 Open Journal of Geology, 2011, 1, 37-44 http://dx.doi.org/10.4236/ojg.2011.13004 Published Online October 2011 (http://www.SciRP.org/journal/ojg) Copyright © 2011 SciRes. OJG A New Species of Fossil Mus (Muridae, Mammalia) from the Late Quaternary Deposits of Narmada Valley, Central India Bahadur Singh Kotlia1, M ou li sh re e Jos hi 2, Lalit Mohan Joshi1 1Department of Geology, Kumaun University, Nainital, India 2Faculty of Petroleum and Renewable Energy Engineering, Universiti Teknologi Malaysia (UTM), Johor Bahru, Malaysia E-mail: Bahadur.kotlia@gmail.com Received April 18, 2011; revised July 21, 2011; accepted August 29, 2011 Abstract A new species of fossil Mus (Muridae, Rodentia) is described from the Pleistocene fluviatile deposits of the Narmada valley (Central India). The species, Mus narmadaensis sp. Nov., has a comparatively smaller lower molar which is characterized by a narrow molar with well connected cusps, small anterior expansion of lin- gual anteroconid, protoconid and metaconid, reduced posterior cingulum in addition to hypoconid and ento- conid nearly at the same level. The large M3 has centrally placed bulbous hypoconid. Among the extant spe- cies, the present one is closest to M. shortridgei in having similarly placed protoconid and metaconid in M1 and a well developed hypoconid in M3. Keywords: Fossil Mus, Late Quaternary, Narmada basin, Central India 1. Introduction Considered to be the most successful groups of living mammals, the murid rodents were originated in the In- dian sub- continent about 14 ma ago. At present, they are found all over the world with ability to adapt themselves to varied environmental conditions and show marked species diversity. Today more than 70% of the murid species are found in the Indo-Australian region, whereas, 26% murid taxa are found in Africa [1]. The oldest known fossil murid, Antemus chinjiensis was evolved from a cricetid Potwarmus primitivus and was recovered from the Chinji Formation (Siwalik sub-group) in the Potwar Plateau [2]. The study of fossil murids in the In- dian subcontinent was initiated by [3-6] and followed by [2] who made significant contribution to the study of Pakistan Siwalik by describing various murid taxa. Sub- sequently, a sizeable work on the Afghanistan murids was done by [7-11]. As far as the Indian murids are con- cerned, a number of researches, e.g. [12-26] have shown that the murids were widespread in the country from the Pliocene onwards. Among the murids, the genus Mus has been reported from various parts of India, e.g., from Kurnool caves [27], Saketi [19], Kashmir basin [22], Narmada valley [21,25,28], Upper Pleistocene of Bhimtal [23,24], and Dulam [25]. The great diversity of Mus both in terms of number and taxa indicates that the probable place of its origin was the Indian subcontinent. However, an early stock migration to the African continent during Mio- Pliocene time has been suggested [29]. Elsewhere in Asia, Mus has been described from China [30], Crete [31], former USSR [32], Hungary [33], Japan [34] and Thailand [35]. We report here the lower molars of a new species of Mus from the Devakachar section of the Hirdepur For- mation of the Narmada deposits. 2. Area of Study, Litho-Chronology and Fossil Material Narmada, the largest river in the Central India, originates at the plateau of Amarkantak (22º40’N; 81º40’E) and after traversing across the middle of the Indian sub conti- nent, it joins the Gulf of Cambay near Baroda. The cour- se of the river is controlled by the east-west lineament. Between Bhedaghat (23º8’N; 79º48’E) and Hoshan- gabad (22º45’N; 77º45’E), the river forms a trough in 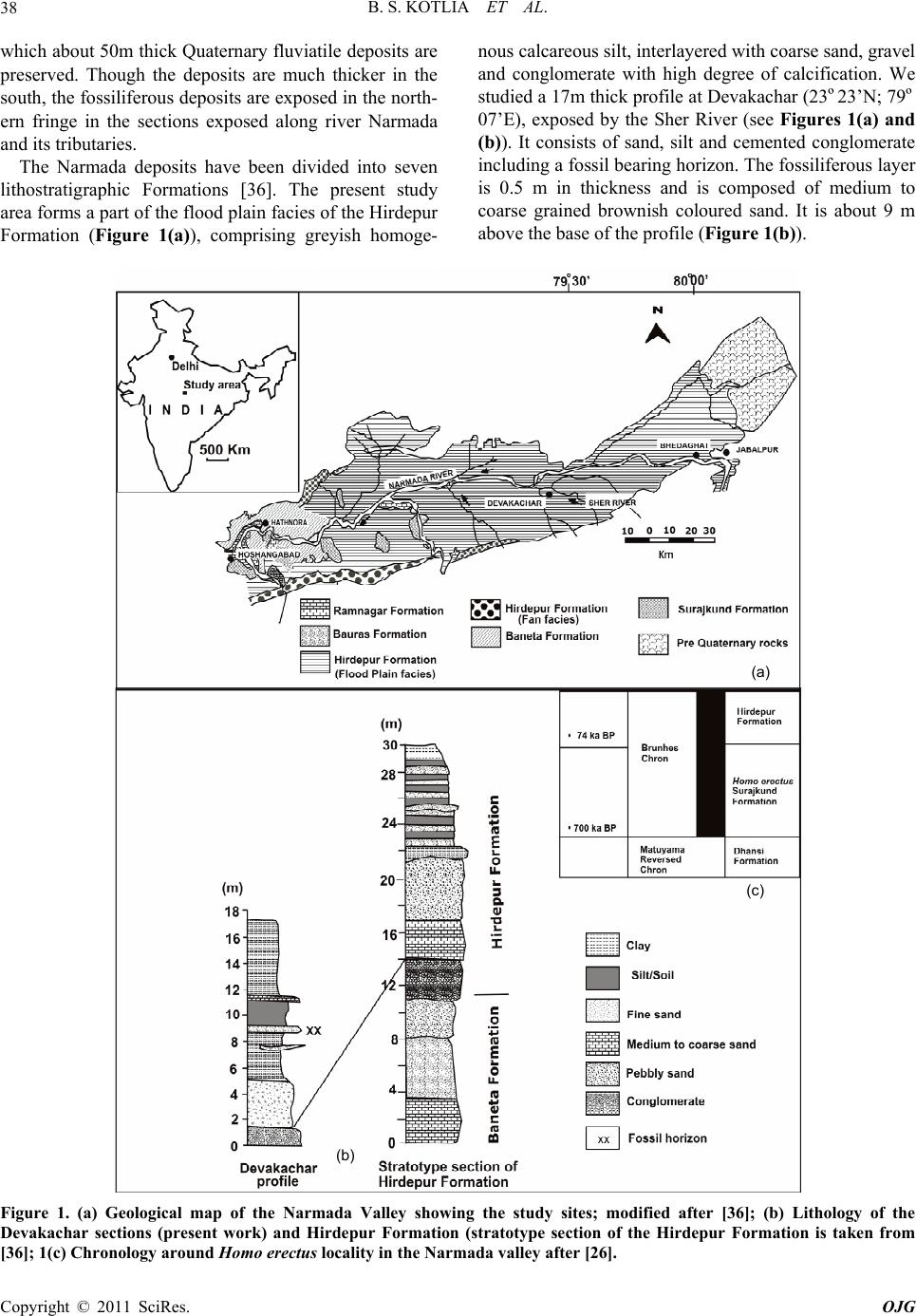 B. S. KOTLIA ET AL. 38 which about 50m thick Quaternary fluviatile deposits are preserved. Though the deposits are much thicker in the south, the fossiliferous deposits are exposed in the north- ern fringe in the sections exposed along river Narmada and its tributaries. The Narmada deposits have been divided into seven lithostratigraphic Formations [36]. The present study area forms a part of the flood plain facies of the Hirdepur Formation (Figure 1(a)), comprising greyish homoge- nous calcareous silt, interlayered with coarse sand, grav el and conglomerate with high degree of calcification. We studied a 17m thick profile at Devakachar (23º23’N; 79º 07’E), exposed by the Sher River (see Figures 1(a) and (b)). It consists of sand, silt and cemented conglomerate including a fossil bearing horizon. The fossiliferous layer is 0.5 m in thickness and is composed of medium to coarse grained brownish coloured sand. It is about 9 m above the base of the profile (Figure 1(b)). (a) (b) (c) Figure 1. (a) Geological map of the Narmada Valley showing the study sites; modified after [36]; (b) Lithology of the Devakachar sections (present work) and Hirdepur Formation (stratotype section of the Hirdepur Formation is taken from [36]; 1(c) Chronology around Homo erectus locality in the Narmada valley after [26]. Copyright © 2011 SciRes. OJG 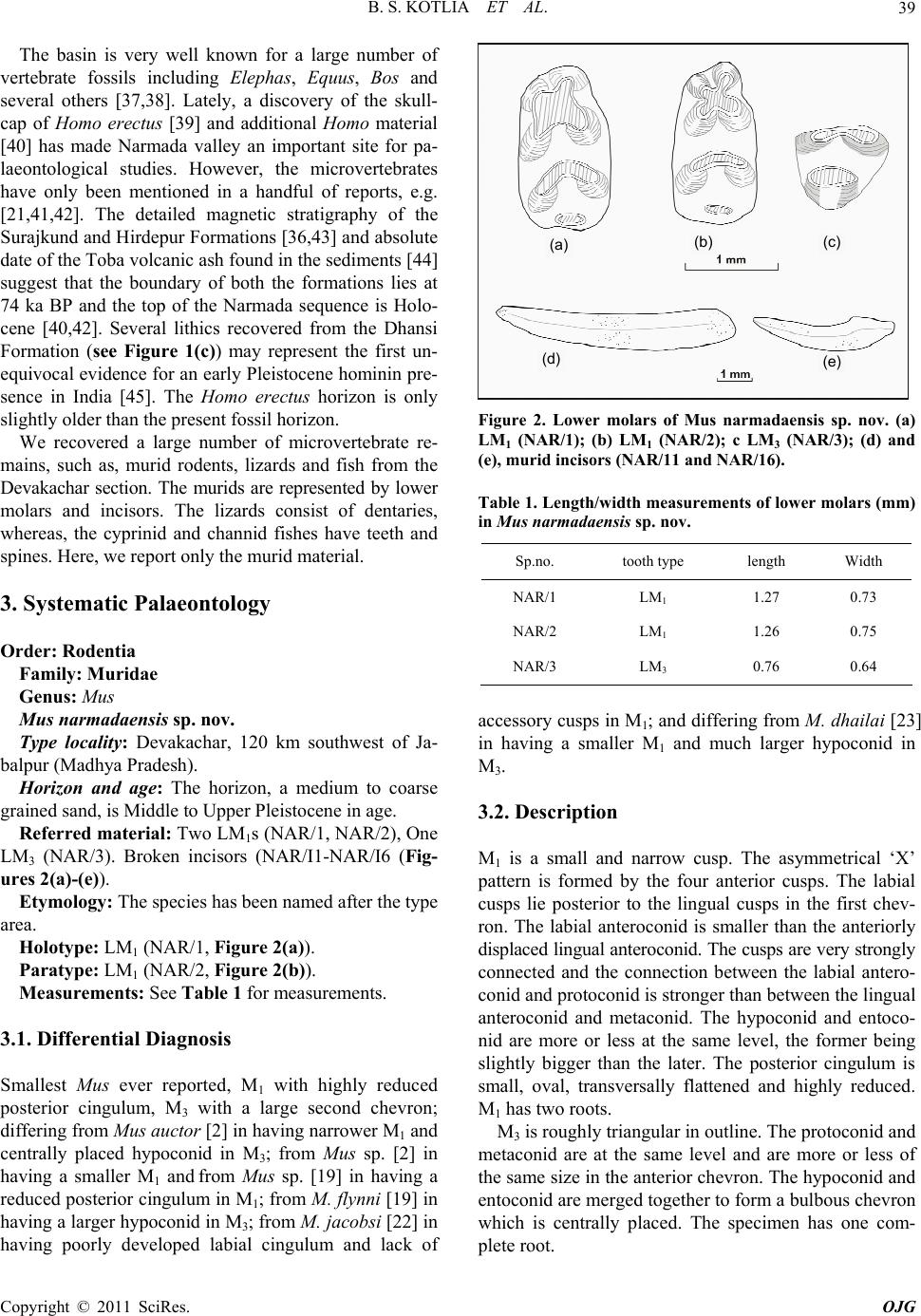 B. S. KOTLIA ET AL. Copyright © 2011 SciRes. OJG 39 The basin is very well known for a large number of vertebrate fossils including Elephas, Equus, Bos and several others [37,38]. Lately, a discovery of the skull- cap of Homo erectus [39] and additional Homo material [40] has made Narmada valley an important site for pa- laeontological studies. However, the microvertebrates have only been mentioned in a handful of reports, e.g. [21,41,42]. The detailed magnetic stratigraphy of the Surajkund and Hirdepur Formations [36,43] and absolute date of the Toba volcanic ash found in the sediments [44] suggest that the boundary of both the formations lies at 74 ka BP and the top of the Narmada sequence is Holo- cene [40,42]. Several lithics recovered from the Dhansi Formation (see Figure 1(c)) may represent the first un- equivocal evidence for an early Pleistocene hominin pre- sence in India [45]. The Homo erectus horizon is only slightly older than the present fossil horizon. We recovered a large number of microvertebrate re- mains, such as, murid rodents, lizards and fish from the Devakachar section. The murids are represented by lower molars and incisors. The lizards consist of dentaries, whereas, the cyprinid and channid fishes have teeth and spines. Her e, we report only the murid material. 3. Systematic Palaeontology Order: Rodentia Family: Muridae Genus: Mus Mus narmadaensis sp. nov. Type locality: Devakachar, 120 km southwest of Ja- balpur (Madhya Pradesh). Horizon and age: The horizon, a medium to coarse grained sand, is Middle to Upper Pleistocene in age. Referred material: Two LM1s (NAR/1, NAR/2), One LM3 (NAR/3). Broken incisors (NAR/I1-NAR/I6 (Fig- ures 2(a)-(e ) ). Etymology: The species has been named after the type area. Holotype: LM1 (NAR/1, Figure 2(a)). Paratype: LM1 (NAR/2, Figure 2(b)). Measurements: See Table 1 for measurements. 3.1. Differential Diagnosis Smallest Mus ever reported, M1 with highly reduced posterior cingulum, M3 with a large second chevron; differing from Mus auctor [2] in having narrower M1 and centrally placed hypoconid in M3; from Mus sp. [2] in having a smaller M1 and from Mus sp. [19] in having a reduced posterior cingulum in M1; from M. flynni [19] in having a larger hypoconid in M3; from M. jacobsi [22] in having poorly developed labial cingulum and lack of (a) (b) (c) (e) (d) Figure 2. Lower molars of Mus narmadaensis sp. nov. (a) LM1 (NAR/1); (b) LM1 (NAR/2); c LM3 (NAR/3); (d) and (e), murid incisors (NAR/11 and NAR/16). Table 1. Length/width measurements of lower molars (mm) in Mus narmadaensis sp. nov. Sp.no. tooth type length Width NAR/1 LM1 1.27 0.73 NAR/2 LM1 1.26 0.75 NAR/3 LM3 0.76 0.64 accessory cusps in M1; and differing from M. dhailai [23 ] in having a smaller M1 and much larger hypoconid in M3. 3.2. Description M1 is a small and narrow cusp. The asymmetrical ‘X’ pattern is formed by the four anterior cusps. The labial cusps lie posterior to the lingual cusps in the first chev- ron. The labial anteroconid is smaller than the anteriorly displaced lingual anteroconid. The cusps are very strongly connected and the connection between the labial antero- conid and protoconid is stronger than between the lingual anteroconid and metaconid. The hypoconid and entoco- nid are more or less at the same level, the former being slightly bigger than the later. The posterior cingulum is small, oval, transversally flattened and highly reduced. M1 has two roots. M3 is roughly triangular in outline. The protoconid and metaconid are at the same level and are more or less of the same size in the anterior chevron. The hypoconid and entoconid are merged together to form a bulbous chevron which is centrally placed. The specimen has one com- plete root. 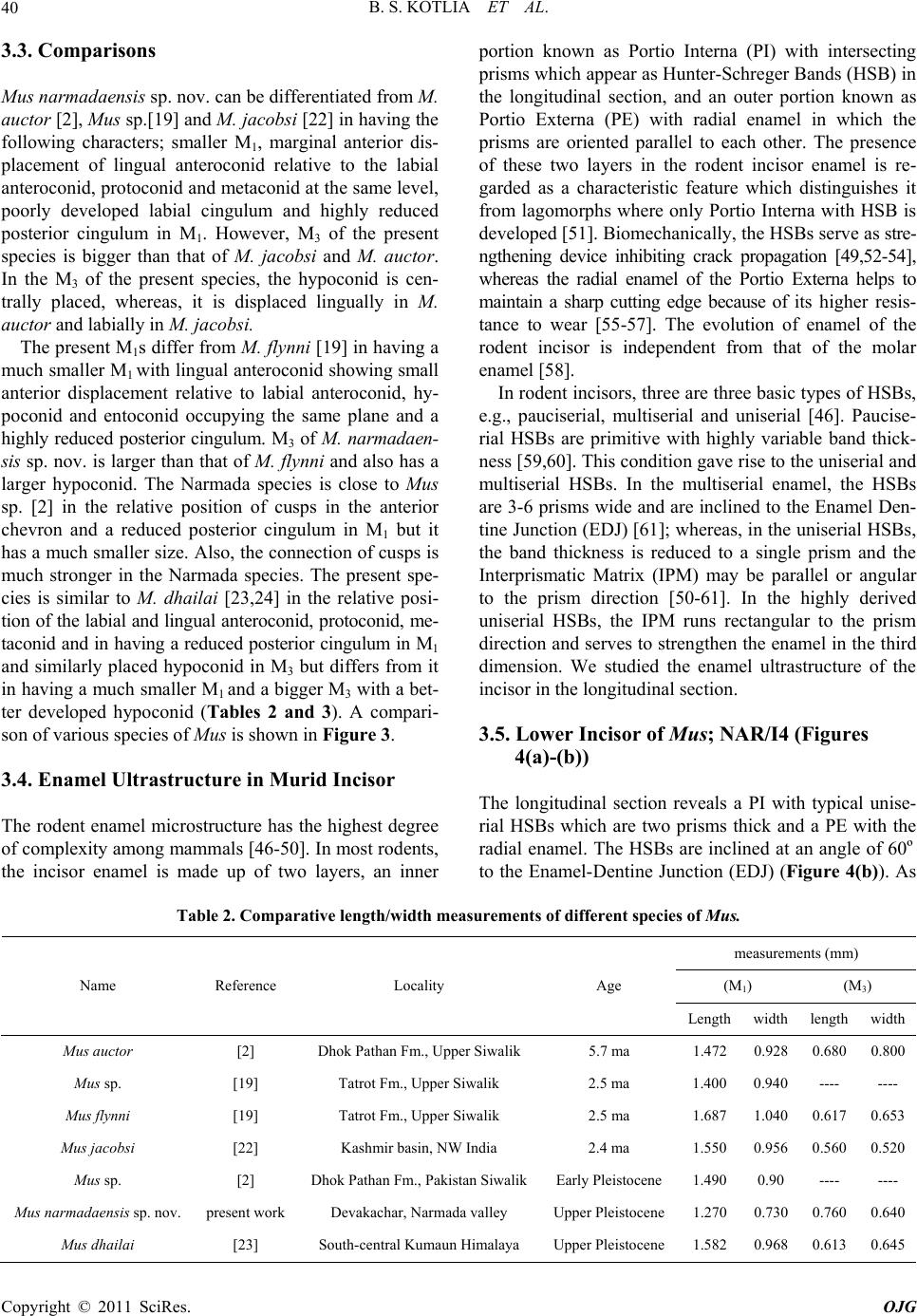 B. S. KOTLIA ET AL. 40 3.3. Comparisons Mus narmadaensis sp. nov. can be differentiated from M. auctor [2], Mus sp.[19] and M. jacobsi [22] in having the following characters; smaller M1, marginal anterior dis- placement of lingual anteroconid relative to the labial anteroconid, protoconid and metaconid at the same level, poorly developed labial cingulum and highly reduced posterior cingulum in M1. However, M3 of the present species is bigger than that of M. jacobsi and M. auctor. In the M3 of the present species, the hypoconid is cen- trally placed, whereas, it is displaced lingually in M. auctor and labially in M. jacobsi. The present M1s differ from M. flynni [19] in having a much smaller M1 with lingual anteroconid sh owing small anterior displacement relative to labial anteroconid, hy- poconid and entoconid occupying the same plane and a highly reduced posterior cingulum. M3 of M. narmadaen- sis sp. nov. is larg er than that of M. flynni and also ha s a larger hypoconid. The Narmada species is close to Mus sp. [2] in the relative position of cusps in the anterior chevron and a reduced posterior cingulum in M1 but it has a much smaller size. Also, the connection of cusps is much stronger in the Narmada species. The present spe- cies is similar to M. dhailai [23,24] in the relative posi- tion of the labial and lingual anteroconid, protoconid, me- taconid and in having a reduced posterior cingulum in M1 and similarly placed hypoconid in M3 but differs from it in having a much smaller M1 and a bigger M3 with a bet- ter developed hypoconid (Tables 2 and 3). A compari- son of various species of Mus is shown in Figure 3. 3.4. Enamel Ultrastructure in Murid Incisor The rodent enamel microstructure has the highest degree of complexity among mammals [46-50]. In most rodents, the incisor enamel is made up of two layers, an inner portion known as Portio Interna (PI) with intersecting prisms which appear as Hunter-Schreger Bands (HSB) in the longitudinal section, and an outer portion known as Portio Externa (PE) with radial enamel in which the prisms are oriented parallel to each other. The presence of these two layers in the rodent incisor enamel is re- garded as a characteristic feature which distinguishes it from lagomorphs where only Portio Interna with HSB is developed [51]. Biomechanically, the HSBs serve as stre- ngthening device inhibiting crack propagation [49,52-54], whereas the radial enamel of the Portio Externa helps to maintain a sharp cutting edge because of its higher resis- tance to wear [55-57]. The evolution of enamel of the rodent incisor is independent from that of the molar enamel [58]. In rodent incisors, three are three basic types of HSBs, e.g., pauciserial, multiserial and uniserial [46]. Paucise- rial HSBs are primitive with highly variable band thick- ness [59,60]. This condition gave rise to the uniserial and multiserial HSBs. In the multiserial enamel, the HSBs are 3-6 prisms wide and are inclined to the Enamel Den- tine Junction (EDJ) [61]; whereas, in the uniserial HSBs, the band thickness is reduced to a single prism and the Interprismatic Matrix (IPM) may be parallel or angular to the prism direction [50-61]. In the highly derived uniserial HSBs, the IPM runs rectangular to the prism direction and serves to strength en the enamel in the third dimension. We studied the enamel ultrastructure of the incisor in the longitudinal section. 3.5. Lower Incisor of Mus; NAR/I4 (Figures 4(a)-(b)) The longitudinal section reveals a PI with typical unise- rial HSBs which are two prisms thick and a PE with the radial enamel. The HSBs are inclined at an angle of 60º to the Enamel-Dentine Junction (EDJ) (Figure 4(b)). As Table 2. Comparative length/width measurements of different species of Mus. measurements (mm) (M1) (M3) Name Reference Locality Age Length width lengthwidth Mus auctor [2] Dhok Pathan Fm., Upper Si wa li k 5.7 ma 1.472 0.928 0.6800.800 Mus sp. [19] Tatrot Fm., Upper Siwalik 2.5 ma 1.400 0.940 ---- ---- Mus flynni [19] Tatrot Fm., U p pe r Siwalik 2.5 ma 1.687 1.040 0.6170.653 Mus jacobsi [22] Kashmir basin, NW India 2.4 ma 1.550 0.956 0.5600.520 Mus sp. [2] Dhok Pathan Fm., Pakistan SiwalikEarly Pleistocene1.490 0.90 ---- ---- Mus narmadaensis sp. nov. present work Devakachar, Narmada valley Upper Pleistocene1.270 0.730 0.7600.640 Mus dhailai [23] South-central Kumaun Himalaya Upper Pleistocene1.582 0.968 0.6130.645 Copyright © 2011 SciRes. OJG 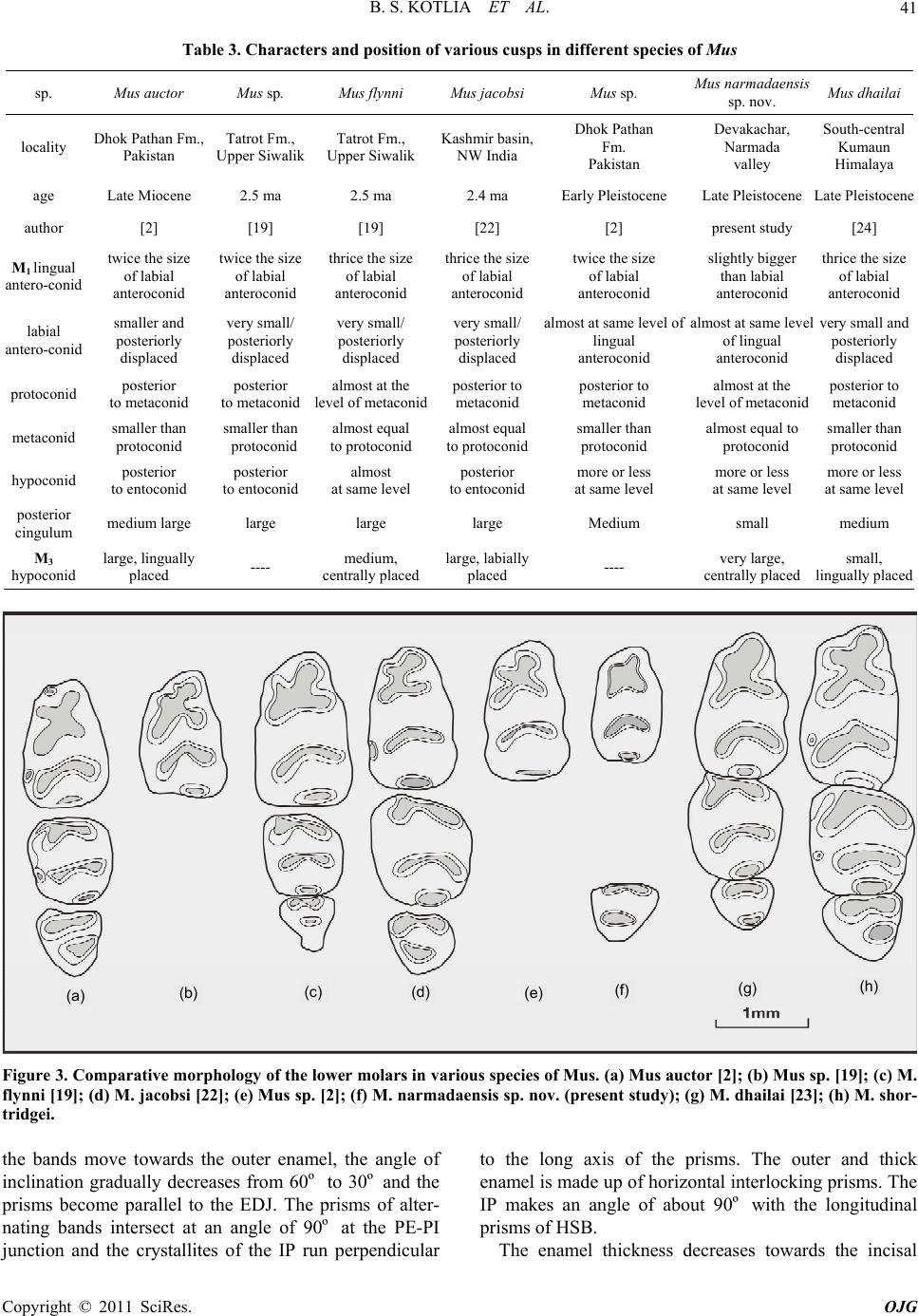 B. S. KOTLIA ET AL.41 Table 3. Characters and position of various cusps in different species of Mus sp. Mus auctor Mus sp. Mus flynni Mus jacobsiMus sp. Mus narmadaensis sp. nov. Mus dhailai locality Dhok Pathan Fm., Pakistan Tatrot Fm., Upper Siwalik Tatrot Fm., Upper Siwalik Kashmir basin, NW India Dhok Pathan Fm. Pakistan Devakachar, Narmada valley South-central Kumaun Himalaya age Late Miocene 2.5 ma 2.5 ma 2.4 ma Early Pleistocene Late Pleistocene Late Pleistocene author [2] [19] [19] [22] [2] present study [24] M1 lingual antero-conid twice the size of labial anteroconid twice the size of labial anteroconid thrice the size of labial anteroconid thrice the size of labial anteroconid twice the size of labial anteroconid slightly bigger than labial anteroconid thrice the size of labial anteroconid labial antero-conid smaller and posteriorly displaced very small/ posteriorly displaced very small/ posteriorly displaced very small/ posteriorly displaced almost at same level of lingual anteroconid almost at same level of lingual anteroconid very small and posteriorly displaced protoconid posterior to metaconid posterior to metaconid almost at the level of metaconidposterior to metaconid posterior to metaconid almost at the level of metaconid posterior to metaconid metaconid smaller than protoconid smaller than protoconid almost equal to protoconid almost equal to protoconidsmaller than protoconid almost equal to protoconid smaller than protoconid hypoconid posterior to entoconid posterior to entoconid almost at same level posterior to entoconidmore or less at same level more or less at same level more or less at same level posterior cingulum medium large large large large Medium small medium M3 hypoconid large, lingually placed ---- medium, centrally placed large, labially placed ---- very large, centrally placed small, lingually placed (a) (b) (c) (e) (d) (f) (g) (h) Figure 3. Comparative morphology of the lower molars in various species of Mus. (a) Mus auctor [2]; (b) Mus sp. [19]; (c) M. flynni [19]; (d) M. jacobsi [22]; (e) Mus sp. [2]; (f) M. narmadaensis sp. nov. (present study); (g) M. dhailai [23]; (h) M. shor- tridgei. the bands move towards the outer enamel, the angle of inclination gradually decreases from 60º to 30º and the prisms become parallel to the EDJ. The prisms of alter- nating bands intersect at an angle of 90º at the PE-PI junction and the crystallites of the IP run perpendicular to the long axis of the prisms. The outer and thick enamel is made up of horizontal interlocking prisms. The IP makes an angle of about 90º with the longitudinal prisms of HSB. The enamel thickness decreases towards the incisal Copyright © 2011 SciRes. OJG  B. S. KOTLIA ET AL. Copyright © 2011 SciRes. OJG 42 ab Figure 4. Longitudinal views of the enamel ultrastructure in the lower incisor in Mus (Bar represents 1 mm). direction (Figure 4(a)). Near the tip of the incisor, the HSBs are more closely spaced just below the outer enamel and the IPM is dense below the PE. The PI is reduced towards the incisal end. At the tip of th e incisor, only the radial enamel of the PE is present (Figure 3(a)). The crystallites of the IPM are rectangular and serve to strengthen the enamel in a third dimension. This feature is generally seen in the derived species of murids. 4. Discussion The reduced posterior cingulum in M1 in the present specimen points to its affinity with Pahari section of Mus [13]. In India, Mus is represented by three subgenera, Mus with M. booduga and M. dunni; Pyromys with M. saxicola, M. shortridgei and M. platythrix; and Coelomys with M. mayori, M. pahari and M. crociduroides [14]. Coelomys section includes Asiatic species such as M. mayori, M. pahari, M. crociduroides and M. shortridgei [13]. The Narmada Mus resembles M. pahari in general outline and the placement of cusps in the anterior chev- ron of M1. However, the second chevron in M3 of M. pahari is weakly developed and shows a small lingually placed hypoconid. M. crociduroides has a weak second chevron in M3 and is therefore different from the present species. M. mayori differs from M. narmadaensis sp . nov. in having a posteriorly displaced protoconid in M1 and a lingually placed and weakly developed hypoconid in M3. Among the extant species, M. narmadaensis sp. nov. shows closest resemblance with M. shortridgei in having similarly placed protoconid and metaconid in M1 and a well developed hypoconid in M3. A very small size of the M. narmadaensis sp. nov. may be attributed to its getting iso lated from the stock at the onset of glacial age during the Pleistocene period. Murids are very sensitive to the climatic changes and it is believed that the onset of cold climatic conditions wiped out several species of murids while some migrated to warmer regions [25]. It may be postulated that M. nar- madaensis sp. nov. was one of those species that mi- grated towards Central India from the Lesser Himalayan region at the onset of glaciation. It may have lived there in isolation for a considerable period due to which it could not evolve more progressively although its enamel shows some derived characters as much as in other Pleistocene species. 5. Acknowledgements The study was spon sored by the University Grants Com- mission and Council of Scientific and Industrial Resear- ch, New Delhi. The laboratory facilities were provided by the Department of Geology, Kuma un University , Naini tal. 6. References [1] R. Patnaik, “Tooth Enamel Microstructure in Rattus rat- tus: Functional Implications”, EMSI Bulletin, Vol. 1, No. 1, 2000, pp. 9-15. [2] L. L. Jacobs, “Fossil Rodents (Rhizomydae and Muridae) from Neogene Siwalik Deposits, Pakistan,” Museum of Northern Arizona Press Bulletin Series, Vol. 52, 1978, pp. 1-103. [3] P. T. Cautley, “Letter Noticing the Discovery of Further Fossils in Vast Quantity in the Siwalik Range,” Journal Asiatic Society of Bengal, Vol. 4, 1835, pp. 585-587. [4] E. H. Colbert, “Siwalik Mammals in American Museum of Natural History,” Trans American Philosophical Soci- ety Natural History, Vol. 26, 1935, pp. 1-401.  B. S. KOTLIA ET AL.43 [5] G. E. Lewis, “Siwalik Fossil Mastomys,” American Jour- nal of Science, Vol. 237, No. 4, 1939, pp. 341-344. doi:10.2475/ajs.237.5.341 [6] C. C. Black, “Review of Fossil Rodents from Neogene Siwalik Beds of India and Pakistan,” Pale ont olog y, Vol. 15, No. 2, 1972, pp. 238-266. [7] S. Sen, “Rongeurs et Lagomorphes du gisement Pliocene de Pul-e Charkhi, Basin de Kabul, Afghanistan,” Bulletin Museum National History Natural, 5th Edition, Vol. 5, No. 1, 1983, pp. 33-74. [8] S. Sen, M. Sabatier, M. Brunet and E. Heintz, “Decouverte de Rongeurs <<Africans>> dans le Pliocene d’ Afghanistan (basin de Sarobi) Implications Paleo- Biogeographiques et Stratigraphiques,”Bulletin Museum National History Natural, 4th Edition, Vol. 1, No. 1, 1979, pp. 65-75. [9] L. D. Brandy, “Etude de Rongeurs muroides du Neogene superieur et du Quaternaire d’Europe, d’ Afrique du Nord et d’Afghanistan, Evolution, biogeographie, correlations”, These, 3e cycle University Science Technical Language Montpellier, 1979, pp. 1-190. [10] L. D. Brandy, “Rongeurs Muroides du Neogene Supe- rieur d’Afghanistan. Evolution, Biogeographie, Correla- tions,”Palaeovertebrata, Vol. 11, No. 4, 1981, pp.133- 179. [11] L. D. Brandy, L. D. Sabatier and J. J. Jaeger, “Implication Phylogenetique et. Biogeographiques des Derniers de- couvertes de Muridae en Afghanistan, au Pakistan et en Ethiopie,”Geobios, Vol. 13, No. 4, 1980, pp. 639-643. doi:10.1016/S0016-6995(80)80007-6 [12] J. R. Ellerman, “Mammalia Rodentia. The fauna of India including Pakistan, Burma and Ceylon,” Zoological Sur- vey of India, Vol. 3, 1961, pp.1-864. [13] X. Misonne, “African and Indo-Australian Muridae. Evolutionary,” Annals Museum Africa Centrale Zoologi- cal Series, Vol. 8, No. 172, 1969, pp. 1-219. [14] J. T. Marshall, “A synopsis of Asian Species of Mus (Ro- dentia, Muridae),” American Museum Natural History Bulletin, Vol. 158, 1977, pp. 173-220. [15] T. J. Roberts, “The Mammals of Pakistan,” Ernest Benn Limited, London, 1977, p.361. [16] R. Gaur, “First Report on a Fossil Rattus (Murinae, Ro- dentia) from the Pinjor Formation of Upper Siwalik of India,” Current Science, Vol. 55, No. 11, 1986, pp. 542-544. [17] G. G. Musser, “The Occurrence of Hadromys (Rodentia, Muridae) in Early Pleistocene Siwalik Strata in Northern Pakistan and Its Bearing on Biogeographic Affinities be- tween Indian and Northeastern African Murinae Faunas,” American Museum Novitites, Vol. 2883, 1987, pp. 1-36. [18] P.Raghavan, “Palaeoenvironment of the Pinjor Formation Based on Microfossil Assemblages and Enamel Uultra- structure of Pinjor and Other Siwalik Primates,” Ph.D Thesis, Panjab University, Chandigarh, 1989. [19] R. Patnaik, “Micropalaeontology, Biostratigraphy and Palaeoenvironmental Analysis of the Siwalik Se- quence in the Saketi Nahan Area (Himachal Pradesh),” Ph.D. Thesis, Panjab University, Chandigarh, 1991. [20] R. Patnaik, M. Bahadur, T. Sharma and A. Sahni, “A Com- parative Analysis of the Molars of Mus booduga, Mus dunni and Fossil Mus of the Indian Subcontinent, Phy- logenetic and Palaeobiogeographic Implications,” Cur- rent Science, Vol. 65, 1993, pp. 782-785. [21] R. Patnaik, G. L. Badam and V. Sathe, “Discovery of Microvertebrates from the Pleistocene Deposits of Cen- tral Narmada Valley, India,” Current Science, Vol. 68, No. 8, 1995, pp. 828-830. [22] B. S. Kotlia, “Pliocene Muridae (Rodentia, Mammalia) from Kashmir Basin, Northwestern India,” Neues Jahr- buch fϋr Geologie und Paläeontologie Abhandlungen, Vol. 184, No. 3, 1992, pp. 339-357. [23] B. S. Kotlia, “Upper Pleistocene Soricidae and Muridae from Bhimtal-Bilaspur Deposits, Kumaun Himalaya, In- dia,” Journal of Geological Society of India, Vol. 46, No. 2, 1995, pp. 177-190. [24] B. S. Kotlia, “First Upper Pleistocene Mus (Muridae, Rodentia) from the Indian Subcontinent,” Acta Zoologica Cracoviensia, Vol. 39, No.1, 1996, pp.251-259. [25] B. S. Kotlia, “A New Species of Fossil Mus (Rodentia, Muridae) from the Indian Himalaya: Evolutionary and Phylogenetic Implications,” Palaeoworld, Vol. 17, 2008, pp.47-56. [26] B. S. Kotlia and M. Joshi, “Taphonomy of Late Pleisto- cene Micromammalian Fauna of Narmada Valley Central India,” Palaeoworld, Vol. 20, No. 1, 2011, pp. 84-91. doi:10.1016/j.palwor.2010.09.017 [27] M. L. K. Murty, “Late Pleistocene Fauna of Kar- nool Caves, South India,” In: A.T. Clason, Ed., Archaeo- logical Studies, North Holland Publishing Company Am- sterdam, New York, 1975, pp. 132-138. [28] M. Joshi, “Palaeontology and Palaeoenvironment of Plei- stocene-Holocene Deposits of Narmada Valley between Jabalpur and Hoshangabad, Madhya Pradesh,” Ph.D the- sis, Kumaun University, Nainital, 2003. [29] D. Gerrads, “Rongeurs et Lagomorphs du Pleistocene moyen de la Grotte des Rhinoceros, Carriere Oulalpd Hamida la Casablanca, Maroc,” Neues Jahrbuch fϋr Ge- ologie und Paläeontologie Abhandlungen, Vol. 191, No. 2, 1994, pp. 147-172. [30] W. C. Pie, “On the Mammalian Remains from Lo cality 3 at Choukoutien,” Palaeontologia Sinica, Vol. 7, No. 5, 1936, pp. 1-120. [31] D. M. A. Bate, “New Pleistocene Muridae from Crete,” Annales Magazine Natural History, Vol. 11, No. 9, 1942, pp. 41-49. [32] I. M. Gromov, “Upper Quaternary Rodents of the Samara Bend and Conditions of Preservation,” Transactions of Institute of Zoological Academic Sciences, Vol. 22, 1957, pp. 112-150. [33] D. Janossy, “Vorlaufige Mittelpleis tozane vertebraten- fauna der Tarko-Felsnische (N.O. Ungarn),”Annale His- tory National Museum National History, Vol. 54, 1962, pp. 155-176.” [34] K. Kowalski and Y. Hasegawa, “Quaternary Rodents Copyright © 2011 SciRes. OJG  B. S. KOTLIA ET AL. Copyright © 2011 SciRes. OJG 44 from Japan,” Bulletin of National Science Museum Series, Vol. 2, No. 1, 1976, pp. 31-66. [35] L. Ginsberg, R. Ingavat and S. Sen, “Decouverte d’une fauna d’age Pleistocene Moyen Terminal (loangien) dans le nord de la Thailande,”Comptets Rend Academic Sci- ence of Paris, Vol. 294, 1982, pp. 295-297. [36] M. P. Tiwari and H. Y. Bhai, “Quaternary Stratigraphy of the Narmada Valley,” Geological Survey of India (Spe- cial Publication), Vol. 46, 1997, pp.33-63. [37] G. L. Badam, R. K. Ganjoo and Salahuddin, “Preliminary Taphonomical Studies of Some Pleistocene Fauna from the Central Narmada Valley, Madhya Pradesh, India,” Palaeogeography, Palaeoclimatology, Palaeoecology, Vol. 53, No. 2-4, 1986, pp. 335-348. doi:10.1016/0031-0182(86)90067-2 [38] S. Biswas, “Fossil Mammalia of the Quaternary Sequence of the Narmada Valley: Their Affinity, Age and Ecol- ogy,” Geological survey of India (Special Publication), Vol. 46, 1997, pp.91-104 [39] A. Sonakia, “The Skull-Cap of Early Man and Associated Mammalian Fauna from Narmada Valley Alluvium, Ho- shangabad Area, Madhya Pradesh (India),” Journal of Geological Society of India, Vol. 113, No. 6, 1984, pp. 159-171. [40] A. R. Sankhyan, “New Fossils of Early Stone Age Man from Central Narmada Valley,” Current Science, Vol. 88, No. 5, 2005, pp. 704-707. [41] R. Patnaik and A. Sahni, “Record of a Bird Humerus from Upper Pleistocene Narmada Valley Sediments,” Palaeontological Society of India, Vol. 39, 1995, pp. 77-79. [42] B. S. Kotlia and M. Joshi, “Reconstruction of Late Pleis- tocene Palaeoecology of the Upper Narmada Valley (Central India) Using Fossil Communities,” Palaeoworld, Vol. 17, 2008, pp.153-159. doi:10.1016/j.palwor.2008.06.006 [43] K. V. Rao, S. Chakraborti, K. J. Rao, M. S. V. Ramani, S. D. Marathe and B. T. Borkar, “Magnetostratigraphy of the Quaternary Fluvial Sediments,” Geological Survey of India (Special Publication), Vol. 46, 1997, pp. 65-78. [44] S. K. Acharyya and P. K. Basu, “Toba Ash on the Indian Subcontinent and Its Implication for the Correlation of late Pleistocene Alluvium,” Quaternary Research, Vol. 40, No. 1, 1993, pp. 10-19. doi:10.1006/qres.1993.1051 [45] R. Patnaik, P. R., Chauhan, M. R. Rao, B. A. B. Black- well, A. R. Skinner, A. Sahni, M. S. Chauhan and H. S. Khan, “New Geochronological, Paleoclimatological, and Archaeological Data from the Narmada Valley Hominin Locality, Central India,” Journal of Human Evolution, Vol. 56, No. 2, 2009, pp.114-133. doi:10.1016/j.jhevol.2008.08.023 [46] V. A. Korvenkontio, “Mikroskopische Untersuchungen an Nagerincisiven, und Hinweise auf die Schmelzstruktur der Backenzahne,”Annales Zoologici Societatis Zoolo- gicae-Botanica, Fennicae, Vanamo, Vol. 2, 1934, pp. 1-274. [47] J. H. Wahlert, “Variability of Rodent Incisor Enamel as Viewed in the Thin Section, and the Microstructure of the Enamel in Fossil and Recent Rodent Groups,” Breviora, Vol. 309, 1968, pp. 1-18. [48] A. Boyde, “Development of the Structure of the Enamel of the Incisor Teeth in the Three Classical SubOrdinate Groups of the Rodentia,” In: P. M. Butler and K.Y. Joy- sey, EdS., Development, Function and Evolution of Teeth, 1978, pp. 43-58. [49] W. V. Koenigswald, “Schmelzmuster and Morphology in the Molars of Arvicolidae (Rodentia) ,” Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft, Vol. 539, 1980, pp. 1-129. [50] W. V. Koenigswald, “Evolutionary Trends in the Enamel of Rodent Incisors,” In: W. P. Luckett, and J. L. Harten- berger, Eds., Evolutionary Relationships among Rodents, a Multidisciplinary Analysis, A Life Sciences, NATO ASI Series, 1985, pp. 403-422. [51] J. Tomes, “On the Structure of the Dental Tissue of the Order Rodentia,” Philosophical Transactions of the Royal Society, London, Vol. 140, 1850, pp. 529-567. doi:10.1098/rstl.1850.0029 [52] J. Lehner and H. Plenck, “Die Zahne,” In: W. V. Mollen- dorff, Ed., Handbush de mikroskopischen Anatomie des Menschen, Julius Springer, Berlin, 1936, pp. 449-708. [53] H. U. Pfretzschner, “Structural Reinforcement and Crack Propagation in Enamel,” In: D. E. Russel, et al., Ed., Memories Museum National History Natural Paris, Vol. 53, 1988, pp. 133-143. [54] W. V. Koenigswald and H. U. Pfretzschner, “Biome- chanics in the Enamel of Mammalian Teeth,” In: N. Schmidt-Kittler and K. Vogel, Eds., Constructional Mor- phology and Evolution, 1991, pp. 113-125. [55] J. M. Rensberger and W. V. Koenigswald. “Functional and Phylogenetic Interpretation of Enamel Microstructure in Rhinoceros,” Palaeobiology, Vol. 6, No. 4, 1980, pp. 477-495. [56] M. Fortelius, “Vertical Decussating of Enamel Prisms in Lophodont Ungulates,” In: R. W. Fearnhead and S. Suga, Eds., Tooth Enamel IV, 1984, pp. 427-431. [57] M. Fortelius, “Ungulate Cheek Teeth: Developmental, Functional and Evolutionary Interrelations,” Acta Zo- ologica Fennica, Vol. 180, 1985, pp. 1-76. [58] W. V. Koenigswald, “Enamel Modification in Enlarged Front Teeth among Mammals and the Various Possible Reinforcements of the Enamel,” Memoires du Muse’um National d’ Histoire Naturelle, Vol. C 53, 1988, pp.148- 165. [59] T. Martin, “Schmelzmikrostructur in den Inzisiven Alt- und Neuweltlicher Hystricognather Nagetiere,” Palae- over-tebrata, 1992, p. 1-168. [60] T. Martin, “Early Rodent Incisor Enamel Evolution: Phy- logenetic Implications,” Journal of Mammalian Evolution, Vol. 1, No. 4, 1993, pp. 227-254. doi:10.1007/BF01041665 [61] A. Sahni, “SEM Studies of Eocene and Siwalik Rodent Enamels,” Geoscience Journal, Vol. 12, 1980, pp. 21-30.
|