 Advances in Chemical Engi neering and Science , 2011, 1, 208-214 doi:10.4236/aces.2011.14030 Published Online October 2011 (http://www.SciRP.org/journal/aces) Copyright © 2011 SciRes. ACES Melt Rheology of Poly(Lactic Acid)/Low Density Polyethylene Polymer Blends Kotiba Hamad*, Mosab Kaseem, Fawaz Deri Faculty of Science, Department of Chemistry, Laboratory of Materials Rheology (LMR), University of Damascus, Damascus, Syria E-mail: *kotibahamad@yahoo.com Received June 28, 2011; revised July 26, 2011; accepted September 23, 2011 Abstract In this work, rheological properties of poly(lactic acid) (PLA), low density polyethylene (LDPE) polymer blends were investigated in the molten state. The experiments were carried on a capillary rheometer. The effect of shear stress, temperature and blending ratio on the flow activation energy at a constant shear stress and melt viscosity of the blends are described. The results showed that the PLA/LDPE polymer blends are pseudo plastic in nature, where there viscosity decreases with increasing shear stress. Also it was found the melt viscosity of the blends decreases with increasing PLA content in the blend. Keywords: PLA, LDPE, Polymer Blends, Melt Viscosity, Flow Activation Energy 1. Introduction Two or more existing polymers may be blended for various reasons. One reason is to achieve a material that has a combination of the properties of the constituents, e.g. a blend of two polymers, on e of which is chemically resistant and the other tough. Another reason is to save costs by blending a high-performance polymer with a cheaper material. Blending also benefits the manufac- turer by offering: 1) Improved processability, product uniformity, and scrap reduction. 2) Quick formulation changes. 3) Plant flexibility and high productivity. 4) Reduction of the number of grades that need to be manufactured and stored. 5) Inherent recyclability, etc [1]. Poly(lactic acid) (PLA) is well known aliphatic poly- esters derived from corn and sugar beets, and degrades into nontoxic compounds in landfill. It can be synthe- sized from direct condensation of lactic acid or by ring-opening polymerization of cyclic lactide [2], in this way, intermediate lactide (cyclic dimer of lactic acid) is formed in the first step, and after removing condensation product water, the PLA oligomer is depolymerized to lactide, in the second step, purified lactide converted into corresponding high-molecular weight polyesters by cata- lytic ring-opening polymerization. These new techniques in synthesizing PLA, which allow economical production of high molecular weight PLA polymer, have broadened its uses. PLA was chosen for its high biocompatibility and biodegradability. It has b ecome an alternative to tra- ditional commodity plastics for everyday applications as an environmental friendlily polymer due to its some unique properties such as high strength, high stiffness and resistance to fats and oil. However, brittleness and other properties such as low viscosity, low thermal sta- bility, high moisture sensitivity, medium gas barrier properties, high cost (comparing with PE, PP, PS…) and low solvents resistance (e.g., against water) of the pure polymer are often insufficient for a lot of applications. Properties of PLA can be modified by polymer blend- ing techniques, where it was blended with several syn- thetic and biopolymers in efforts to enhance its proper- ties and also to obtain novel materials. PLA have been blended with rubbers [3], thermoplastic starch (TPS) [4-9], poly(butylene succinate) (PBS) [10], poly(butylene succinate adipate) (PBSA) [11], poly(butylene adipate- co-terephthalate) (PBAT) [12-15], acrilontryl- butadiene- styrene (ABS) [16], polypropylene ( PP) [17-19], and po- lystyrene (PS) [20,21], to obtain materials with lower cost and improved properties. Polyolefin’s industry is still a very dynamic business, where low density polyethylene (LDPE) plays a key role and it has a wide range of applications. LDPE has good properties such as, low cost, processability, resilience and moisture insensitivity. However, the use of LDPE causes serious environmental concerns because it is not  K. HAMAD ET AL. 209 biodegradable; i.e., it is not biodegraded by the microor- ganisms present in the environment. Even though recy- cling has become an alternative, not all post-consumer products may be recycled and, in some cases, this proc- ess is not economically sustainable. The waste manage- ment problem has prompted the development of, at least, partially degrad able plastics, a fact that may be achieved by blending with biodegradable fillers, which can effec- tively reduce the volume of plastic waste by partial deg- radation. Therefore, LDPE was blended with native starch or thermoplastic starch (TPS) in efforts to enhance its degradability [22], and it was found that LDPE’s de- gradability can be improved when starch is added to it, although the pure LDPE is not biodegradable. However, the addition of TPS might negatively affect the proper- ties of LDPE. In this case PLA, which has good me- chanical properties, can be used as alternative to TPS. Studies on PLA/PE blends were reported by many au- thors [23-25]. Blends PLA with LDPE offer cost-performance bene- fits, and enhance the degrad ability o f LDPE. In th is work PLA/LDPE blends were prepared using a laboratory scale single screw extruder and characterized in terms of the rheological properties. Such works are very impor- tant in the development of composites and blends from biodegradable polyesters. No attention has been given in the past to rheological characterization of PLA/LDPE polymer blends. 2. Experimental 2.1. Materials Poly(lactic acid) (PLA) (ESUN™ A-1001) [density = 1.25 g/cm3 (21.5˚C), MFI = 12.5 g/10 min (190˚C/2.18 Kg)] was supplied by Bright China Industrial Company. Ltd (Shenzhen, China), the selected grade is an extrusion material; it was dried at 70˚C for 6 h before using. Low density polyethylen e (LDPE) (SABIC®LDPE2308TN00) [density = 0.924 g/cm3, MFI = 7.5 g/10 min (190˚C/2.16 Kg)] was supplied by Sabic (KSA). 2.2. Blends Compounding Blends of PLA/LDPE in different ratios were com- pounded using a laboratory scale single screw extruder (SSE) (D = 20 mm, L/D = 25) [SHAM EXTRUDER 25D Performance: Kreem Industrial Establishment, Da- mascus-Syria], it could be operated at different speeds, varied from 0 to100 rpm. The screw has a fluted type mixing device located before the metering zone [26], which can extend th e compounding ab ility of the SSE, in this type of mixers the material is forced to pass at a high shear stress. This brings in some level of dispersing ac- tion besides reorienting the interfacial area and increas- ing the imposed total strain. The flight depth of screw in the metering zone was 1.5 mm, and the helix angle 17.7˚. PT124G-124 melt pressure transducer (Shanghai Zhao- hui Pressure Apparatus Co., Ltd-China) was located in the die head for measuring the melt pressure. The screw speed was set at 70 rpm in the blends preparation, and the extruder temperature profile along the barrel was 180˚C, 190˚C, 200˚C, 210˚C (from feed zone to die). The blends were extruded through a multi holes die (3 mm), the extrudates were then fed into a granulator, which converted them into granules. The ob- tained granules were dried at 70˚C for 6 h before study- ing. The compositions of the blends are shown in Table 1. 2.3. Rheology Rheological properties of the blends were studied using a constant pressure circular capillary rheometer. The melt is extruded by the use of dead weights (i.e. constant pressure) rather than constant plunger speed. This in- strument, popularly known as the Melt Flow Indexer (MFI), is very popular in the thermoplastics industry due to its ease of operation and low cost, which more than compensates for its lack of sophistication. The parameter measured through the melt flow indexer contains mixed information of the elastic and viscous effects of the polymer. The rheological experiments were carried out at 175˚C, 180˚C, 185˚C and 190˚C, and by using L/R = 8, 15, 25 and 36 capillaries. Bagley’s correction [27,28] was performed by using the data from the four capillary dies. The apparent shear rate (γa) is given by: 3 4 a Q R (1) where R is the capillary radius, and Q is the volumetric flow rate. The true shear rate (γr) is given by: 31 . 4 r n na (2) where n is the non-Newtonian index depending on tem- perature, the term ( 314nn) was the Rabinowitsch correction factor. The apparent shear stress (τa) is given by: 2 a RP L (3) Table 1. Compositions of PLA/LDPE blends. Sample PLA100PLA70 PLA50 PLA30PLA0 PLA (wt%) LDPE (wt%) 100 0 70 30 50 50 30 70 0 100 Copyright © 2011 SciRes. ACES  210 K. HAMAD ET AL. where P is the pressure at the capillary en trance, and L is the capillary length. The true shear stress (τr) is given by: 2 r P LR e (4) where e is the Bagley’s correction factor. The true vis- cosity (ηr) is given by: r r r (5) The values of flow activation energy at a constant shear stress (Eτ) were determined by using Arrhenius equation form: e T rA (6) where A is the consistency related to structure and for- mulation and R is the gas constant (8.314 J/mo lK). 3. Results and Discussion 3.1. Flow Curves The flow curves, ie plots of shear stress versus shear rate for PLA/LDPE blends covering the whole composition range have been measured over a temperature range from 175˚C to 190˚C in steps of 5˚C. A typical result for the plot at 175˚C is shown in Figure 1. It can be seen that these curves apparently deviate from linear relationship inclining to the axis of shear rate, which means that the homopolymers of PLA, LDPE and their blends are pseudo plastic non-Newton fluids similar to most polymeric melts, and they obey the power law: n K (7) where K is the consistency index and n is the non- New- tonian index, which can be calculated from the slope of Figure 1. Flow curves of the blends at 175˚C. the lines in Figure 1. dlog dlog n (8) Figure 2 shows the values of n for PLA/LDPE blend melts over blending ratio at 175˚C. It could be noted from Figure 2, th at the values of n were less than 1, im- plying that PLA/LDPE blend melts were pseudo plastic; similar to most polymer melts. Also it could be noted from Figure 2, that the n values of the blends are less than those of the homopolymers, PLA and LDPE. The value of n describes the deviation from the Newtonian fluids about flow behavior, so it is also called the flow behavior index. A higher value of n reveals less influ- ence of shear rate on flow behavior. In other words, the changes in viscosity upon shear rate are not obvious, so it could be said that the flow behavior of the blend melts is more sensitive to shear rate as compared with the ho- mopolymers. Also it should be noted from Figure 2, that the PLA/LD PE blends f ound to be incompatib le, wher e they show a drop in n values and PLA50 is the most non- Newtonian (n = 0.52). The compatible polymer blends show high non-Newtonian indexes which are closed to that of their major phase (PLA and LDPE). 3.2. Melt Viscosity Figure 3 shows plots of the melt viscosity versus shear stress for homopolymers PLA, LDPE and the blends at different temperatures. It shows that the viscosity of PLA, LDPE and the blends decreases with increasing shear stress, showing a typical property of pseudo-plastic non-Newton fluids, this behavior was attributed to the alignment or arrangement of chain segments of polymers in the direction of applied shear stress [29]. Figure 2. Non-newtonian inde x of the blends at 175 ˚C. Copyright © 2011 SciRes. ACES  K. HAMAD ET AL. Copyright © 2011 SciRes. ACES 211 In order to observe how the melt viscosity varies with the blending ratio at different temperatures, cross plots of Figure 3 are given in Figure 4. The high viscosity at a low shear rate provide the integrity of the extrudate dur- ing extrusion, and the low viscosity at a high shear rate enables low injection pressure, high injection speed and less time of the injection cycle. It could be noted from Figure 4 that the melt viscosity of the blend decreases with increasing PLA at 175˚C, and this behavior can be attributed to the low melt vis- Figure 3. Variation of melt viscosity with shear stress for PLA/LDPE blends. Figure 4. Variation of melt viscosity with blending ratio for PLA/LDPE blends. 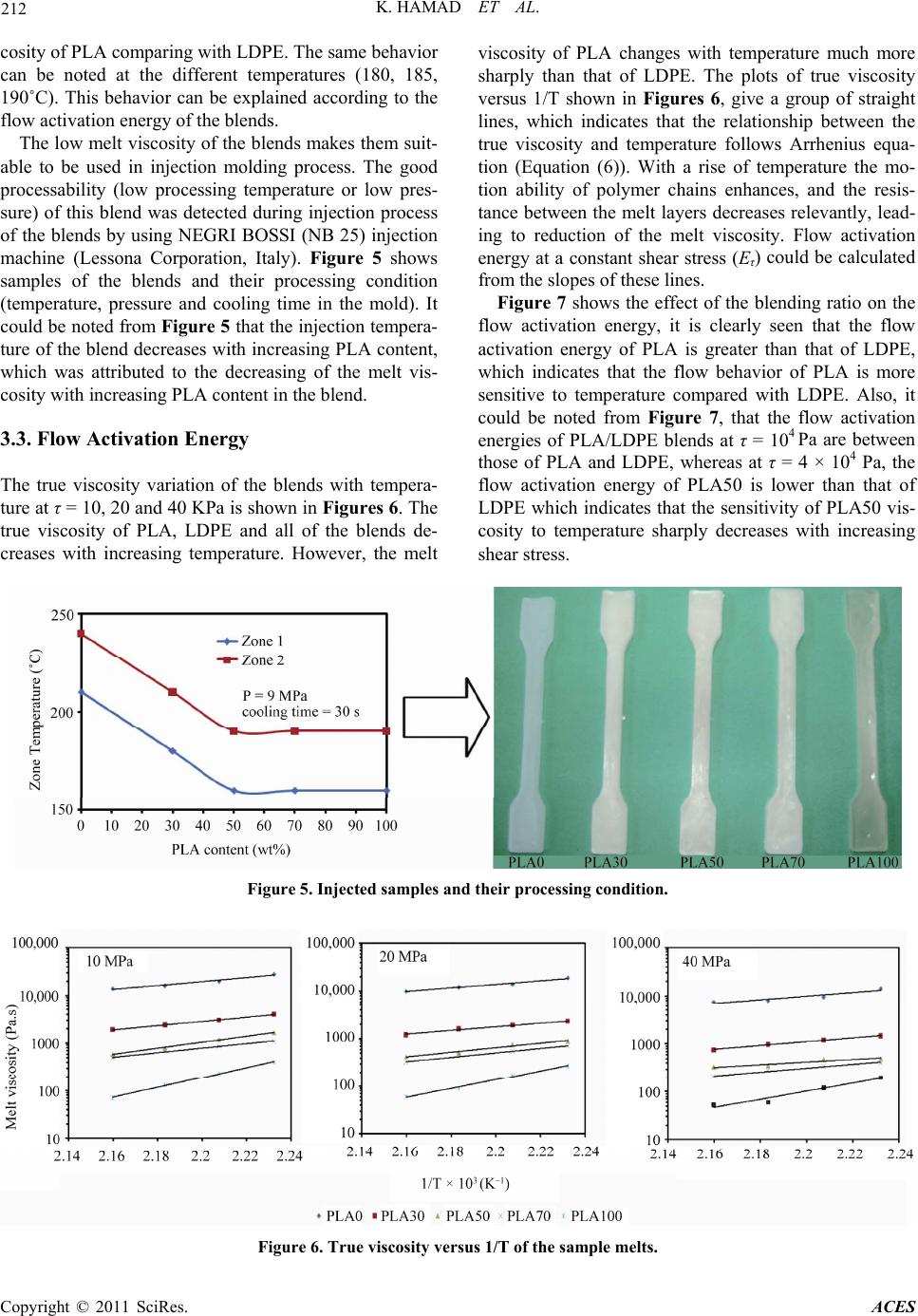 K. HAMAD ET AL. Copyright © 2011 SciRes. ACES 212 cosity of PLA co mparing wi th LDPE. The same behavi or can be noted at the different temperatures (180, 185, 190˚C). This behavior can be explained according to the flow activation energy of the blends. The low melt viscosity of the blends makes them suit- able to be used in injection molding process. The good processability (low processing temperature or low pres- sure) of this blend was detected during injection process of the blends by using NEGRI BOSSI (NB 25) injection machine (Lessona Corporation, Italy). Figure 5 shows samples of the blends and their processing condition (temperature, pressure and cooling time in the mold). It could be noted from Figure 5 that the injection tempera- ture of the blend decreases with increasing PLA content, which was attributed to the decreasing of the melt vis- cosity with increasing PLA content in the blend. 3.3. Flow Activation Energy The true viscosity variation of the blends with tempera- ture at τ = 10, 20 and 40 KPa is shown in Figures 6. The true viscosity of PLA, LDPE and all of the blends de- creases with increasing temperature. However, the melt viscosity of PLA changes with temperature much more sharply than that of LDPE. The plots of true viscosity versus 1/T shown in Figures 6, give a group of straight lines, which indicates that the relationship between the true viscosity and temperature follows Arrhenius equa- tion (Equation (6)). With a rise of temperature the mo- tion ability of polymer chains enhances, and the resis- tance between the melt layers decreases relevantly, lead- ing to reduction of the melt viscosity. Flow activation energy at a constant shear stress (Eτ) could be calculated from the slopes of these lines. Figure 7 shows the effect of the blending ratio on the flow activation energy, it is clearly seen that the flow activation energy of PLA is greater than that of LDPE, which indicates that the flow behavior of PLA is more sensitive to temperature compared with LDPE. Also, it could be noted from Figure 7, that the flow activation energies of PLA/LDPE blends at τ = 104 Pa are between those of PLA and LDPE, whereas at τ = 4 × 104 Pa, the flow activation energy of PLA50 is lower than that of LDPE which indicates that the sensitivity of PLA50 vis- cosity to temperature sharply decreases with increasing shear stress. Figure 5. Injected samples and their processing condition. Figure 6. True viscosity versus 1/T of the sample melts. 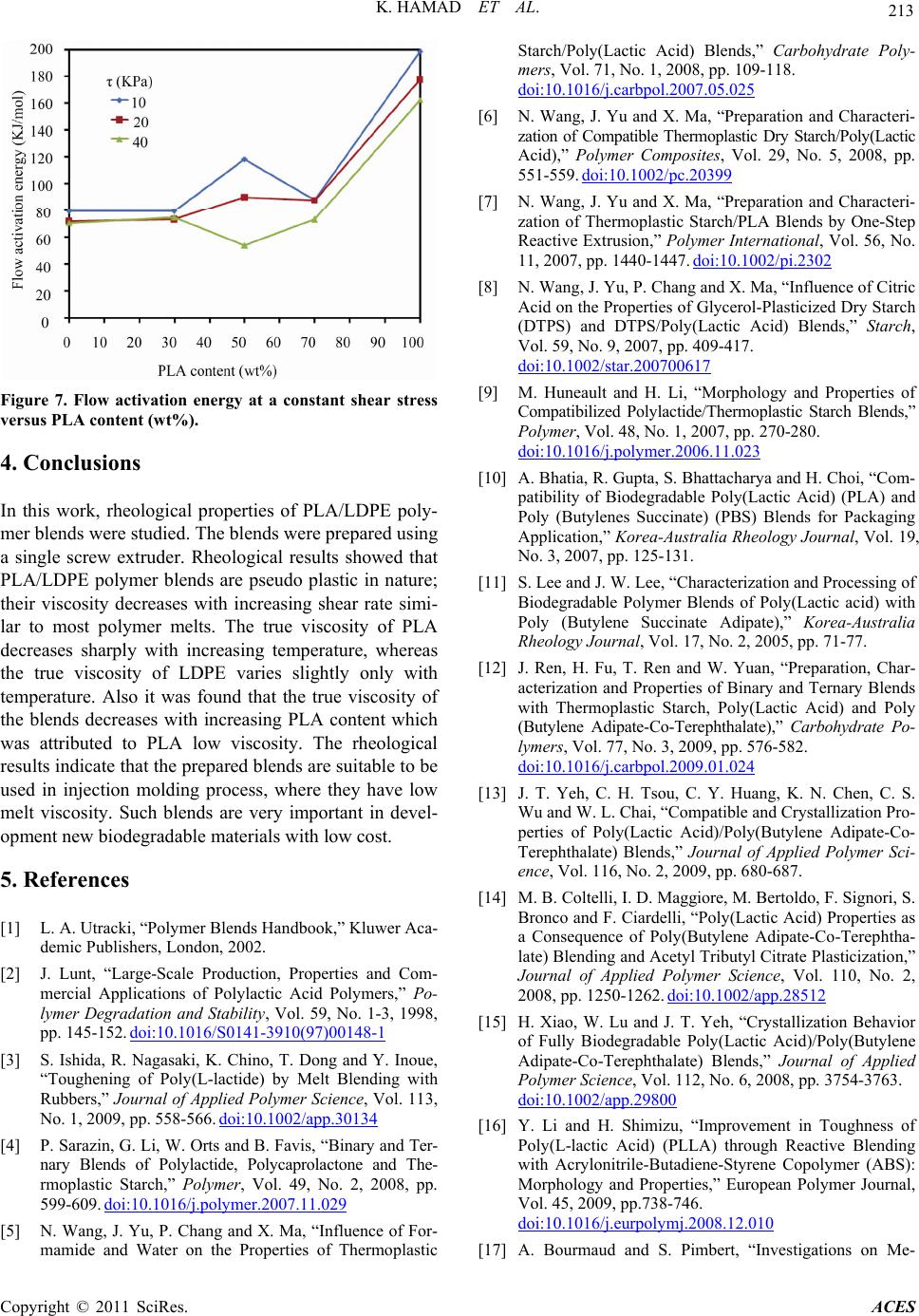 K. HAMAD ET AL. Copyright © 2011 SciRes. ACES 213 Figure 7. Flow activation energy at a constant shear stress versus PLA content (wt%). 4. Conclusions In this work, rheological properties of PLA/LDPE poly- mer blends were studied. The blends were prepared using a single screw extruder. Rheological results showed that PLA/LDPE polymer blends are pseudo plastic in nature; their viscosity decreases with increasing shear rate simi- lar to most polymer melts. The true viscosity of PLA decreases sharply with increasing temperature, whereas the true viscosity of LDPE varies slightly only with temperature. Also it was found that the true viscosity of the blends decreases with increasing PLA content which was attributed to PLA low viscosity. The rheological results indicate that the prepared blends are suitable to be used in injection molding process, where they have low melt viscosity. Such blends are very important in devel- opment new biodegradable materials with low cost. 5. References [1] L. A. Utracki, “Polymer Blends Handbook,” Kluwer Aca- demic Publishers, London, 2002. [2] J. Lunt, “Large-Scale Production, Properties and Com- mercial Applications of Polylactic Acid Polymers,” Po- lymer Degradation and Stability, Vol. 59, No. 1-3, 1998, pp. 145-152. doi:10.1016/S0141-3910(97)00148-1 [3] S. Ishida, R. Nagasaki, K. Chino, T. Dong and Y. Inoue, “Toughening of Poly(L-lactide) by Melt Blending with Rubbers,” Journal of Applied Polymer Science, Vol. 113, No. 1, 2009, pp. 558-566. doi:10.1002/app.30134 [4] P. Sarazin, G. Li, W. Orts and B. Favis, “Binary and Ter- nary Blends of Polylactide, Polycaprolactone and The- rmoplastic Starch,” Polymer, Vol. 49, No. 2, 2008, pp. 599-609. doi:10.1016/j.polymer.2007.11.029 [5] N. Wang, J. Yu, P. Chang and X. Ma, “Influence of For- mamide and Water on the Properties of Thermoplastic Starch/Poly(Lactic Acid) Blends,” Carbohydrate Poly- mers, Vol. 71, No. 1, 2008, pp. 109-118. doi:10.1016/j.carbpol.2007.05.025 [6] N. Wang, J. Yu and X. Ma, “Preparation and Characteri- zation of Compatible Thermoplastic Dry Starch/Poly(Lactic Acid),” Polymer Composites, Vol. 29, No. 5, 2008, pp. 551-559. doi:10.1002/pc.20399 [7] N. Wang, J. Yu and X. Ma, “Preparation and Characteri- zation of Thermoplastic Starch/PLA Blends by One-Step Reactive Extrusion,” Polymer International, Vol. 56, No. 11, 2007, pp. 1440-1447. doi:10.1002/pi.2302 [8] N. Wang, J. Yu, P. Chang and X. Ma, “Influence of Citric Acid on the Properties of Glycerol-Plasticized Dry Starch (DTPS) and DTPS/Poly(Lactic Acid) Blends,” Starch, Vol. 59, No. 9, 2007, pp. 409-417. doi:10.1002/star.200700617 [9] M. Huneault and H. Li, “Morphology and Properties of Compatibilized Polylactide/Thermoplastic Starch Blends,” Polymer, Vol. 48, No. 1, 2007, pp. 270-280. doi:10.1016/j.polymer.2006.11.023 [10] A. Bhatia, R. Gupta, S. Bhattacharya and H. Choi, “Com- patibility of Biodegradable Poly(Lactic Acid) (PLA) and Poly (Butylenes Succinate) (PBS) Blends for Packaging Application,” Korea-Australia Rheology Journal, Vol. 19, No. 3, 2007, pp. 125-131. [11] S. Lee and J. W. Lee, “Cha racterization and Processing of Biodegradable Polymer Blends of Poly(Lactic acid) with Poly (Butylene Succinate Adipate),” Korea-Australia Rheology Journal, Vol. 17, No. 2, 2005, pp. 71-77. [12] J. Ren, H. Fu, T. Ren and W. Yuan, “Preparation, Char- acterization and Properties of Binary and Ternary Blends with Thermoplastic Starch, Poly(Lactic Acid) and Poly (Butylene Adipate-Co-Terephthalate),” Carbohydrate Po- lymers, Vol. 77, No. 3, 2009, pp. 576-582. doi:10.1016/j.carbpol.2009.01.024 [13] J. T. Yeh, C. H. Tsou, C. Y. Huang, K. N. Chen, C. S. Wu and W. L. Chai, “Compatible and Crystallization Pro- perties of Poly(Lactic Acid)/Poly(Butylene Adipate-Co- Terephthalate) Blends,” Journal of Applied Polymer Sci- ence, Vol. 116, No. 2, 2009, pp. 680-687. [14] M. B. Coltelli, I. D. Maggiore, M. Bertoldo, F. Signori, S. Bronco and F. Ciardelli, “Poly(Lactic Acid) Properties as a Consequence of Poly(Butylene Adipate-Co-Terephtha- late) Blending and Acetyl Tributyl Citrate Plasticization,” Journal of Applied Polymer Science, Vol. 110, No. 2, 2008, pp. 1250-1262. doi:10.1002/app.28512 [15] H. Xiao, W. Lu and J. T. Yeh, “Crystallization Behavior of Fully Biodegradable Poly(Lactic Acid)/Poly(Butylene Adipate-Co-Terephthalate) Blends,” Journal of Applied Polymer Science, Vol. 112, No. 6, 2008, pp. 3754-3763. doi:10.1002/app.29800 [16] Y. Li and H. Shimizu, “Improvement in Toughness of Poly(L-lactic Acid) (PLLA) through Reactive Blending with Acrylonitrile-Butadiene-Styrene Copolymer (ABS): Morphology and Properties,” European Polymer Journal, Vol. 45, 2009, pp.738-746. doi:10.1016/j.eurpolymj.2008.12.010 [17] A. Bourmaud and S. Pimbert, “Investigations on Me-  214 K. HAMAD ET AL. chanical Properties of Poly(Propylene) and Poly(Lactic Acid) Reinforced by Miscanthus Fibers,” Composites Part A: Applied Science and Manufacturing, Vol. 39, No. 9, 2008, pp. 1444-1454. doi:10.1016/j.compositesa.2008.05.023 [18] N. Reddy, D. Nama and Y. Yang, “Polylactic Acid/ Poly- propylene Polyblend Fibers for Better Resistance to Deg- radation,” Polymer Degradation and Stability, Vol. 93, No. 1, 2008, pp. 233-241. doi:10.1016/j.polymdegradstab.2007.09.005 [19] K. Hamad, M. Kaseem and F. Deri, “Rheological and Me- chanical Characterization of Poly(Lactic Acid)/ Polypro- pylene Polymer Blends,” Journal of Polymer Research, Vol. 65, No. 5, 2011, pp. 509-519. [20] G. Biresaw and C. J. Carriere, “Compatibility and Me- chanical Properties of Blends of Polystyrene with Biode- gradable Polyesters,” Composites Part A: Applied Science and Manufacturing, Vol. 35, No. 3, 2004, pp. 313-320. doi:10.1016/j.compositesa.2003.09.020 [21] K. Hamad, M. Kaseem and F. Deri, “Rheological and Mechanical Properties of Poly(Lactic Acid)/Polystyrene Polymer Blend,” Polymer Bulletin, Vol. 65, No. 5, 2010, pp. 509-519. doi:10.1007/s00289-010-0354-2 [22] H. M. Park, S. R. Lee, S. R. Chowdhury, T. K. Kang, H. K. Kim, S. H. Park and C. S. Ha, “Tensile Properties, Morphology, and Biodegradability of Blends of Starch with Various Thermoplastics,” Journal of Applied Poly- mer Science, Vol. 86, No. 11, 2002, pp. 2907-2915. doi:10.1002/app.11332 [23] Y. F. Kim, C. N. Choi, Y. D. Kim, K. Y. Lee and M. S. Lee, “Compatibilization of Immiscible Poly(L-lactide) and Low Density Polyethylene Blends,” Fibers and Polymers, Vol. 5, No. 4, 2004, pp. 270-274. doi:10.1007/BF02875524 [24] H. Balakrishnan, A. Hassan and M. U. Wahit, “Mechani- cal, Thermal, and Morphological Properties of Polylactic Acid/Linear Low Density Polyethylene Blends,” Journal of Elastomers and Plastics, Vol. 42, No. 3, 2010, pp. 223- 239. doi:10.1177/0095244310362403 [25] G. Singh, H. Bhunia, A. Rajor, R. N. Jana and V. Choud- hary, “Mechanical Properties and Morphology of Poly- lactide, Linear Low-Density Polyethylene, and Their Blends,” Journal of Applied Polymer Science, Vol. 118, No. 1, 2010, pp. 496-502. [26] Z. Tadmor and C. G. Gogos, “Principles of Polymer Pro- cessing,” John Wiley & Sons, Inc., Hoboken, 2006. [27] C. D. Han, “Rheology and Processing of Polymeric Mate- rials (Polymer Processing),” Oxford University Press, New York, 2007. [28] E. B. Bagley, “End Corrections in the Capillary Flow of Polyethylene,” Journal of Applied Physics, Vol. 28, No. 5, 1957, pp. 624-627. doi:10.1063/1.1722814 [29] W. Sinthavathavorn, M. Nithitanakul, B. Grady and R. Mangaraphan, “Melt Rheology and Die Swell of PA6/ LDPE Blends by Using Lithium Ionomer as a Compatibi- lizer,” Polymer Bulletin, Vol. 63, No. 1, 2009, pp. 23-35. doi:10.1007/s00289-009-0063-x Copyright © 2011 SciRes. ACES
|