 American Journal of Plant Sciences, 2011, 2, 554-560 doi:10.4236/ajps.2011.24066 Published Online October 2011 (http://www.SciRP.org/journal/ajps) Copyright © 2011 SciRes. AJPS Conocephalum conicum (L.) Dumort. (Snake Liverwort) Threatened in Bhaderwah (J & K) due to Environmental Shock Mudassar Iqbal1, Anima Langer1, Afroz Alam2 1Department of Botany University of Jammu, Jammu, India; 2Department of Bioscience and Biotechnology, Banasthali University, Rajasthan, India. Email: Mudassar2010.2010@rediffmail.com Received December 28th, 2010; revised May 13rd, 2011; accepted June 6th, 2011. ABSTRACT Paper includes information on population status of Conocephalum conicum collected from diverse habitats in Bhad er- wah (J & K state). Taxon exhibits tremendous diversity in morphoanatomical details of various gametophytic charac- ters. Isozyme analysis was performed to find out whether variability in the taxon is only environmental or has genetic basis as well. Keywords: Bryophyta, Liverwort, Marchantiales, Conocephalum, Jammu & Ka shmir 1. Introduction Bryophytes constitute one of the largest groups of land plants among the plant kingdom. Himalayas rank first at national [1] and third at global level in bryo-diversity [2]. During the last few decades, however, Himalayas have suffered tremendously due to various tourism and ur- banization related anthropogenic activities leading to the disappearance of many of their inhabitants including bryophytes. On basis of an observations [3] on occur- rence of several hepatics in Western Himalaya (Nainital) over the years, 9 threatened taxa [Athalamia pinguis, Dumortiera hirsuta, Cryptomitrium himalayensis, Re- boulia hemispherica, Stephensoniella brevipedunculata, Weisnerella denudata, Fossombronia himalayensis, Sewardiella tuberifera and Conocephalum conicum] which were had observed to grow luxuriantly in the yes- teryears, but had disappeared or undergone extreme re- duction in population size, from the area. The suggested specific reasons include anthropogenic activities like rapid urbanization, land clearance, road construction, deforestation, indiscriminate and wreck less collection etc., as factors which could have contributed to the habi- tat destruction and consequently, the disappearance of these taxa. She, therefore, voice was raised for their con- servation. Against this backdrop, work on intraspecific variabil- ity was initiated on three, Reboulia hemispherica [4], Dumortiera hirsuta [5] and Conocephalum conicum [6] of the nine hepatic taxa enlisted by Pant [1] in Depart- ment of Botany, at University of Jammu, Jammu. Present communication deals with our observations on popula- tion status of Conocephalum conicum collected from Bhaderwah in Jammu region, one of the areas of the North-west Himalaya which used to host rich bryo-di- versity in the yesteryears, because of the undisturbed, stable habitats due to the inaccessibility of the area. Bhaderwah lies between 32˚08′ N - 35˚52′ N latitude and 75˚48′ E - 75˚32′ E longitude having an altitudinal range of 821 - 4341 m. The climate of Bhaderwah is tem- perate; temperature varies from minimum mean 17.3˚C to maximum mean 29.9˚C in summers and minimum mean –0.9˚C to maximum mean 02.8˚C in winters. It experiences severe cold during winters with snowy sea- son between the months of December to March with temperature remaining subzero. The snow melts off by April and weather becomes pleasant and remains so up to May. In June and July, temperature increases upto the maximum 29.9˚C. During August and September, the temperature subsides and it again becomes cold after- wards. The annual rainfall is up to 249.6 mm and maxi- mum humidity is 84% [7]. In recent years, Bhaderwah has been subjected to tre- mendous developmental and tourism related pressures, like construction of bridges, highways, parks, roads,  Conocephalum conicum (L.) Dumort. (Snake Liverwort) Threatened in Bhaderwah (J & K) 555 due to Environmental Shock tourism huts, deforestation, destruction of rocks by quar- rying etc. Furthermore, a University Campus has recently being established at Bhaderwah over a vast area. These activities are likely to lead to habitat destruction and de- pletion/disappearance of many of the inhabitants of the area. Under such circumstances, only those organisms which have accumulated genetic variability are likely to survive, whereas genetically homogenous taxa are des- tined to be wiped out by the natural selection. Cono- cephalum conicum, a species reported to have disappeared elsewhere in Western Himalaya in response to similar reasons, was therefore selected for the present study. 2. Materials and Methods Conocephalum conicum (L.) Dumort. is commonly called snake liverwort is the study material collected from Bhaderwah, Jammu & Kashmir, India. 3. Results and Discussion Conocephalum conicum is commonly called snake liv- erwort due to hexagonal areolae on its dorsal surface (Figure 1(a)). It is also called cone headed liverwort because of its conical archegoniophores (Figure 1(b)). It is worldwide in distribution, as it is known to be distrib- uted in America, Algeria, Alaska, Azores, Bhutan, Cau- casus, Canary, China, Europe, Florida, India, Ireland, Italy, Japan, Korea, Kuriles, Liukium Maderia, Nepal, Pakistan, Rubki-Neylong, Seghalim and Siberia [8]. From India, the taxon is reported from Eastern [9-11] and Western Himalaya [12-16]. In Jammu and Kashmir (North West Himalaya), it has so far been collected from Poonch [17], Udhampur and Kathua as stated by [18] districts of Jammu region and Ladakh [19]. During present investigations, 46 accessions of C. conicum have been collected growing at different sites ranging in altitude from 1230 m - 2600 m. Populations were collected growing on substrates with pH ranging between 6.5 - 6.9. Plants have been collected from di- verse habitats, such as epilithic (rock crevices, caves; Figures 1(c) and (d)), under dripping chilled water (Fig- ure 1(e)), under snow cover (Figure 1(f)), stream banks (Figure 1(g)), submerged under water (Figure 1(h)), non-epilithic (cool and moist shady soil, loamy soil) (Figure 1(i)), and epiphytic on decaying log of Cedrus deodara (Figure 1(j)). Thalli of various populations displayed variability in morpho-anatomy of almost every gametophytic character (thallus size, rhizoids, scales, air pores, vegetative anat- omy etc.) studied, that has been communicated separately. In this paper focus is mainly on reproductive biology of the taxon. Figure 1. (a) A part of Thallus showing hexagonal areolae on its dorral surface. (b) Thallus of C. conicum showing conical archegoniophore. (c) & (d) Various habitats from where populations of Conocephalum conicum have been collected. (e) Under dripping chilled water. (f) Under snow cover. (g) Stream banks. Submerged under water. Non-epilithic. (h) Epiphytic. (i) On rotten log of Cedrus deodara. C. conicum reproduces both, asexually as well as sexu- ally; asexual reproduction occurs usually by regeneration from thallus fragments; thallus being light in weight, is easily transported by water; thallus produces ventral in- novations, both apical and lateral (Figure 2(a)). During Copyright © 2011 SciRes. AJPS  Conocephalum conicum (L.) Dumort. (Snake Liverwort) Threatened in Bhaderwah (J & K) 556 due to Environmental Shock Figure 2. (a) Thalli of Conocephalum conium showing apical and lateral innovations. (b) & (c) Male plants with 2 - 3 receptacles. (d) Female thalli with young (Note upto 4 re- ceptacles/thallus). (e) & (f) Mature receptacles (1 in Figure 2(e) and 2 in Figure 2(f)). (g) Female thallus with healthy receptacle and stalk. (h) Female thalli of Acc. MI 37 and MI 38 showing unhealthy/shrivelled re ceplacles and stalk. present investigation, only 15 accessions were recorded in fertile form, of which 12 comprised both male and female thalli growing together, while remaining three had only female plants. Dioecious; male receptacles ses- sile, green, disciform, disc slightly raised above main thallus, surrounded by thin growth of thallus; terminal or subterminal; receptacles oval or circular having con- spicuous papillae on their upper surface; borne on main thallus as well as on apical innovations; number of re- ceptacles per thallus varied from 2 - 3 (Figures 2(b) and (c)); initiation of male receptacles occurred in April, and they disappeared in July. Although female thalli were recorded presently in 15 accessions, mature receptacles could be observed only from 3; in the remaining 12 accessions, receptacles were observed at young stages only, as they degenerated be- fore reaching maturity. Female receptacle terminal or slightly sub-terminal, 1 to 4 per thallus, sunken when young in a cup formed by dorsal growth of the thallus (Figure 2(d)); receptacles appeared in first week of May; one flask shaped archegonium present per lobe with broad venter and long neck; post fertilization changes recorded after mid June, when neck of archegonium de- generated; receptacles started emerging out of sunken cup, stalk elongated slowly till it reached maximum of 11 cm (Figures 2(e)-(g)) in March next year. Mature recep- tacles conical; raised on long stalk. Receptacles were symmetric, healthy and 5 - 8 lobed in single accession (Acc. MI 46; Figures 2(e)-(g)) and unhealthy and asymmetric up to 5 lobed in two (Acc. MI 37 and MI 38; Figures 2(h)-3(d)). Stalk stout and healthy in Acc. MI 46 (Figures 2(e)-(g)) but unhealthy and shriveled in Acc. MI 37 and MI 38 (Figures 2(h)-3(d)). Mature sporophytes were present only in 1 and/or 2 lobes per receptacle in Acc. MI 37 and MI 38; remaining lobes being extremely reduced, whereas in Acc. MI 46 mature sporophytes were recorded in a maximum of 5 lobes out of 8 (Figures 3(e)-(h)). One sporophyte per lobe, having foot, reduced seta and pyri- form capsule (Figure 3(i)). Sporophytes excised from developed lobes of unhealthy receptacles exhibited re- duced capsules (Figure 3(j)). Mature spores observed only in Acc. MI 46 (Figure 4(a)); capsules of Acc. MI 37 and MI 38 possessed very few viable spores (Figure 4(b); Table 1). Capsules from even the fully mature receptacles of these unhealthy populations possessed developing sporocytes and elaters; ratio between the two being around 0.4:1. Capsules showed very low spore output as compared to that of elaters. Like the other two populations spore-elater ratio in the healthy population (Acc. no. MI 46) was around 0.4:1 (Table 3). Out of the spores formed in Acc. MI 46, another 10% were non viable. These were small (16 µm - 20 µm) × (12 µm - 16 µm), triangular and shriveled as compared to viable ones which were large (44.5 µm - 102.3 µm) × (25 µm - 55.4 µm), green and healthy (Ta- ble 2). Elaters brown, healthy, bi- and tri- spiral, unbranched and bluntly fusiform in Acc. MI 46 (Figure 4(c); Table 2). Elaters of abnormal morphology (branched and blackish) were also present in capsules of Acc. MI 37 Copyright © 2011 SciRes. AJPS  Conocephalum conicum (L.) Dumort. (Snake Liverwort) Threatened in Bhaderwah (J & K) 557 due to Environmental Shock Figure 3. (a) A female thallus of Acc. no MI 37 showing asymmetric receptacles and stalk. (b-d) Female thallus of Acc. no MI 37 and MI 38 with unhealthy/shrivelled stalk. (e-h) Female thalli of Acc. no MI 46 bearing receplacles with 1 (e), 2 (f), 4 (g) and 5 (h) sporophytes. (i) & (j) Healthy (i) and unhealthy (j) Sporophyte exised from receptacle lobes of MI 46 (i) and M 37 (j). and MI 38 (Figure 4(d); Table 3). Size of elaters var- Figures 4. (a) A few viable and non-viable spores excised from matures capsule of healthy population. (b) Developing sporo- cytes and claters, excised from capsule of unhealthy population (Note the abundance of elaters). (c) Whole mount of healthy elaters. (d) Whole moun t of abnormal elaters. ied between (50 µm - 150 µm) × (15 µm - 70 µm) (Table 3). Occurrence of elaters is a characteristic unique to the liverworts. In contrast, they are totally lacking in mosses. Archesporium throughout the class hepaticopsida is endothecial in origin and differentiates into sporocytes and elaterocytes; former undergo reduc- tional division, each giving rise to 4 spores and the later differentiating into elaters. Since both, sporocytes and elaterocytes belong to the same generation, basic ratio between spores and elaters expected is 4:1. This ratio exists in a number of Marchantialean taxa like Reboulia, Mannia, Targionia, Conocephalum etc. During the course of evolution there has been a trend towards the increase in spore output and consequently, higher spore-elater ratios. Marchantia polymorpha displays highest ratio (128:1) among Marchantiales. Schistochila (Jungermanniales) exhibits still higher (200:1) spore-elater ratio (Table 2). Sporocytes of all such taxa where spore-elater ratio is higher than 4:1, undergo 1 or more mitotic divisions, prior to meiosis. Interestingly, these divisions have been accompanied by the reduction in spore size. Production of large number of small sized spores is an adaptation for wind dispersal of spores and subsequently, successful under terrestrial habitat. Al- though Conocephalum, Reboulia, Mannia and Tar- gionia are theoretically expected to show 4:1 ratio, actual atios are sometimes lower. Bischler (1998) re- r Copyright © 2011 SciRes. AJPS  Conocephalum conicum (L.) Dumort. (Snake Liverwort) Threatened in Bhaderwah (J & K) due to Environmental Shock Copyright © 2011 SciRes. AJPS 558 Table 1. Number of viable/non-viable spores in receptacles with different sporophytes. Size (µm) Oval Circular Number of Viable/Non-Viable Spores in Receptacles with Different Sporophytes Acc. No. L B diameter 1 2 3 4 5 6 7 8 MI 37 42.5 - 115 (89.5) 22 - 52 (49.1) 22.5 - 52.5 (38.2) 2848 - 2861 (2672) 5344 - 5675 (5545) ― ― ― ― ― ― MI 38 42 - 110 (93.3) 25.5 - 49 (37.4) 22 - 50 (37.4) 2789 - 2915 (2845) 5125 - 5478 (5344) ― ― ― ― ― ― Table 2. Variation recorded in spore characters. Size (µm) Oval Circular Number of Viable/Non-Viable Spores in Receptacles with Different Sporophytes Acc. No. L B diameter 1 2 3 4 5 6 7 8 MI 37 42.5 - 115 (89.5) 22 - 52 (49.1) 22.5 - 52.5 (38.2) 2848 - 2861 (2672) 5344 - 5675 (5545) ― ― ― ― ― ― MI 38 42 - 110 (93.3) 25.5 - 49 (37.4) 22 - 50 (37.4) 2789 - 2915 (2845) 5125 - 5478 (5344) ― ― ― ― ― ― M1 46 Viable Non-viable 44.5 - 120.3 (95.7) 16 - 20 (18.3) 25 - 25.4 (44.3) 12 - 16 (14.5) 23.2 - 55.3 (33.4) ― 2485 - 2725 (2560) 50 - 110 (85) 5000 - 5350 (5130) 150 - 225 (180) 7787 - 7985 (7785) 315 - 375 (350) 9704 - 10492 (10263) 400 - 425 (415) 12500 - 13505 (13126) 450 - 480 (465) ― ― ― ― ― ― Table 3. Variation recorded in elaters’ output. Size (µm) Number of elaters in receptacle with different sporophytes No. of spirals Spore/Elater ratio Acc.no. L B 1 2 3 4 5 67823 4 1 2 3 4 5 MI 37 45 - 105 (91.5) 15 - 75 (35.3) 6106 - 6504 (6305.2) 12212 - 12895 (12710.3) ― ― ― –––+– + 0.42:1 0.43:1 ― ―― MI 38 43 - 350 (320.5) 12 - 65 (25.5) 5895 - 5990 (5935.1) 12427 - 12895 (12610.1) ― ― ― –––+– + 0.47:1 0.42:1 ― ―― MI 46 50 - 350 (250.5) 15 - 70 (45.5) 6335 - 6660 (6410.1) 12317 - 12910 (12730.4) 18318 - 18602 (18415.3) 24920 - 25689 (25220.1) 30982 - 31670 (31525.5) –––++ – 0.39:1 0.40:1 0.42:1 0.40:10.41:1 corded a similar situation in C. conicum and Pellia sp., and attributed it to endosporic, precocious spore germi- nation in the species. Although in these species ratio was lower than 4:1 yet the number of spores was far more than elaters. Data obtained presently are in total contrast to the expected numbers. Here, elaters were more abun- dant than spores, leading to the ratio between spores and elaters as low as 0.4:1 (Table 2). This is attributable to the fact that a large number of sporocytes do not undergo reduction division and therefore, the number of spores does not multiply. This clearly indicates that although apparently, C. conicum seems to be well established un- der various habitats in the study area, all the populations are asexually propagated only. Although female recepta- cles were formed in 15 accessions, they reached maturity only in 3 and out of these too, a few viable spores are formed only in one. 4. Population Status It can be inferred from the data obtained for various re- productive characters (female gametangia, sporocytes and spore-elater ratios) that there is minimal (if any) contribution of sexual reproduction to the variability re- corded among various populations. In order to further assess the nature of variability, five populations of C. conicum collected from diverse habitats i.e., epilithic (under dripping chilled water, under snow cover), non epilithic (moist shady soil, sandy soil) and epiphytic (Cedrus deodara) were subjected to Polyacrylamide gel electrophoresis for three enzyme systems (peroxidases, estrases and phosphatases). The zymograms, thus ob- tained revealed homogeneity in the occurrence of bands. A total of 6 bands were obtained for phosphatases (Fig- ures 5(a)-(d)), 5 for esterases (Figures 5(e)-(f)), one 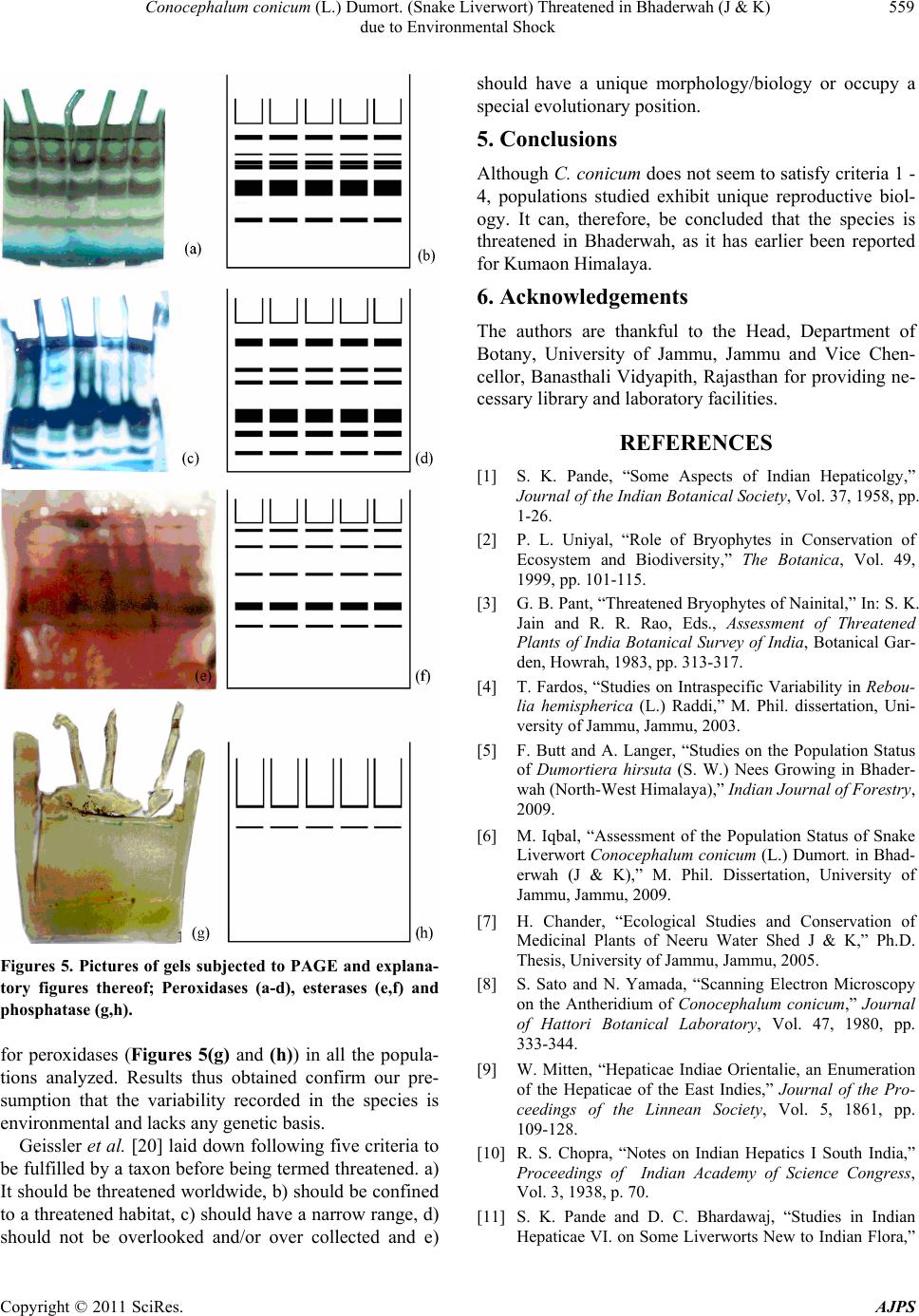 Conocephalum conicum (L.) Dumort. (Snake Liverwort) Threatened in Bhaderwah (J & K) 559 due to Environmental Shock Figures 5. Pictures of gels subjected to PAGE and explana- tory figures thereof; Peroxidases (a-d), esterases (e,f) and phosphatase (g,h). for peroxidases (Figures 5(g) and (h)) in all the popula- tions analyzed. Results thus obtained confirm our pre- sumption that the variability recorded in the species is environmental and lacks any genetic basis. Geissler et al. [20] laid down following five criteria to be fulfilled by a taxon before being termed threatened. a) It should be threatened worldwide, b) should be confined to a threatened habitat, c) should have a narrow range, d) should not be overlooked and/or over collected and e) should have a unique morphology/biology or occupy a special evolutionary position. 5. Conclusions Although C. conicum does not seem to satisfy criteria 1 - 4, populations studied exhibit unique reproductive biol- ogy. It can, therefore, be concluded that the species is threatened in Bhaderwah, as it has earlier been reported for Kumaon Himalaya. 6. Acknowledgements The authors are thankful to the Head, Department of Botany, University of Jammu, Jammu and Vice Chen- cellor, Banasthali Vidyapith, Rajasthan for providing ne- cessary library and laboratory facilities. REFERENCES [1] S. K. Pande, “Some Aspects of Indian Hepaticolgy,” Journal of the Indian Botanical Society, Vol. 37, 1958, pp. 1-26. [2] P. L. Uniyal, “Role of Bryophytes in Conservation of Ecosystem and Biodiversity,” The Botanica, Vol. 49, 1999, pp. 101-115. [3] G. B. Pant, “Threatened Bryophytes of Nainital,” In: S. K. Jain and R. R. Rao, Eds., Assessment of Threatened Plants of India Botanical Survey of India, Botanical Gar- den, Howrah, 1983, pp. 313-317. [4] T. Fardos, “Studies on Intraspecific Variability in Rebou- lia hemispherica (L.) Raddi,” M. Phil. dissertation, Uni- versity of Jammu, Jammu, 2003. [5] F. Butt and A. Langer, “Studies on the Population Status of Dumortiera hirsuta (S. W.) Nees Growing in Bhader- wah (North-West Himalaya),” Indian Journal of Forestry, 2009. [6] M. Iqbal, “Assessment of the Population Status of Snake Liverwort Conocephalum conicum (L.) Dumort. in Bhad- erwah (J & K),” M. Phil. Dissertation, University of Jammu, Jammu, 2009. [7] H. Chander, “Ecological Studies and Conservation of Medicinal Plants of Neeru Water Shed J & K,” Ph.D. Thesis, University of Jammu, Jammu, 2005. [8] S. Sato and N. Yamada, “Scanning Electron Microscopy on the Antheridium of Conocephalum conicum,” Journal of Hattori Botanical Laboratory, Vol. 47, 1980, pp. 333-344. [9] W. Mitten, “Hepaticae Indiae Orientalie, an Enumeration of the Hepaticae of the East Indies,” Journal of the Pro- ceedings of the Linnean Society, Vol. 5, 1861, pp. 109-128. [10] R. S. Chopra, “Notes on Indian Hepatics I South India,” Proceedings of Indian Academy of Science Congress, Vol. 3, 1938, p. 70. [11] S. K. Pande and D. C. Bhardawaj, “Studies in Indian Hepaticae VI. on Some Liverworts New to Indian Flora,” Copyright © 2011 SciRes. AJPS  Conocephalum conicum (L.) Dumort. (Snake Liverwort) Threatened in Bhaderwah (J & K) due to Environmental Shock Copyright © 2011 SciRes. AJPS 560 Journal of the Indian Botanical Society, Vol. 28, 1949, pp. 1-13. [12] F. Stephani, “Enumeration des Hepatiques Connues Dons les Iles de la Societe (Principalement a Tahiti) et Dans les Iles Marquises, In: E. Bescherella,”Journal de Botany, Vol. 12, 1898, pp. 136-150. [13] S. R. Kashyap, “Liverworts of Western Himalaya and Punjab Plains. Part I,” The Chronica Botanica, Delhi, 1929. [14] N. S. Parihar, “An Annotated Revised Census of Indian Hepatics,” University of Allahabad Studies (India), Bot- any Section, 1961-1962, pp. 1-56. [15] N. S. Parihar, B. Lal and N. Katiyar, “Hepatics and An- thocerotes of India. A new Annotated Checklist,” Central Book Depot, Allahabad, 1994. [16] H. Bischler, “Systematics and Evolution of the Genera of the Marchantiales,” Bryophytorum Bibliotheca, Vol. 51, 1998 pp. 1-201. [17] M. Tanwir, “Studies on the Diversity of Hepatic Flora of District Poonch (North-West Himalaya),” Ph. D. Disser- tation, University of Jammu, Jammu, 2005. [18] M. Tanwir, A. Langer and M. Bhandari, “Liverwort and Hornwort of Patnitop and Its Adjoining Areas (J&K), Western Himalaya, India,” Geophytology, Vol. 37, No. 1-2, 2008, pp. 35-41 [19] M. Tanwir and A. Langer, “Liverworts of Ladakh, J & K State (North-West Himalaya), India,” Journal of the In- dian Botanical Society, Vol. 85, 2006, pp. 71-73. [20] P. Geissler, B. C. Tan and T. Hallingback, “Additions to the World Red List of Bryophytes,” The Bryological Times, Vol. 93, 1997, pp. 1-7.
|