003, pp. 1199-1204. doi:10.1046/j.1365-3040.2003.01041.x [56] T. R. Sinclair, L. C. Purcell, C. A. King, C. H. Sneller, P. Chen and V. Vadez, “Drought Tolerance and Yield In- crease of Soybean Resulting from Improved Symbiotic N2 Fixation,” Field Crops Research, Vol. 101, No. 1, 2007, pp. 68-71. doi:10.1016/j.fcr.2006.09.010
|