Open Access Library Journal
Vol.05 No.02(2018), Article ID:82578,14 pages
10.4236/oalib.1104365
Spreading Dynamic of a PLSGP Giving up Smoking Model on Scale-Free Network
Yanling Fei*, Xiongding Liu
School of Electronics and Information, Yangtze University, Jingzhou, China
Copyright © 2018 by authors and Open Access Library Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: January 23, 2018; Accepted: February 20, 2018; Published: February 23, 2018
ABSTRACT
A new PLSGP (potential smokers-light smokers-persistent smokers-giving up smokers-potential smokers) model with birth and death rates on complex heterogeneous networks is presented. Using the mean-field theory, we obtain the basic reproduction number and find that basic reproduction number for constant contact is independent of the topology of the underlying networks. When , the smoking-free equilibrium is globally asymptotically stable, then the smoking will disappear. When , the smoking-present equilibrium is global attractivity, then the number of smoker will remain stable and smoking will become endemic. Numerical simulations illustrated theoretical results. Our result shows that the model is very important to control the spread of the smoking.
Subject Areas:
Complex Network Models, Dynamical System, Network Modeling and Simulation, Numerical Mathematics, Numerical Methods
Keywords:
Spreading Dynamic, Smoking, Basic Reproduction Number, Equilibrium, Scale-Free Network

1. Introduction
Smoking is closely related to health, and smoking ranks fourth among the top 10 risk factors for health, according to the World Health Organization report. Tobacco has been identified as a primary carcinogen around the world. Smokers are 10 to 30 times more likely to develop lung cancer than non-smokers. Smoking problem of people has become a significant public health concern. The behavior of smoking often causes a range of negative consequences. Long-term smoking produces negative changes in the heart, such as heart rate and blood pressure rise. Smoking damages almost all parts of the human body and contributes to a number of human diseases including lung cancer, respiratory disease, heart disease, alimentary canal effect and eventually death. Due to the increasing in the number of smokers, tobacco use is also as a disease to be treated.
In recent years, many types of epidemic models are discussed, such as virus dynamics models [1] [2] , tuberculosis models [3] [4] , and HIV models [5] [6] . One of the famous representative works in this area was done by Pastor-Satorras and Vespignani [7] . They presented a detailed analytical and numerical study on an SIS epidemic model in the highly heterogeneous networks (i.e., scale-free networks). The most striking result is that they found the absence of the epidemic threshold in these networks. That is, the threshold approaches zero in the limit of a large number of edges and nodes, and even a quite small infectious rate can produce a major epidemic outbreak. Liu XD et al. [8] presented an SIS (susceptible-infected-susceptible) epidemic model with infective medium and feedback mechanism on scale-free networks. They found that the epidemic is not only spread between individuals by direct contacts but also transmitted by medium, people’s initial response when epidemic disease outbreaks have been also considered. Moreno et al. [9] also found these similar conclusions for an SIR (susceptible-infected-removed) epidemic model on scale-free networks. The epidemic model is constantly evolving, such as SIRS (susceptible-infected-removed- susceptible), SEIR (susceptible-infected-exposed-removed), SIQRS (susceptible-infected-quarantined-recovered-susceptible) [10] [11] [12] [13] [14] .
Studying of smoking behavior has attracted the attention of many scholars and researchers recently. In order to explore the spread rule of smoking, some models are developed. Castillo-Garsow et al. [15] proposed a mathematical model for giving up smoking in the first time. In this model, a total constant population was divided into three states: potential smokers, that is, people who do not smoke yet but might become smokers in the future (P), smokers (S), and quit smokers (Q). Zaman [16] extended the work of Castillo-Garsow et al. [15] by adding the population of occasional smokers in the model, and presented qualitative behavior of the model. Zaman [17] presented the optimal campaigns in the smoking dynamics. They consider two possible control variables in the form of education and treatment campaigns oriented to decrease the attitude towards smoking and first showed the existence of an optimal control for the control problem.
However, in real life, some potential smokers may become light smoker since they contact with light smokers or persistent smokers. Some quit smokers may be only temporary quit smokers, so they will become potential smokers again. Enlightening by the previously mentioned cases, we present a PLSGP giving up smoking model on scale-free network. The paper is organized as follows: The model is formulated in Section 2. The basic reproduction number and existence of smoking equilibriums are calculated in Section 3. In Section 4, we analyze the stability of the equilibria. In Section 5, sensitivity analysis and numerical simulations are illustrated. In Section 6, we give some conclusions and discussions.
2. The Model Formulation
In this paper, we establish the giving up smoking model as Figure 1. From Figure 1, the total population is divided into four compartments, namely, the potential smokers compartment (P), light or occasion smokers compartment (L), persistent smokers compartment (S), and quit smokers group (G). The total recruitment number into this homogeneous social mixing community is b. Transmission coefficient from the potential smokers compartment to the light smokers compartment is , Transmission coefficient from the potential smokers compartment to the persistent smokers compartment is , Transmission coefficient from the light smokers compartment to the persistent smokers compartment is , The permanent quit smoking rate is , The relapse rate of which temporary quit people to become potential smokers is . Naturally death rate is . The total population size is . Let , , , be the relative densities of potential smokers, light smokers, persistent smokers and quit smokers nodes of degree k at time t, respectively.
With these assume, the dynamic mean-field equations of the model can be written as follows:
(2.1)
where
(2.2)
Figure 1. Transfer diagram for somking model.
where, is the probability that a node has degree k and thus , denotes the average degree. . Clearly, these variables obey the normalization condition:
. (2.3)
The initial conditions for system can be given as follows , , , . In this model, we assumed equal to p.
(2.4)
3. The Basic Reproduction Number and Equilibrium
Theorem 1. Consider system (2.1). Define . There always exists the smoking-free equilibrium . When , the system has an occasion smoking equilibrium .
Proof. To get the information-prevailing equilibrium solution , we need to make the right side of system equal to zero, it should satisfy
(3.1)
where , we follow from (3.1) that
(3.2)
Obviously, satisfies (3.1). Hence, and is an equilibrium of (2.1), which is called the smoking-free equilibrium.
Substituting and of (3. 2) into
Let
Clearly, is a solution of equation. To ensure the equation has a nontrivial solution, the following condition must should satisfied
and . (3.3)
We can obtain the reproductive number
.
4. Stability Analysis of the Equilibrium
Theorem 2. When , the smoking-free equilibrium of system (2.1) is globally asymptotically stable.
Proof. The Jacobian matrix of the smoking-free equilibrium of system (2.1), which is a matrix, can be written as follows:
where
A direct calculation leads to the characteristic polynomial of the smoking-free equilibrium in the following from:
,
where and .
Note that is equivalent to and that also implies: , which means . Note that is equivalent to and that also implies and, which means . Therefore, there exists a unique positive eigenvalue of if and only if , otherwise, if , all real-valued eigenvalues of are negative. By the Perron-Frobenius theorem, it implies that the maximal real part of all eigenvalues of is positive if and only if . Then, a theorem of Lajmanovich and York [18] yields the results of this theorem. The proof is thus completed.
Next, the globally attractivity of positive endemic equilibrium is discussed. The main result is given in the following theorem.
Lemma 1. [19] if , and , when and , we have , if , and , when and , we have .
Theorems 3. Suppose that is a solution of (2.4), with , , and . If , then , where is the unique smoking equilibrium of (2.4) for .
Proof: In the following, k is fixed to be any integer in . There exists a sufficiently small constant and a larger enough constant such that and for , therefore for . Submit this into the first equation of (2.4) gives
By Lemma 1, for any given constant , there exists a , such that for , where
(4.1)
From the second equation of (2.4), it follows that
(4.2)
Hence, for any given constant , there exists a , such that for , where
(4.3)
Then, it follows from the third equation of (2.4),
(4.4)
Similarly, for any given constant , there exists a , such that for , where
(4.5)
Since , we substitute this into the first equation of (2.4)
(4.6)
So for any given enough small constant , there exists a , such that for , where
(4.7)
It follows that
(4.8)
So for any given enough small constant , there exists a , such that for , where
(4.9)
From the third equation of (2.1) implies that
(4.10)
So for any given enough small constant , there exists a , such that for , where
(4.11)
Due to is a small positive constant, we can derive that , and . Let
(4.12)
We can easily get , .
Again, from the first equation of (2.1), it has
(4.13)
Hence, for any given constant
, there exists a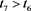 , such that
, such that
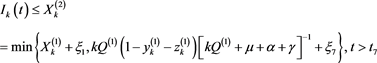 (4.14)
(4.14)
Then, from the second equation of (2.1), we have
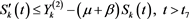 (4.15)
(4.15)
So, for any given constant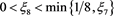 , there exists a
, there exists a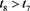 , such that
, such that
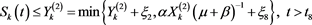 (4.16)
(4.16)
Consequently, from the third equation of (2.1), we have
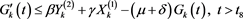 (4.17)
(4.17)
Hence, for any given constant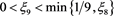 , there exists a
, there exists a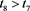 , such that
, such that
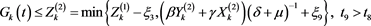 (4.18)
(4.18)
Turning back, one has
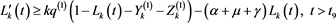 (4.19)
(4.19)
So, for any given enough small constant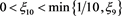 , there exists a
, there exists a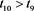 , such that
, such that 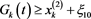 for
for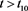 , where
, where
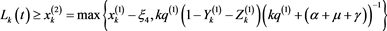 (4.20)
(4.20)
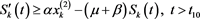 (4.21)
(4.21)
So for any given enough small constant




From the third equation of (2.1) implies that

So, for any given enough small constant




Repeating the above analyses and calculation, we get six sequences









We can easy get that

Since the sequential limits of (4.26) exist, let


Noting that










Substituting (4.27) and (4.28) into q and Q, respectively, one has

where


By subtracting the above two equations, it arrives at

It is obviously that






Finally, substituting 



5. Numerical Simulations
In this section, some sensitivity analyses are presented to illustrate the result of the smoking model (2.1). We consider the system (2.1) on a scale-free network with the degree distribution 



Parameters used in the simulations list as follows: in Figure 2, we choose























Figure 4 and Figure 5 show the dynamic behavior of light problem smoking and heavier problem smoking with different degree when

6. Conclusion
In this paper, we propose a PLSGP giving up smoking model on scale-free network.
Figure 2. The relative density of population number when
Figure 3. The relative density of population number when
Figure 4. Dynamic behavior of light smoker with different degree when
Figure 5. Dynamic behavior of light smoker with different degree when
Figure 6. Dynamic behavior of light smoker with different degree when
Figure 7. Dynamic behavior of light smoker with different degree when
We divide the smoker into two groups, light smoker and persistent smoker, considering individual’s birth and death rates. Through the mathematical calculation, we obtain the basic reproduction number and equilibriums. Using the comparison theorem and the iteration principle, we analyze the stability of the smoking free equilibrium, and also give the persistence and global attractivity of the smoking. If

Acknowledgements
This work is supported by the National Natural Science Foundation of China under Grants 61672112 and Project in Hubei province department of education under Grants B2016036.
Cite this paper
Fei, Y.L. and Liu, X.D. (2018) Spreading Dynamic of a PLSGP Giving up Smoking Model on Scale-Free Network. Open Access Library Journal, 5: e4365. https://doi.org/10.4236/oalib.1104365
References
- 1. Korobeinikov, A. (2004) Global Properties of Basic Virus Dynamics Models. Bulletin of Mathematical Biology, 66, 879-883.
https://doi.org/10.1016/j.bulm.2004.02.001 - 2. Souza, M.O. and Zubelli, J.P. (2011) Global Stability for a Class of Virus Models with Cytotoxic Tlymphocyte Immune Response and Antigenic Variation. Bulletin of Mathematical Biology, 73, 609-625.
https://doi.org/10.1007/s11538-010-9543-2 - 3. Huo, H.F., Dang, S.J. and Li, Y.N. (2010) Stability of a Two-Straintuberculosis Model with General Contact Rate. Abstract and Applied Analysis, 2010, Article ID: 293747.
- 4. Castillo-Chavez, C. and Song, B.J. (2004) Dynamical Models of Tuberculosis and Their Applications. Mathematical Biosciences and Engineering, 1, 361-404.
https://doi.org/10.3934/mbe.2004.1.361 - 5. Huo, H.F. and Feng, L.X. (2013) Global Stability for an HIV/AIDS Epidemic Model with Different Latent Stages and Treatment. Applied Mathematical Modelling, 37, 1480-1489.
https://doi.org/10.1016/j.apm.2012.04.013 - 6. Xu, R. (2011) Global Stability of an HIV-1 Infection Model with Saturation Infection and Intracellular Delay. Journal of Mathematical Analysis and Applications, 375, 75-81.
https://doi.org/10.1016/j.jmaa.2010.08.055 - 7. Pastor-Satorras, R. and Vespignani, A. (2001) Epidemic Dynamics and En-demic States in Complex Networks. Physical Review E, 63, Article ID: 066117.
https://doi.org/10.1103/PhysRevE.63.066117 - 8. Liu, X., Li, T., Wang, Y., et al. (2017) An SIS Epidemic Model with Infective Medium and Feedback Mechanism on Scale-Free Networks. Open Access Library Journal, 4: e3598.
https://doi.org/10.4236/oalib.1103598 - 9. Moreno, Y., Pastor-Satorras, R. and Vespignani, A. (2002) Epidemic Outbreaks in Complex Heterogeneous Networks. The European Physical Journal B, 26, 521-529.
- 10. Li, C.H., Tsai, C.C. and Yang, S.Y. (2014) Analysis of Epidemic Spreading of an SIRS Model in Complex Heterogeneous Networks. Communications in Nonlinear Science and Numerical Simulation, 19, 1042-1054.
https://doi.org/10.1016/j.cnsns.2013.08.033 - 11. Chen, L. and Sun, J. (2014) Global Stability and Optimal Control of an SIRS Epidemic Model on Heterogeneous Networks. Physica A: Statistical Mechanics and Its Applications, 410, 196-204.
https://doi.org/10.1016/j.physa.2014.05.034 - 12. Liu, J. and Zhang, T. (2011) Epidemic Spreading of an SEIRS Model in Scale-Free Networks. Communications in Nonlinear Science and Numerical Simulation, 16, 3375-3384.
https://doi.org/10.1016/j.cnsns.2010.11.019 - 13. Li, T., Wang, Y. and Guan, Z.H. (2014) Spreading Dynamics of a SIQRS Epidemic Model on Scale-Free Networks. Communications in Nonlinear Science and Numerical Simulation, 19, 686-692.
https://doi.org/10.1016/j.cnsns.2013.07.010 - 14. Huang, S., Chen, F. and Chen, L. (2017) Global Dynamics of a Net-work-Based SIQRS Epidemic Model with Demographics and Vaccination. Communications in Nonlinear Science and Numerical Simulation, 43, 296-310.
https://doi.org/10.1016/j.cnsns.2016.07.014 - 15. Castillo-Garsow, C., Jordan-Salivia, G. and Rodri-guez-Herrera, A. (2000) Mathematical Models for the Dynamics of Tobacco Use, Recovery, and Replase. Tech. Rep. BU-1505-M, Cornell University, Ithaca.
- 16. Zaman, G. (2011) Qualitative Behavior of Giving up Smoking Models. Bulletin of the Malaysian Mathematical Sciences Society, 34, 403-415.
- 17. Zaman, G. (2011) Optimal Campaign in the Smoking Dynamics. Computation and Mathematical Methods in Medicine, 2011, Article ID: 163834.
- 18. Lajmanovich, A. and Yorke, J.A. (1976) A Deterministic Model for Gonorrhea in a Nonhomogenous Population. Mathematical Biosciences, 28, 221-236.
https://doi.org/10.1016/0025-5564(76)90125-5 - 19. Chen, F. (2005) On a Nonlinear Nonautonomous Predator-Prey Model with Diffusion and Distributed Delay. Journal of Computational and Applied Mathematics, 180, 33-49.
https://doi.org/10.1016/j.cam.2004.10.001









