Engineering
Vol. 2 No. 12 (2010) , Article ID: 3610 , 4 pages DOI:10.4236/eng.2010.212125
The Influence of Microscopic Fungi on Chromated Galvanized Zinc Coatings
1State Research Institute Center for Physical Sciences and Technology, Vilnius, Lithuania
2Institute of Materials Science, Kaunas University of Technology, Kaunas, Lithuania
E-mail: lugauskas@chi.lt
Received October 13, 2010; revised October 26, 2010; accepted November 8, 2010
Keywords: Steel, Zinc Coating, Chromatic Solution, Microscopic Fungi, Influence
ABSTRACT
A solution containing Cr(VI), Cr(NO3)3 and its complex with an organic acid as well as several commercial solutions containing Cr(III) complexes with an organic acid and additionally CO2+, F-, SO42- ions were used for the studies. Results of the studies obtained in the following commercial solutions: Likonda 2AT, Cr(NO3)3 + malonic acid; Likonda 3Cr5 and Likonda 3CrMC are discussed. Steel coated with chromated Zn coatings was contaminated by some microscopic fungi. The variety of fungi on chromated plates diminished, however the propagules of fungi did not disappear completely. The Likonda 3Cr5 solution diminishes fungi contamination on chromated steel most effectively. In water used to rinse the surface of chromated plates the number of fungi propagules was detected to be higher as compared to that on the plate surface. The least quantity of fungi propagules was detected in water used to rinse plates coated in the Likonda 3Cr5 solution. The main part of fungi detected on chromated plates treated in the Likonda 3Cr5 solution were the fungi of Cladosporium species (C.herbarum, C.cladosporioides). The latter species also dominated on chromated plates coated with zinc and treated with the other solution. It should be mentioned, that on these plates chromated in this solution, Actinomycetes of the Streptomyces group were abundant. After comparison of surfaces of the plates treated in four solutions it has been determined that the surface of the plates treated in the Likonda 3Cr5 passivation solution and exposed to modelling conditions changed least of all. It has been noticed that on the subject studied white porous rust accumulates, the intensity of this process on the surface studied determines both the probability of corrosion and the resistance of the used safety means to the external factors.
1. Introduction
Zinc is a low cost material which is resistant to corrosion in rural and marine atmosphere when passivated. Hot dip galvanizing, coatings are thick and not appropriated for conformation operation or for application of organic coatings. On the other hand, electroplated coatings are thin, with a smooth, bright surface, and can be confirmed and painted. The most important property of these coatings is their corrosion resistance. In normal industrial practice, a conversion treatment by immersion in a chemical bath is generally used to form a passive layer over plated zinc [1]. Hexavalent chromium conversion coatings (CCC) have been widely used to enhance the corrosion resistance of electrogalvanized steels. As hexavalent Cr is highly toxic and extremely harmful to the environment, development of alternatives to hexavalent CCC is now all the more important. The properties of CCC are generally closely related to its microstructure that is essential for developing alternatives to hexavalent CCC. The microstructure of hexavalent and trivalent CCC on electrogalvanized steel is characterized using SEM, YRD, XPS and cross-sectional TFM [2].
The mechanism by which conventional CCC’s inhibit the corrosion of metals is not yet fully understood although a large number of studies have been performed and many valuable insights have been obtained. The formation of CCC’s on zinc in a chromate solution takes place in two stages: the first involves the dissolution of zinc into acidic treatment solution and the second stage involves the formation of an adherent precipitate of trivalent chromium [3-5]. This precipitation of insoluble trivalent chromium compounds is assisted by the local pH increase that occurs when hydrogen ions are consumed in the reduction of the hexavalent chromium [6].
Conventional chromate conversion coatings (CCC) have a superior corrosion resistance and self-healing ability but their production has been limited by several environmental legislations because they display high toxicity for human health and cause serious environmenttal pollution. Yet trivalent chromium has low toxicity and the waste water and solid waste from the Cr(III) processes can be treated in a simple and efficient way. Moreover, the industrial implementation of Cr(III) conversion coatings is similar to that of the chromate counterpart. Hence, the Cr(III) conversion coatings treatment is one of the potential alternatives to the CCC process for zinc-coated steels [7,8].
Environmental considerations have been responsible for the increased interest in less toxic trivalent chromium electroplating solutions as an alternative to the conventional highly toxic hexavalent chromium solutions. However, the progress in development of Cr(III) electrolytes has been slow, largely due to the complex nature of the chemistry and electrochemistry of Cr(III) species in aqueous solutions [9,10].
It has been proved scientifically [6,11-13], that in solution of Cr (III) nitrate, malonic acid and Co (II) salt, an organic dicarboxylic acid is the component that has essential influence on zinc dissolution and formation of chromate films and on their decorative and protective properties. The influence of organic acids is directly related to the Cr(III) complex formation in the chromating solution. When Cr(III) ions are in the form of hexaaqua ions, the organic acid increases the quantities of the zinc dissolved and the Cr(III) deposited on the zinc surface. They also predetermine formation of a thick, porous chromate film with big cracks (especially at 60°C).
Its decorative and protective properties are rather poor. When Cr(III) ions are in the form of a complex with organic acids, the quantities of zinc dissolved and the Cr(III) deposited on the zinc surface significantly decrease and thinner chromate films of even surface, good decorative appearance and high corrosion resistance are formed [14-16]. The mechanism of hexavalent chromium detoxification by microorganisms was described in the mentioned references. The process can be used to detoxificate Cr(VI) in the biomass of dead fungi during bioremediation.
Microbially influenced corrosion (MIC) refers to the influence of microorganisms on the kinetics of corrosion processes of metals caused by microorganisms adhering to the interfaces usually called “biofilms” [17-21]. The kinetics of corrosion are determined by the physico-chemical environment at the interface, e.g. by the concentration of oxygen, salts, pH value, redox potential and conductivity. All these parameters can be influenced by microorganisms growing at the interfaces [22,23]. The organisms can attach to surfaces, embed themselves in slime, so called extracellular polymeric substances (EPS) and form layers which are called “biofilms”. These can be very thin (monolayer), but also can reach the thickness of centimetres, as it is the case in microbial mat. MIC can be understood as a kinetic process which depends upon the physico-chemical conditions at the interface, such as pH value, oxygen concentration, redox potential, water content and ionic strength [24,25]. A number of classical and modern methods are available for the detection of microorganisms which may be associated with corrosion [26-29].
The aim of this work was to study the influence of microscopic fungi upon chromated zinc coatings.
2. Materials and Methods
Plates with an area of 50 cm2, 0.7 mm in thickness cut from steel containing C—0.05%, Si—0.02%, Mn—0.29%, S—0.018%, P—0.012% (GOST–1050–88 Russia) were coated with chromate zinc coatings. To obtain the coatings, solutions containing Cr(VI), [Cr(NO3)3]·9H2O and its complex with an organic acid as well as some commercial solutions, containing Cr(III) complexes with organic acids and Co2+, F-, SO42- ions were used. The Cr concentration was 4-5 g×l-1 in all the solution, pH 2.0, the passivation duration was 60 s, at room temperature (18-20°C). Solutions were prepared by dissolving the required quantity of components in distilled water, pH was determined with acid or sodium hydroxide. The solution with Cr6+ ions possessed an activated H+/anion system. In this case SO42- ions acted as an activator. The complex of Cr(III) and organic acid was prepared dissolving the required quantities of substances in distilled water, solution pH was adjusted to 2.0 and the solution was heated for 30 min at a temperature of 70-80°C, the solution was kept in open air for 48 hours and its pH was adjusted to 2.0.
The following commercial solutions were used for studies: Likonda 2AT, Likonda 3Cr5, Likonda 3CrMC. They were prepared by the technological instructions of JSC “Chromtech”.
Prior to the treatment with the solution plates were degreased in Likonda 1 solution by keeping them in it for 5 min. at 55-60°C, then they were washed with cold tap water, mechanically degreased with a mixture of calcium (60%) and magnesium (40%) oxides, rinsed with cold running tap water, after that rinsed thoroughly with distilled water.
Steel plates prepared as described above were coated with a 8-10 μm thick bright zinc coating in the alkaline non-cyanide electrolyte Likonda Zn S-GE (JSC “Chromtech”), rinsed with running tap water and passivated in the studied solution. After passivation the plates were rinsed with running tap water and in distilled water and after that dried in warm air. Plates treated in such a manner were used after 24 hours.
Atmospheric corrosion studies were carried out in the suburb of Vilnius (Visoriai). Corrosion tests were started in april, 2009. Carbon steel samples were exposed at an angle of 45°C to the south. According to the test program, three samples were withdrawn from all test sites after every measuring period. The corrosion rates (mass loss) were determined gravimetrically. The corrosion products were removed according to the ISO 9226 standard using a 3.5 g×l-1 hexametylene tetramine solution for carbon steel and a 200 g×l-1 CrO3 solution for Zn. The time of wetness (TOW), i.e. period in which an electrolyte film was present on the metal surface, was determined with the acid of a humidity tester consisting of thin dielectrically separated electrodes made of all the metals studied, with monitoring the current of electromotive force when the thickness of the aqueous layer was sufficient to accelerate atmospheric corrosion.
Microorganisms were isolated from the metal specimens in atmospheric corrosion test sites in two ways: 1) by direct isolation from the surface of plates, using a sterile metal loop; 2) known area of plates was suspended in 100 saline (0.85% NaCl). This suspension was used to prepare solution series of 1:10; 1:100; 1:1000; 1:10000. Later 0.1 ml of the suspension was set into a Petri dish and 15 ml (45 ± 0.5°C) of the medium was poured over it. Malt agar and Sabourande's medium enriched with 0.5 g×l-1 of chloramphenicol were used. Samples were incubated for 5-7 days in a thermostat at 26 ± 2°C.The results were expressed as colony forming units (cfu) per 1 cm2 area of a specimen. The abundance of mycrobiots was determined by applying the quantitative methods [30].
The colonies of microscopic fungi were subcultured in order to obtain monocultures. In order to achieve this, each isolate was inoculated on three standard agar media: malt extract, synthetic Czapek medium and synthetic medium with maize extract. As the colonies formed, their cultural properties were described, indicating the growth rate, colony structure and appearance, colour of mycelium and reverse of the colony, other properties. A light and scanning electron microscope EVO 50 EP (Carl Zeis SMTAG, Germany) was used to characterize the morphologic peculiarities of each fungal species and the process of conidiogenesis. A systematic position was determined according to various manuals.
The contact of the metal with fungi was investigated: steel plates were placed in Petri dishes filled with a sterile agar medium of malt extract supplied with chloranphenicol (50 mg×l-1). After that the medium with the metal was sown up with the fungi. K2 (reference 2)—the medium with steel plates not contaminated with fungi. K1 (reference 1)—steel exposed to common conditions (room temperature and common humidity) was not contaminated with fungi. The medium with steel plates was cultivated in a thermostat at a temperature of 26 ± 2°C. The intensity of fungi growth and metal oxidation and surface changes were evaluated after 15 and 30 days. The rate of fungal growth was assessed by the naked eye and light microscopy in accordance with the scheme. Methods for corrosion testing of metallic and other inorganic coatings on metallic substrates rating of test specimens and manufactured articles subjected to corrosion test [EN ISO 10289:1999]:
• No fungi growth observed on specimens under a light microscope—1 point;
• Micellium via branched hyphae and possibly sporulation, visible under a light microscope—2 points;
• Growth of fungi, spores not visible to the naked eye, but under a light microscope sporatulation is clearly visible—3 points;
• Growth of fungi clearly evident but covering < 5% of the tested surface—4 points;
• Heavy growth of fungi visible by the naked eye and covering > 25% of the surface—5 points.
Morphological changes were evaluated by using an optical microscope MMU-3 with a CCD camera at 55X magnification, the magnitude of marker was 10 µm per 1 cm of the photograph. Structural surface changes were studied by the XRD method.
A scanning electron microscope EVO 50 EP (Carl Zeis SMTAG, Germany) was used to characterize the morphology of the steel surface too.
For spectroscopical investigations of corrosion products on metal plates, a Nicolet model 5700 Fourier transformation infrared spectrometer (FTIR) in conjunction with a 10 Spec (10 Degree Specular Reflectance Accessory) was used. Samples were placed on the reflectance accessory and spectra were obtained in the reflectance mode. In all cases the spectral range was 4000–400 cm-1 (reciprocal wave length) with a 4 cm-1 resolution and 64 scans. Spectral manipulation included baseline correction, removal of carbon dioxide absorbtion bands and substraction of water vapour interferences. The samples of corroded metal plates were cleaned with dry filter paper. A metal plate stored for up to 30 days without biological interventions was used as reference. In this way, the atmospheric corrosion products from metal plates were eliminated, and IR spectra of the products formed by biological agents were registered [31].
The surface morphology of steel samples after the impact of fungi was examined with a scanning probe microscope Exlorer (VECO Topometrix, USA). The Rq-root-mean Square Roughness (rms) nm was determined. The average roughness is the area between the roughness profile and its mean line or the integral of the absolute value of the roughness profile height over the evolution length Rg is a static parameter, which shows the average square roughness of the steel surface and it is expressed in nm.
The mass loss of coating was determined by the following method: prior to exposure coatings (plates) were rinsed with deionized water, dried in air, 24 hours kept in an exicator with P2O5 (phosphorus pentoxide) and after that they were weighed. The same procedure was repeated after exposure (after 0.5, 1, 1.5 and 2 years). After that the changes in mass were calculated.
To determine the coating mass loss due to corrosion, the products formed during the process were removed by using the methods and the procedure described in the ISO 8407:1991 (E) standard: 1) exposing for 3 min to the CH3COONH4 (ammonium acetate) solution at a temperature of 70°C; 2) rinsing with deionized water; 3) drying in an exicator with P2O5 for 24 hours; 4) weighing.
3. Results and Discussion
Table 1 shows the variety of microscopic fungi on the surface of chromated plates after six months of exposure under atmospheric conditions in the suburb of Vilnius (Visoriai). From the data presented it is seen that some what less quantity of fungi propagules was detected onthe plates chromated in the Likonda 3Cr5 solution, as well as in their rinsing water. However this difference is not that marked as it could be stated that it is due to the anti-fungial influence of the solution. It has been noticed that there were more fungi propagules in rain water as compared to the quantity of propagules on the specimen surface. This suggests, that the majority of the fungi detected on the plates surfaces have found themselves here.
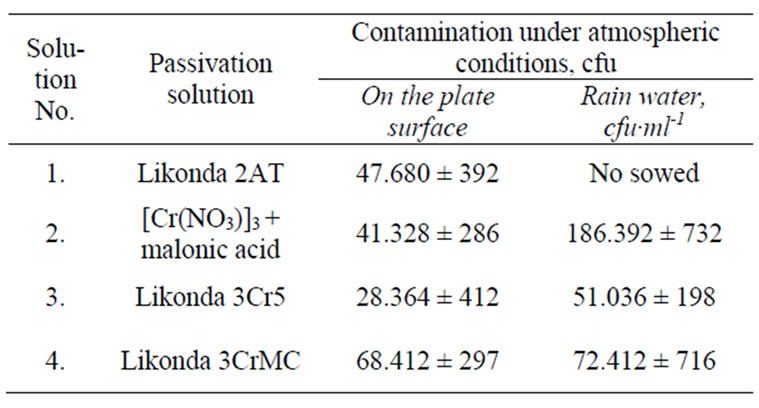
Table 1. Contamination of steel plates coated with the Zn coating chromated in different solutions by microscopic fungi after 6 month exposition to atmospheric conditions (Vilnius,Visoriai).
Accidentally with dust particles and in other ways. The propagules of such fungi are easily washed by rain and they found their way to the collector where they stay until their sow time.
The analysis of composition of the microscopic fungi shows that the fungi of Cladosporium herbarium species were detected in all specimens and their detection frequency is higher than 50%. Unfortunately we failed to determine any regularities in spreading of other fungi on the surfaces of plates chromated in various solutions (Table 2).
The fungi of Aspergillus penicilloides species were detected more often on the surface of plates chromated in Likonda 2AT and [Cr(NO3)3 9H2O] + malonic acid, while Fusarium proliferatum were detected more often on the plates passivated in Likonda 2AT and Likonda 3CrMC. The fungi of Penicillium genus were detected rather often, but their kinds were different in all experimental variants. In all experiments there was abundant mycelium (Mycelia sterilia), and its detection frequency often exceeded 60%. In some experiments bacteria Streptomyces spp. were detected.
The changes in the surface of plates chromated in different solutions are described in Table 3. The bottom side of the chromated plate passyvated in the Likonda 2AT solution was changed most of all as it has a direct contact with the surface of the fungi colony. About 75% of the plate surface was under the grey mycelium. The upper side was less changed. After comparison of the surfaces of plates treated with the fourth solution it has been found that plates passivated in the Likonda 3Cr5 solution and exposed to the mentioned conditions changed the least of all.
During further experiments, plates treated in different chromium solutions were contaminated with mixtures of various fungi species and kept for 60 days under modelled humid conditions at a temperature of 26 ± 2°C. The data obtained are given in Figures 1-4. From the data in Figure 1 it is seen that fungi develop most intensively on the edges of the plates chromated in Likonda 2AT, especially in variation (a) where Chrysosporium merdarium and Aspergillus penicilloides fungi grow, Figure 1(a), in variation (c), where Arthrinium phaeospermum, Aspergillus spp., Chaetomium elatum, Phoma exigua grow on the surface and form a scarse film of a nondescript colour. The reference plates were almost unchanged.
The mentioned fungi are often present in dust of various nature and composition which accumulates on the surface of metal exploited under atmospheric conditions. The fungi easily adapt to the zinc plates chromated in the Likonda 2AT solutions, change their colour and damage their surface.
In Figure 2 views of plates chromated in Cr(NO3)39H2O +
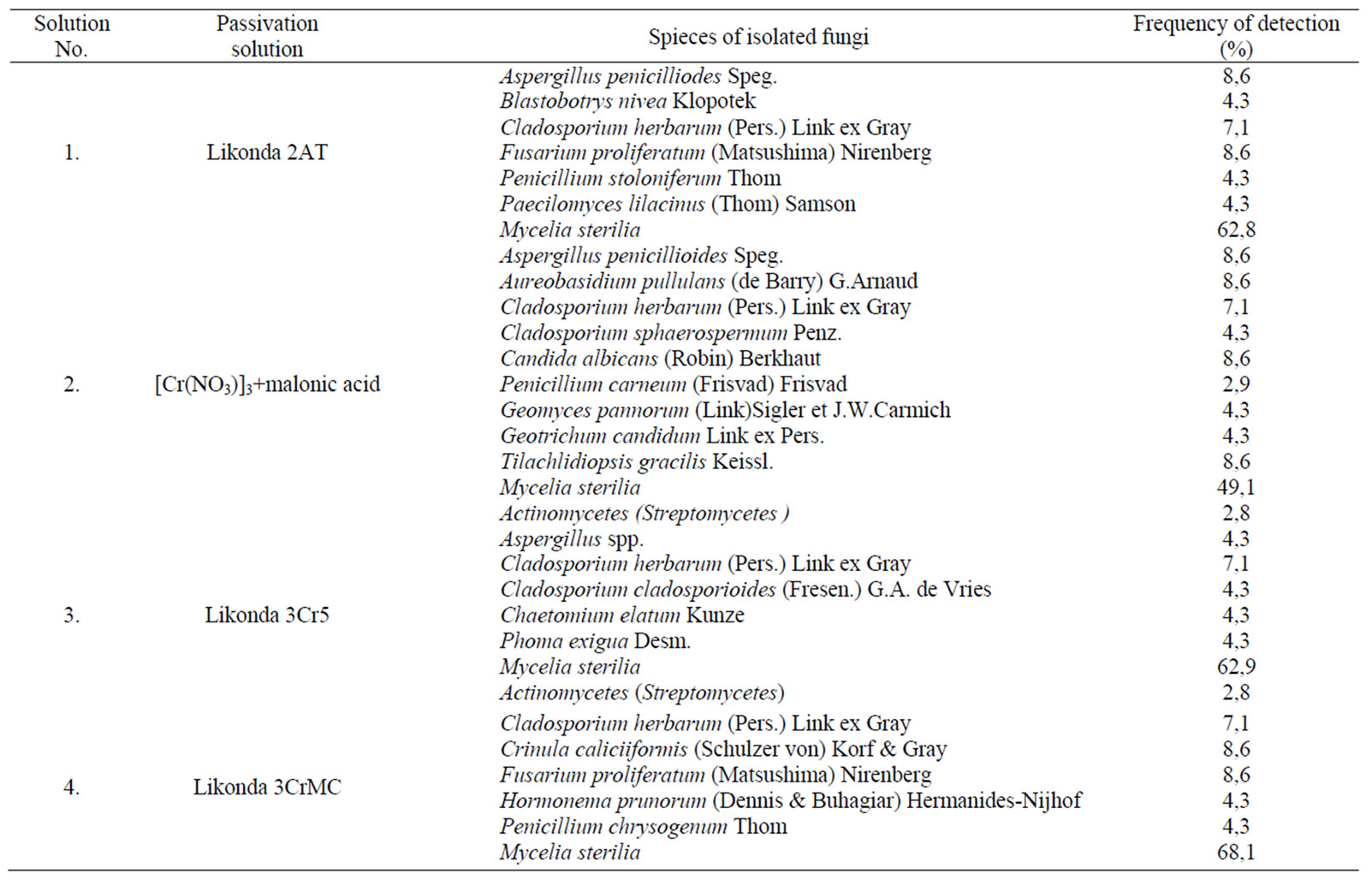
Table 2. Microscopic fungi isolated from the surface of Zn coatings chromated in different solutions after 6 month exposition to atmospheric conditions (Vilnius,Visoriai).

Table 3. Changes in the surface of Zn coatings chromated in different solutions after 6 month exposition to atmospheric condition (Vilnius,Vis-oriai).
malonic acid and influenced by the fungi are presented. In variation (a) intensively developing Chrysosporium merdarium ir Aspergillus penicilloides fungi cover a considerable part of the surface of plates, on the plate in variation (b) Penicillium carneum colonies are formedvariation (c) (Arthrinium phaeospermum) covers the plate surface with a thin cobweb type net.
Plates chromated in the Likonda 3Cr5 solution are intensively attacked by Chrysosporium merdarium, Fusarium proliferatum, Paecilomyces lilacinus fungi (Figure 3(a)), in the other variation of the experiments when plates were passyvated in the Likonda 3Cr5 solution the
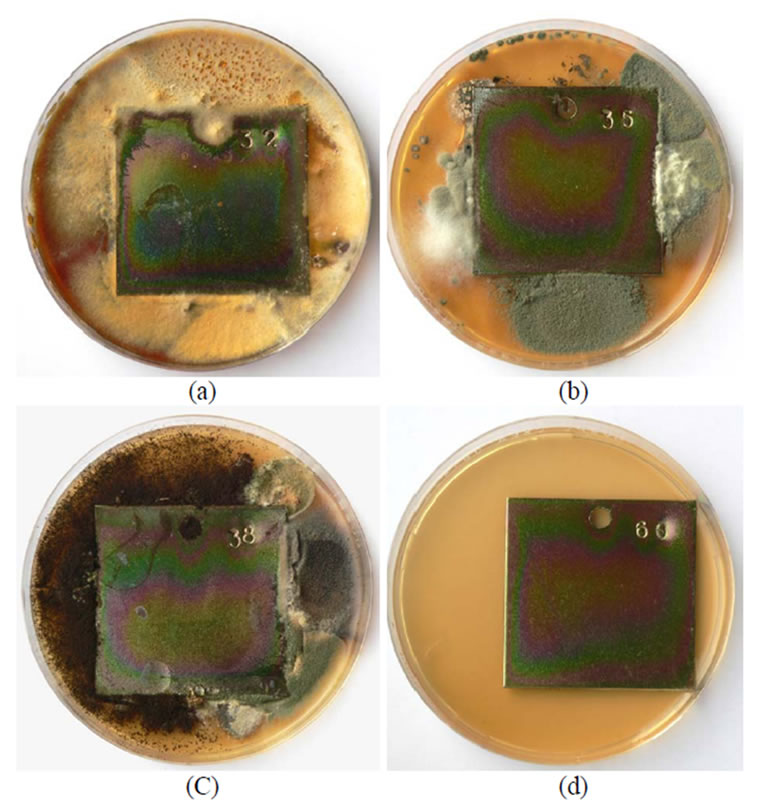
Figure 1. A general view of samples chromated in the solution Likonda 2AT after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Aspergillus penicilloides; (b) Alternaria alternata + Geomyces pannorum + Penicillium carneum + Tilachlidiopsis gracilis; (c) Arthrinium phaeospermum + Aspergillus spp. + Chaetomium elatum + Phoma exigua; (d) A reference sample without contact with fungi.
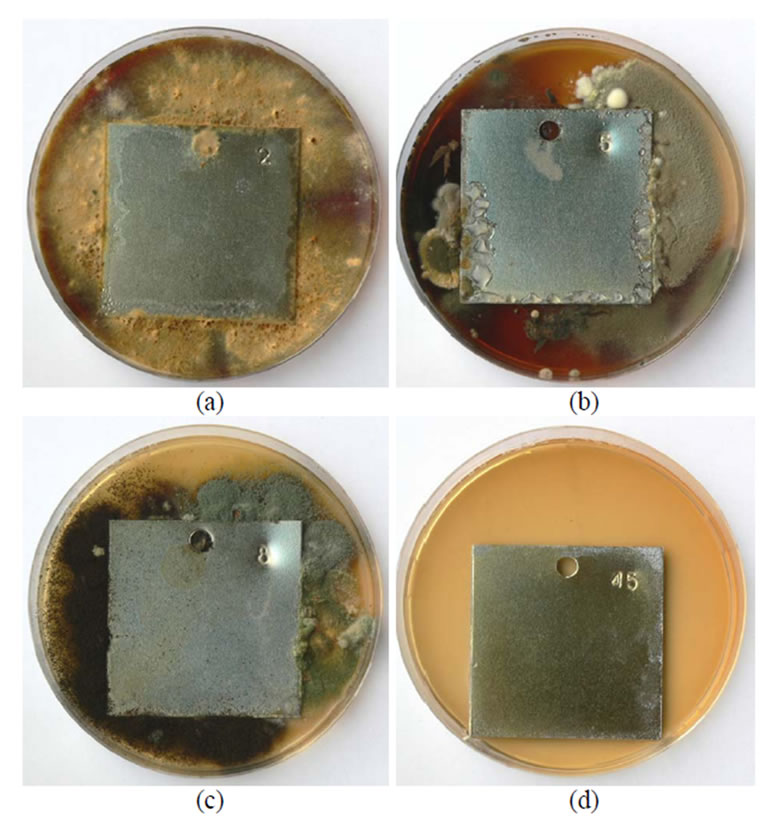
Figure 2. A general view of samples chromated in the solution Cr(NO3)3·9H2O + malonic acid after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Aspergillus penicil-loides; (b) Alternaria alternata + Aspergillus penicillloides + Geotrichum candidum + Penicillium carneum + Aureobasidium pullulans; (c) Arthrinium phaeospermum + Aspergillus spp. + Cladosporium herbarum + Phoma exigua + Penicillium carneum; (d) A reference sample without contact with fungi.
growth of separate fungi is seen only on the edges.
The plates passyvated in Likonda 3CrMC were actively attacked by Chrysosporium merdarium, Fusarium proliferatum, Paecilomyces lilacinus (Figure 4(a)) and Arthrinium phaeospermum, Chaetomium elatum, Phoma exigua (Figure 4(c)), fungi mixtures.
In order to determine the condition of steel plates covered with chromated Zn coatings after 60 days of direct contact with microscopic fungi an optical microscope MMU-3 with the CDD camera was used. The data obtained are presented in Figures 5-8. The surface structure of plates chromated in the Likonda 2AT solution was deteriorated, but not uniformly, depending on the composition of the fungi involved, at times it was split into large pieces (Figures 5 (a,b)), in other cases more fine, oriented structures were observed (Figure 5).
On the surface of the Zn film passivated in the Cr(NO3)3·9H2O + malonic acid solution, the fungi grew differently, in some places (Figure 6(a)) giving tangles
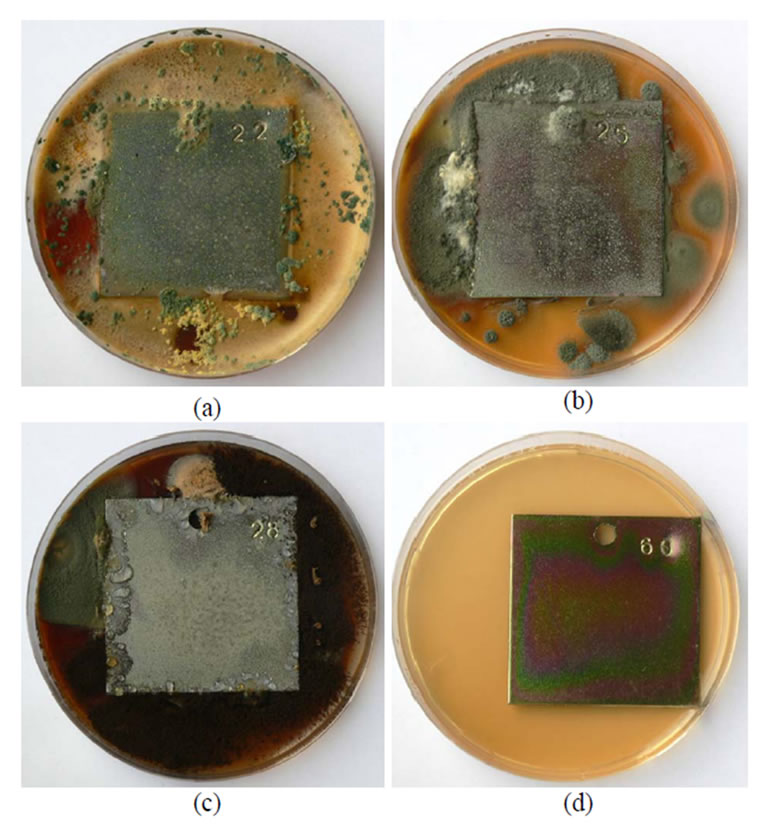
Figure 3. A general view of samples chromated in the solution Likonda 3Cr5 after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Fusarium proliferatum + Paecilomyces lilacinus + Penicillum stoloniferum; (b) Alternaria alternata + Geomyces pannorum + Cladosporium herbarum + Penicillium carneum; (c) Arthrinium phaeospermum + Aspergillus spp. + Cladosporium cladosporioides + Chaetomium elatum; (d) A reference sample without contact with fungi.
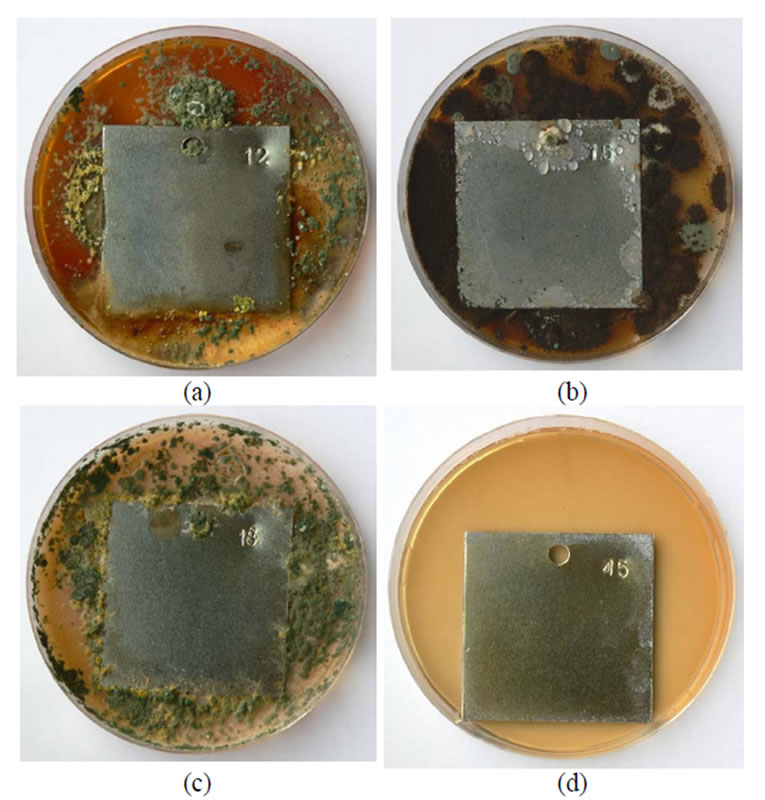
Figure 4. A general view of samples chromated in the solution Likonda 3CrMC after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Fusarium proliferatum + Paecilomyces lilacinus; (b) Alternaria alternata + Aspergillus penicilloides + Cladosporium cladosporioides + Penicillium carneum; (c) Arthrinium phaeospermum + Chaetomium elatum + Phoma exigua; (d) A reference sample without contact with fungi.
of threads, while in other places making abundant conidia (Figure 6(c)) or skeins (Figures 6 (b,d)).
More pronounced changes were observed on the surface of Zn film passivated in the Likonda 3Cr5 solution (Figure 7(c)) which were in contact with Arthrinium-phaeospermum +Aspergillus spp., Cladosporium clado-sporioides+Chaetomium elatum, in other instances this influence was not clearly seen and close to the reference plate (Figure 7).
On the surface of plates chromated in the Likonda 3CrMC solution formation of peculiar structures was observed (Figure 8(a)), as well as tangles of larger and finer threads (Figures 8 (a,b)).
Studies of the plates chromated in various solutions and contaminated with mixtures of various fungi, after that kept for 60 days in contact with developing fungi were carried out using a scanning electronic microscope EVO 50 X VP Carl Zeiss STMAG, Germany and the peculiarities of damage were determined. The data obtained are given in Figures 9-12.
Under the influence of fungi on the surface of plates passivated in Likonda 2AT holes are seen, between them thin hardly visible threads of mycelium were observed. Under the influence of other fungi (Alternaria alternata, Geomyces pannorum, Tilachlidiopsis gracilis) the plate
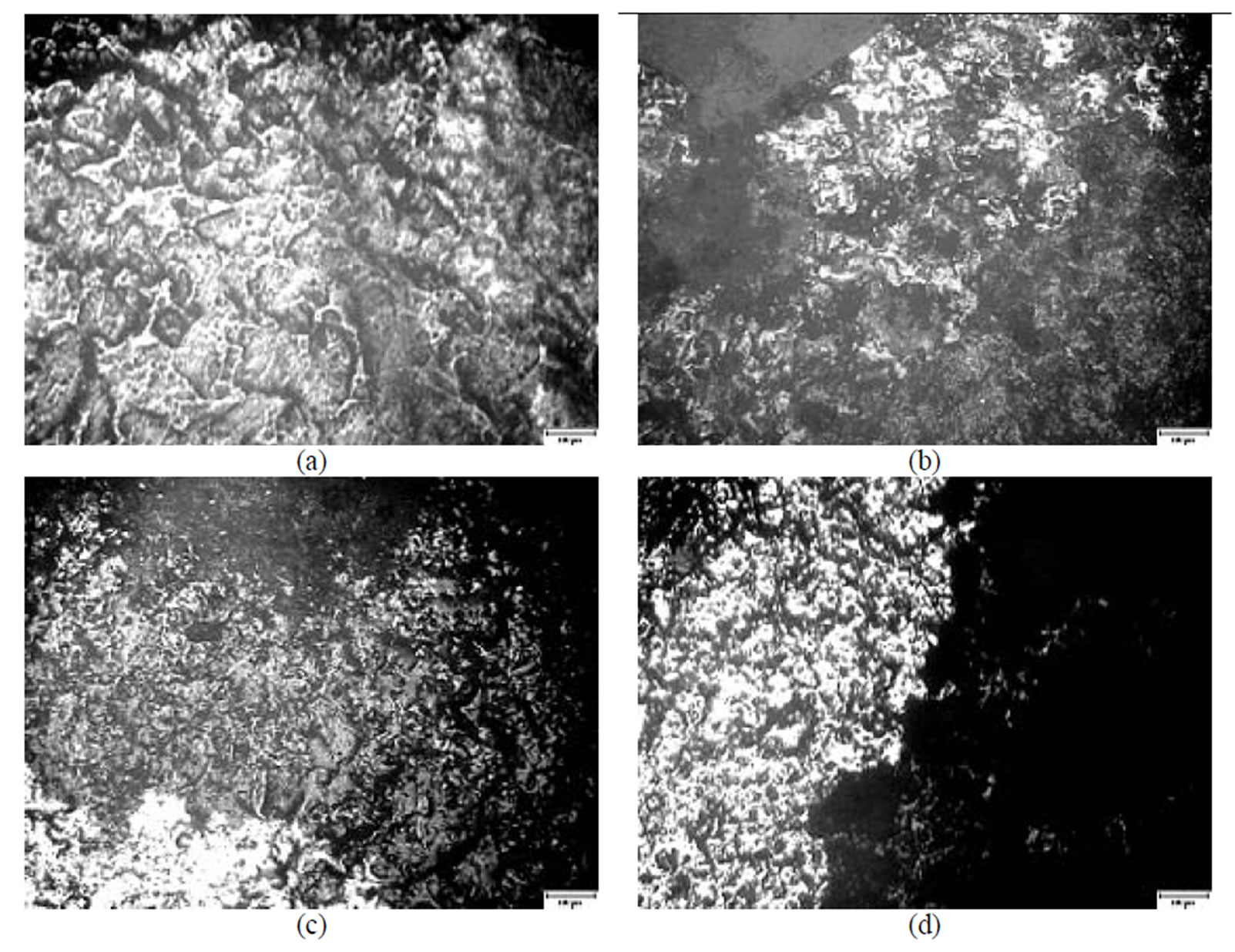
Figure 5. A microscopical view of the surface of plates chromated in the solution Likonda 2 AT after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Aspergillus penicilloides; (b) Alternaria alternata + Geomyces pannorum + Penicillium carneum + Tilachlidiopsis gracilis; (c) Arthrinium phaeospermum + Aspergillus spp. + Chaetomium elatum + Phoma exigua; (d) A reference sample without contact with fungi. Scale 10 µm.
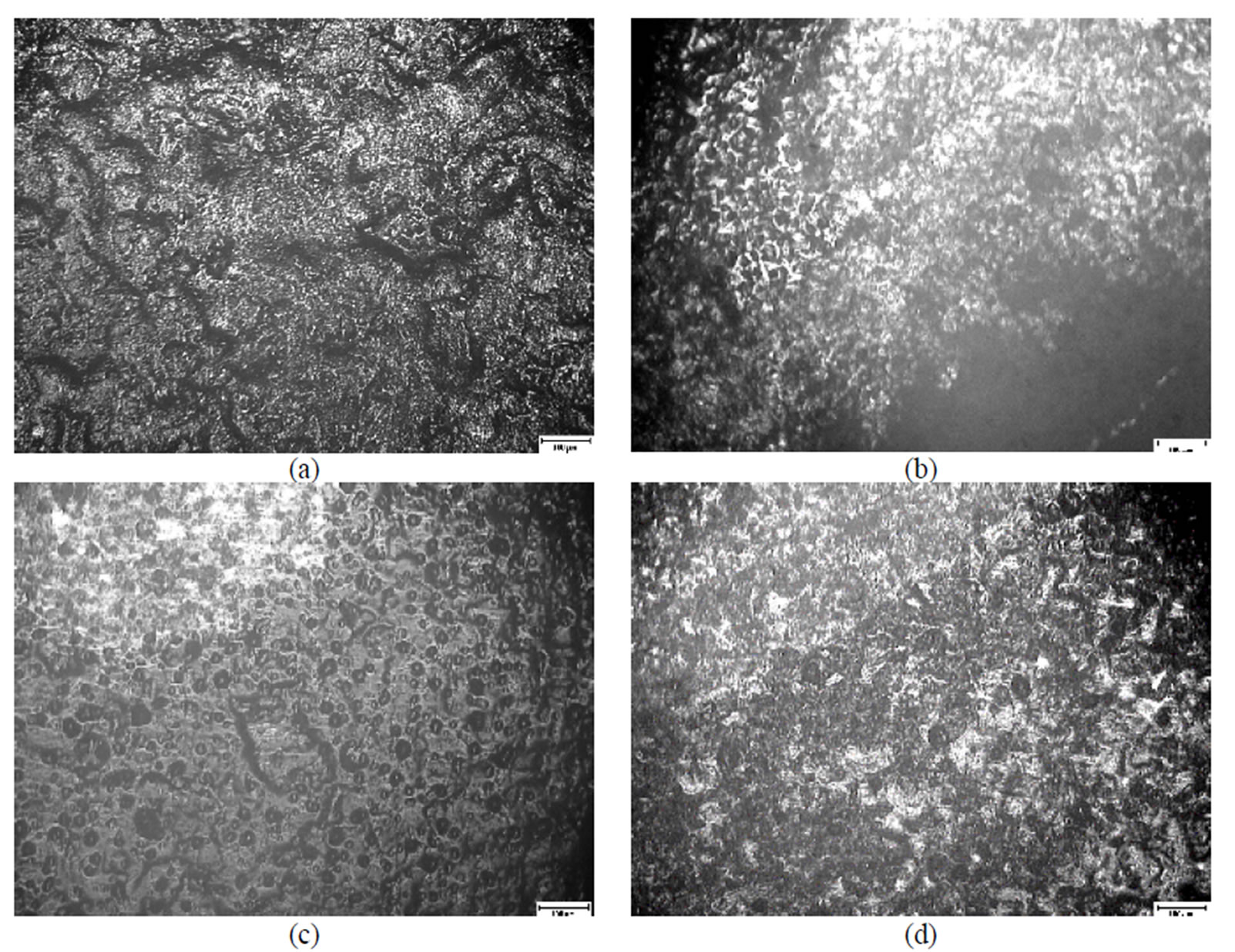
Figure 6. A microscopical view of the surface of plates chromated in the solution Cr(NO3)3·9H2O+malonic acid after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Aspergillus penicilloides; (b) Alternaria alternata + Aspergillus penicillloides + Geotrichum candidum + Penicillium carneum + Aureobasidium pullulans; (c) Arthrinium phaeospermum + Aspergillus spp. + Cladosporium herbarum + Phoma exigua + Penicillium carneum; (d) A reference sample without contact with fungi. Scale 10 µm.
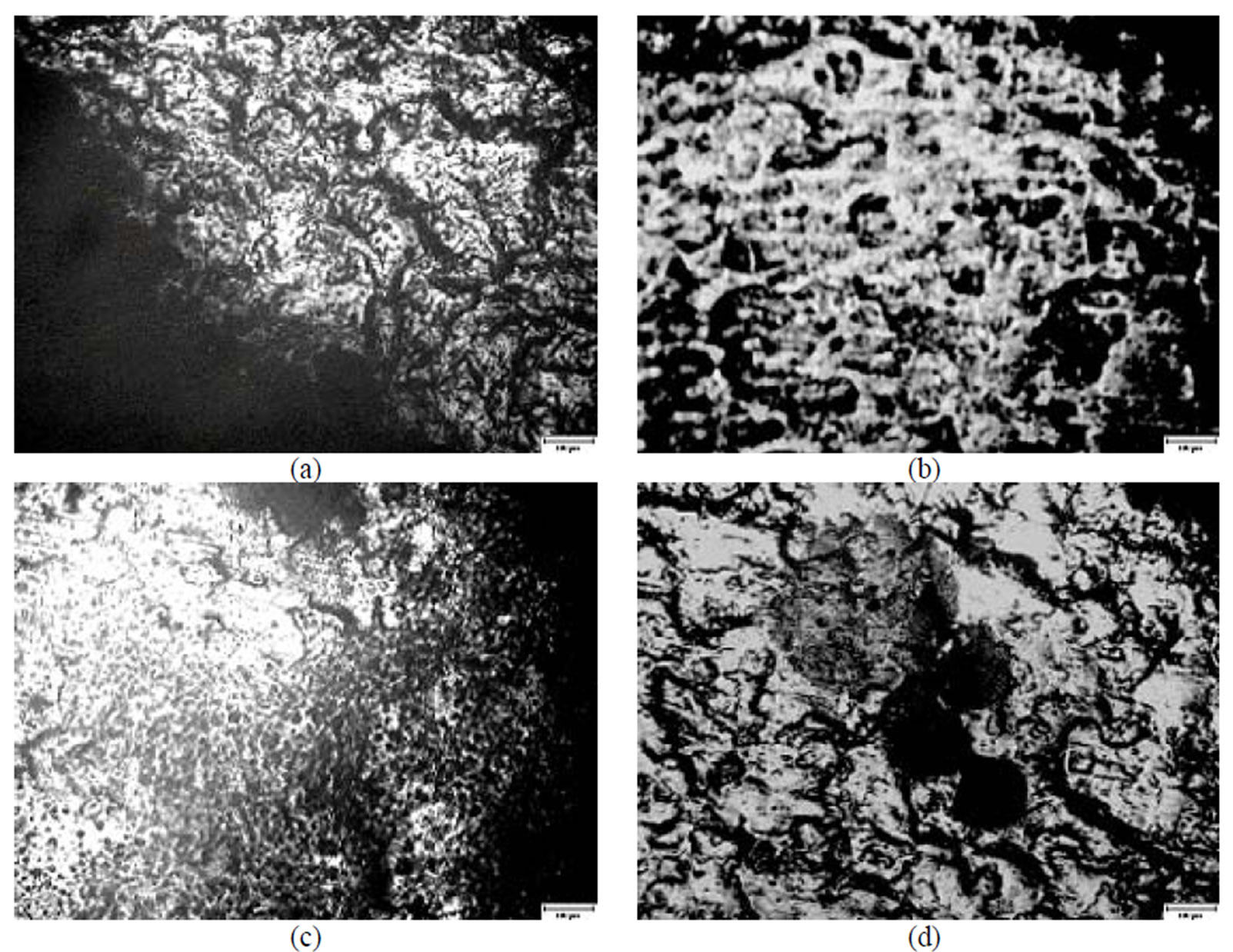
Figure 7. A microscopical view of the surface of plates chromated in the solution Likonda 3Cr5 after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Fusarium proliferatum + Paecilomyces lilacinus + Penicillum stoloniferum; (b) Alternaria alternata + Geomyces pannorum + Cladosporium herbarum + Penicillium carneum; (c) Arthrinium phaeospermum + Aspergillus spp. + Cladosporium cladosporioides + Chaetomium elatum; (d) A reference sample without contact with fungi. Scale 10 µm.
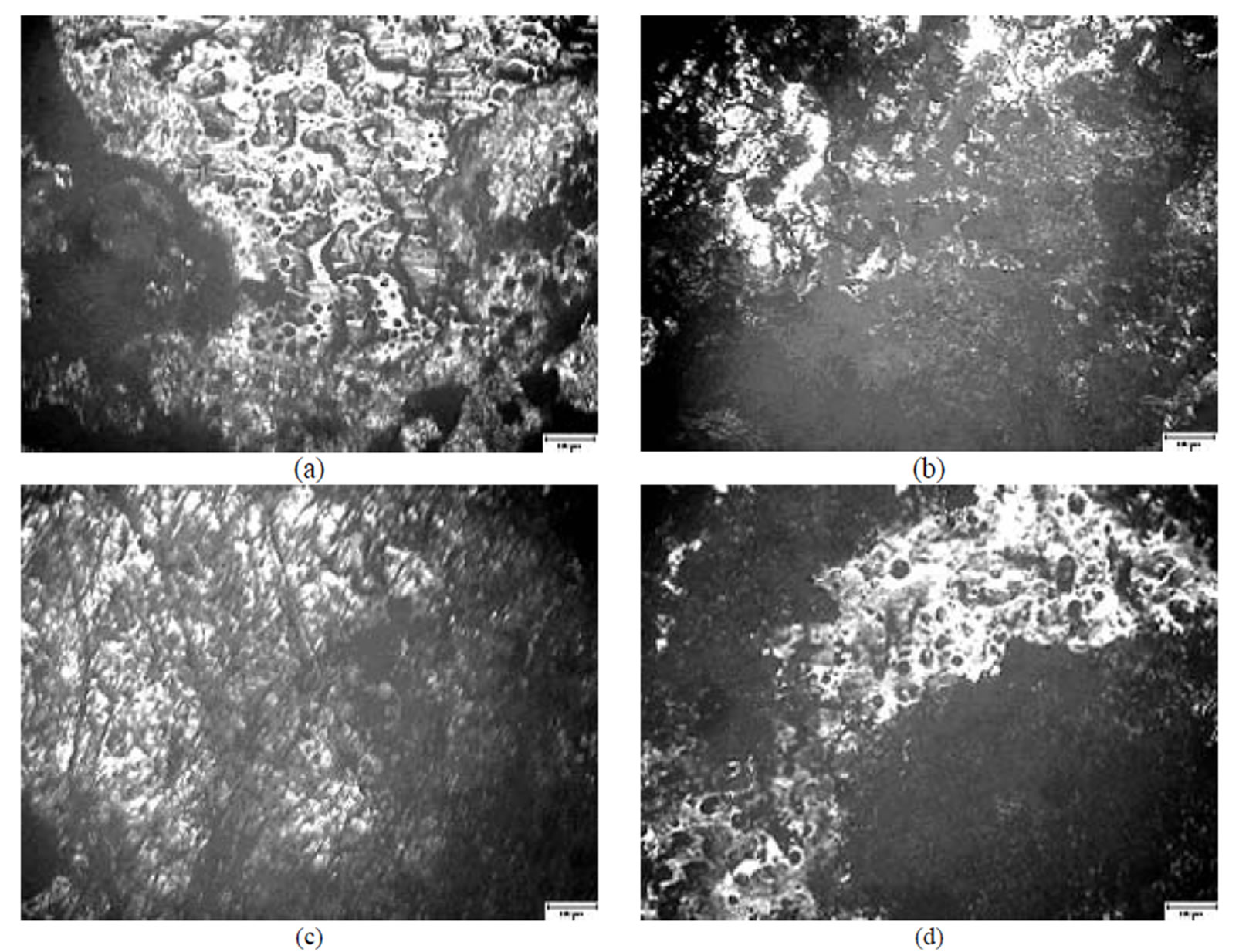
Figure 8. A microscopical view of the surface of plates chromated in the solution Likonda 3CrMC after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Fusarium proliferatum + Paecilomyces lilacinus; (b) Alternaria alternata + Aspergillus penicilloides + Cladosporium cladosporioides + Penicillium carneum; (c) Arthrinium phaeospermum + Chaetomium elatum + Phoma exigua; (d) A reference sample without contact with fungi. Scale 10 µm.
surface is braided with rather thick mycelium threads (Figure 9(b)). Quite a different view is seen when the plate surface is influenced by Arthrinium phaeospermum, Aspergillus spp., Chaetomium elatum and Phoma exigua fungi. In this case mycelium braids the surface and small fungi propagules reminding of conidia are abundunt (Figure 9(c)). The surface of the reference plate is even, not damaged and free from fungi (Figure 9(d)).
Figure 10 presents views of the surface of plates passivated in [Cr(NO3)3]·9H2O + malonic acid after 60 days of contact with fungi. The surface under the influence of Chryisosporium merdarium and Aspergillus penicilloides is damaged with holes possessing damaged edges (Figure 10(a)). Under the influence of the fungi of mixture 3 Arthrinium phaeospermum, Aspergillus spp., Cladosporium herbarum, Phoma exigua, Penicillium carneum, on the surface of coating small fungi conidia dominate, in places they form chains and coherences (Figure 10(c)). The reference specimen is undamaged (Figure 10(d)).
The analysis of the data obtained shows that on the plates chromated in Likonda 3Cr5 it is clearly seen that Chrysosporium merdarium, Fusarium proliferatum, Paecilomyces lilacinus, Penicillium stoloniferum fungi can develop and generate under such conditions and promote formation of holes with clear edges on the surface of coatings (Figure 11(a)). When the same coatings were affected by other fungi group, on their surface were seen large formations remaining of fruit body of some fungi which grew under unfavourable conditions (Figure 11(b)).
On the surface of plate chromated in the Likonda 3Cr5 solution, but exposed to other fungi, formations reminding of perithecium of fungi of Chaetomium species perithecium were seen, the surface was uneven. The surface of the reference sample was without visible changes (Figure 11(d)).
The microscopic view of the surface of plates chromated in the Likonda 3Cr MC solution reminded that of the surface of plates chromated in the Likonda 2AT solution, even though the capacity of some fungi to damage the plate surface, develop and form conidia was clearly visible (Figure 12).
In order to determine the contamination of the surface of steel plates coated with Zn coatings chromated in various solutions with organic substances, the method of FTIR spectra registration was applied.
It has been determined, that moisture accumulates on the surface of specimens chromated in the Likonda 2AT solution after 60 days of contact with different species of microscopic fungi (the peak at about 3600 cm-1), the occurance of the peaks (Figures 16 (b,c)) testifies that the surface studied is covered with an organic layer (Figure 13).
The FTIR spectra of specimens treated with the Cr(NO3)3·9H2O + malonic acid after 60 days of contact with fungi resemble those of reference (Figure 14(d)), and only minor changes were detected for example, a

Figure 9. A general view of the surface of plates chromated in the solution Likonda 2AT after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Aspergillus penicilloides; (b) Alternaria alternata + Geomyces pannorum + Penicillium carneum + Tilachlidiopsis gracilis; (c) Arthrinium phaeospermum + Aspergillus spp. + Chaetomium elatum + Phoma exigua; (d) A reference sample without contact with fungi.
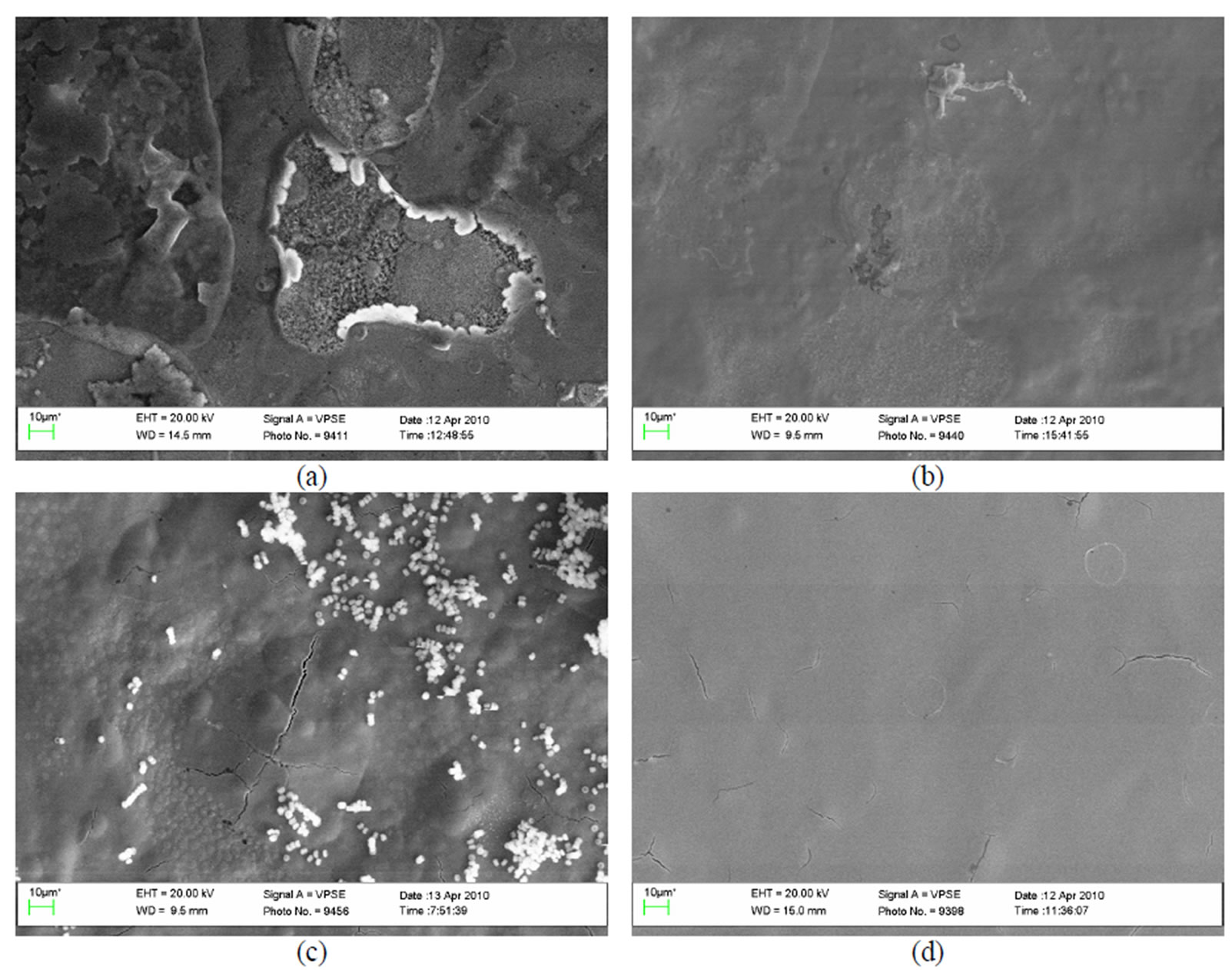
Figure 10. A general view of the surface of plates chromated in the solution Cr(NO3)3·9H2O+malonic acid after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Aspergillus penicilloides; (b) Alternaria alternata + Aspergillus penicillloides + Geotrichum candidum + Penicillium carneum + Aureobasidium pullulans; (c) Arthrinium phaeospermum + Aspergillus spp. + Cladosporium herbarum + Phoma exigua + Penicillium carneum; (d) A reference sample without contact with fungi.
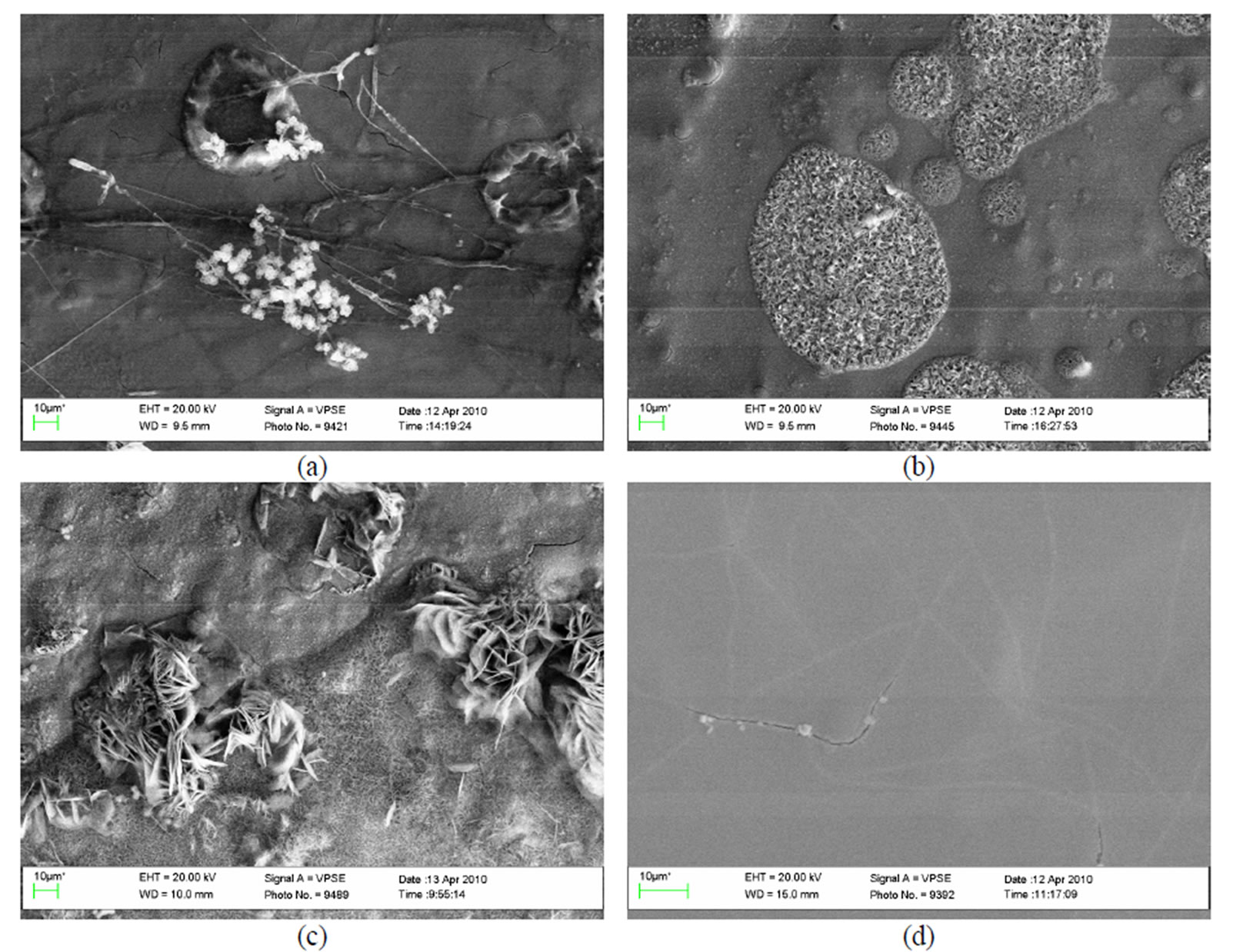
Figure 11. A general view of the surface of plates chromated in the solution Likonda 3Cr5 after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Fusarium proliferatum + Paecilomyces lilacinus + Penicillum stoloniferum; (b) Alternaria alternata + Geomyces pannorum + Cladosporium herbarum + Penicillium carneum; (c) Arthrinium phaeospermum + Aspergillus spp. + Cladosporium cladosporioides + Chaetomium elatum; (d) A reference sample without contact with fungi.
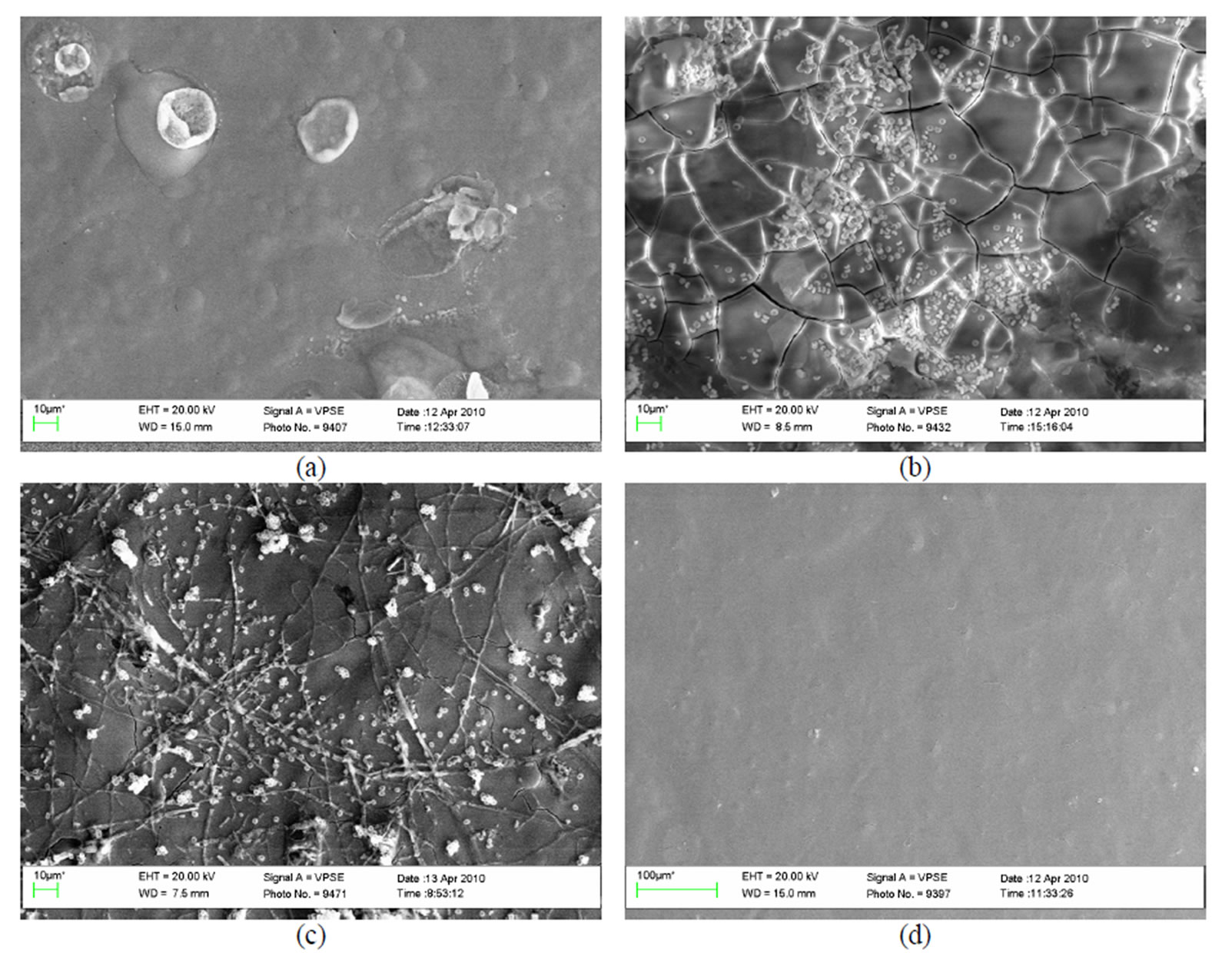
Figure 12. A general view of the surface of plates chromated in the solution Likonda 3CrMC after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Fusarium proliferatum + Paecilomyces lilacinus; (b) Alternaria alternata + Aspergillus penicilloides + Cladosporium cladosporioides + Penicillium carneum; (c) Arthrinium phaeospermum + Chaetomium elatum + Phoma exigua; (d) A reference sample without contact with fungi.
decrease in metal surface contamination (Figure 14(b)).
This testifies, that the Cr solution along with fungi can protect the metal from corrosion damage, because it does not impede the growth of fungi and does not reveal their functional activity.
Specimens passivated in the Likonda 3Cr5 solution and changes detected in them (Figures 15 (a,c)) after 60 days of contact with microscopic fungi were close to those of the reference specimen in (Figure 15(d)). The FTIR spectra of the specimen in (Figure 15(b)) were distinctly different from others, as there were peaks showing organic contamination of the surface.
Figure 16 shows how fungi affected specimens chromated with the Likonda 3CrMC solution. The FTIR spectrum in (Figure 16(a)) differs most markedly as it possesses a few peaks, which are the evidence in favour of a low resistance of the surface of these specimens to fungi action. However, this was not observed in the spectra of other specimens.
The results obtained suggest that the Cr solution possessing FTIR spectra which are less pronounced are the most suitable for metal protection.
From the data obtained it can be stated the Cr solution with less pronounced FTIR spectra (Cr(NO3)3·9H2O + malonic acid) are more suitable for metal protection, while Likonda 3Cr5 is less suitable.
To evaluate the structural changes in surfaces after 60 days of contact with microscopic fungi, the XRD method was applied. It has been determined that the surfaces of specimens (Figure 17) chromated in the Likonda 2AT solution after contact with various species of microscopic fungi changed markedly. The changes can be observed when specimens are compared by fungi species, that means when variants a, b and c are compared, not everywhere the changes are so pronounced, as in the case of comparison of the XRD patterns of mentioned variants with those of the reference variant d.
Data on the XRD spectra recorded while studying the surfaces of specimens chromated in the Cr3(NO3)·9H2O + malonic acid solution after 60 days of contact with microscopic fungi of various species are given in Figure 18. It should be mentioned that in all variants of studies the XRD patterns are very similar, close to those of the reference variant.
This testifies that the surface is little changed, protected from corrosion factors, determining the beginning of corrosion and that the solutions Cr(NO3)3·9H2O + malonic acid and partly Likonda 3Cr5 in the environment of selected fungi can be used to increase the corrosion resistance of metals.
The data in Figure 19 testify that the XRD spectra
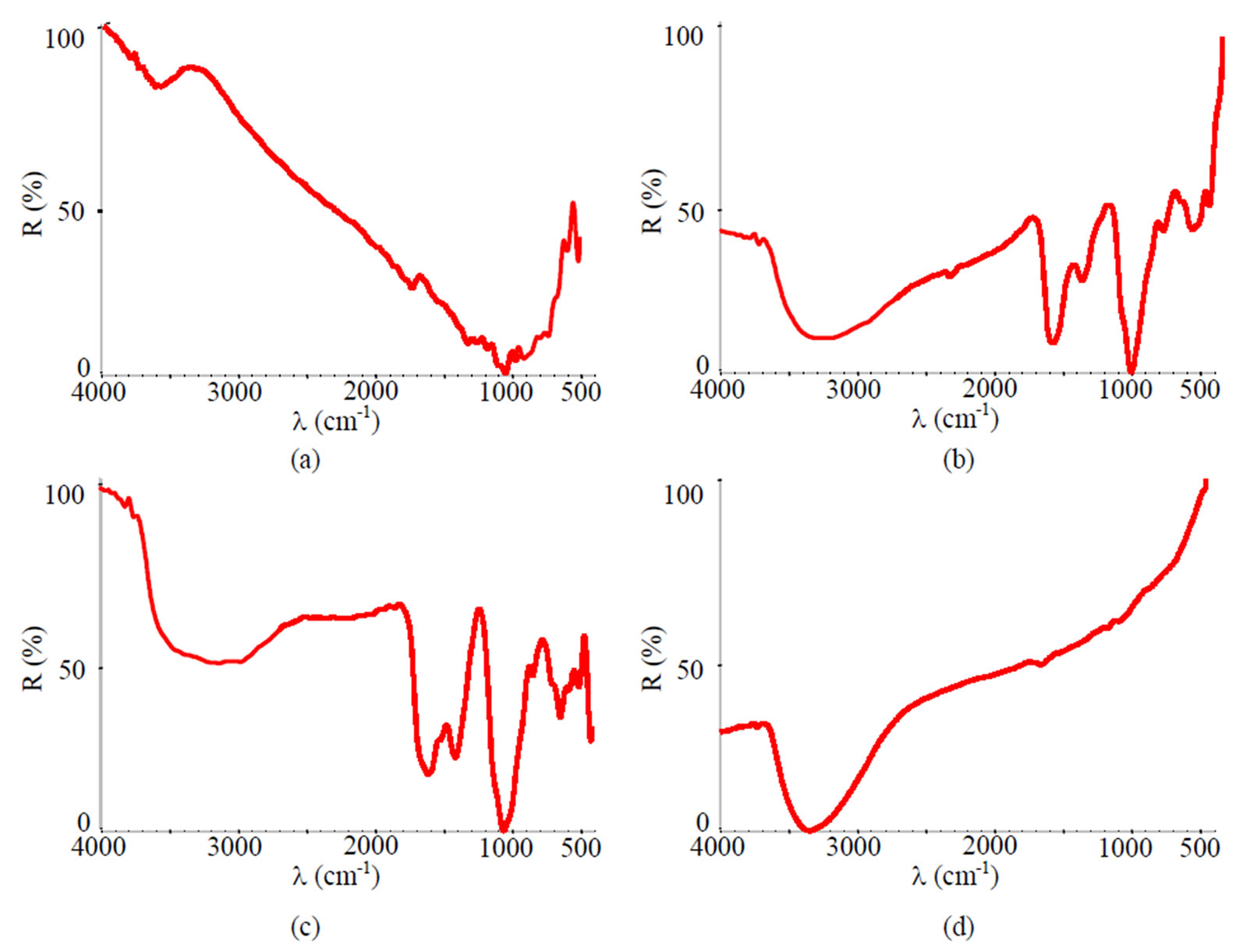
Figure 13. FTIR spectra of samples chromated in the solution Likonda 2AT after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Aspergillus penicilloides; (b) Alternaria alternata + Geomyces pannorum + Penicillium carneum + Tilachlidiopsis gracilis; (c) Arthrinium phaeospermum + Aspergillus spp. + Chaetomium elatum + Phoma exigua; (d) A reference sample without contact with fungi.
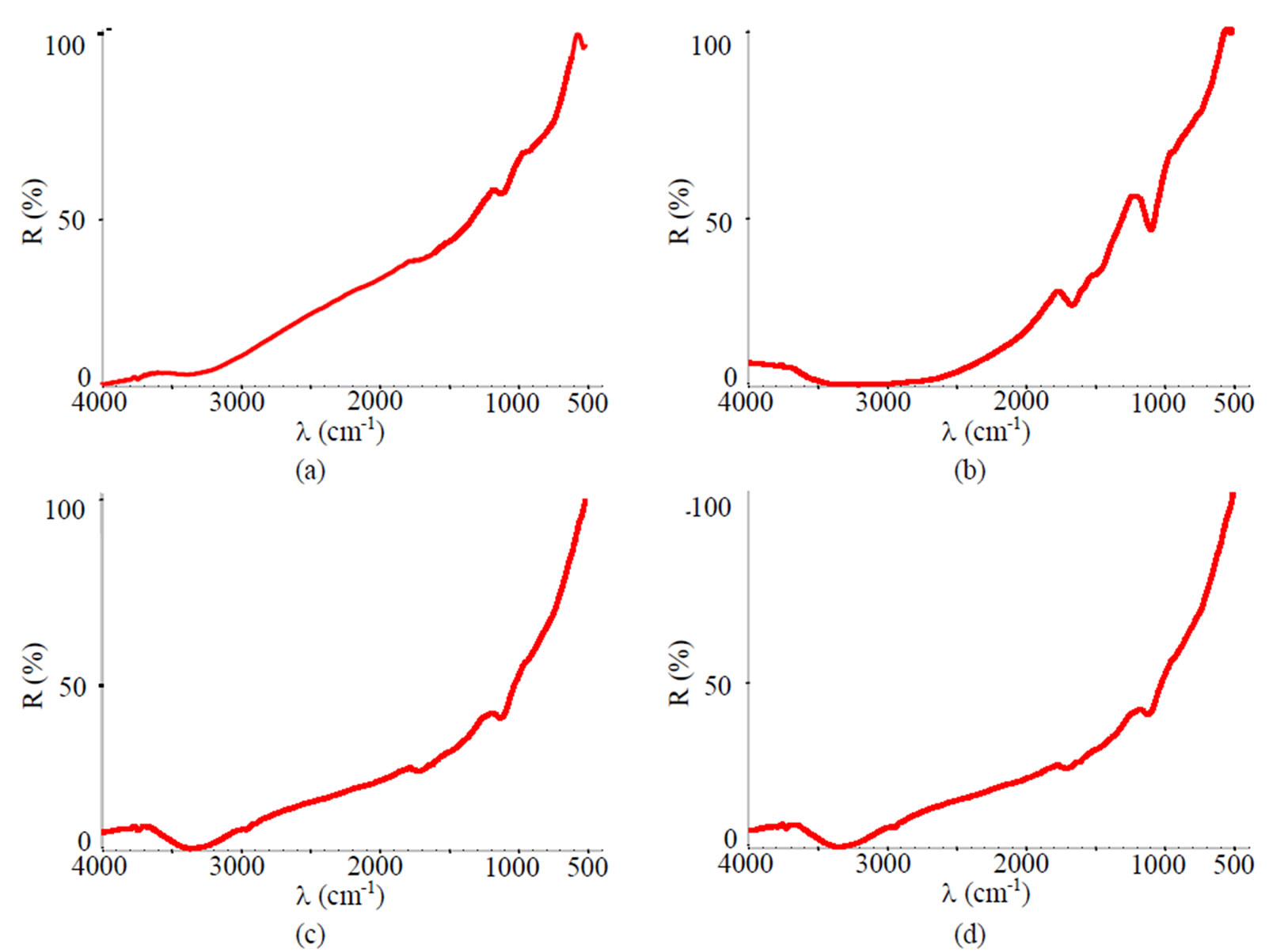
Figure 14. FTIR spectra of samples chromated in the solution Cr(NO3)3·9H2O+malonic acid after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Aspergillus penicilloides; (b) Alternaria alternata + Aspergillus penicillloides + Geotrichum candidum + Penicillium carneum + Aureobasidium pullulans; (c) Arthrinium phaeospermum + Aspergillus spp. + Cladosporium herbarum + Phoma exigua + Penicillium carneum; (d) A reference sample without contact with fungi.
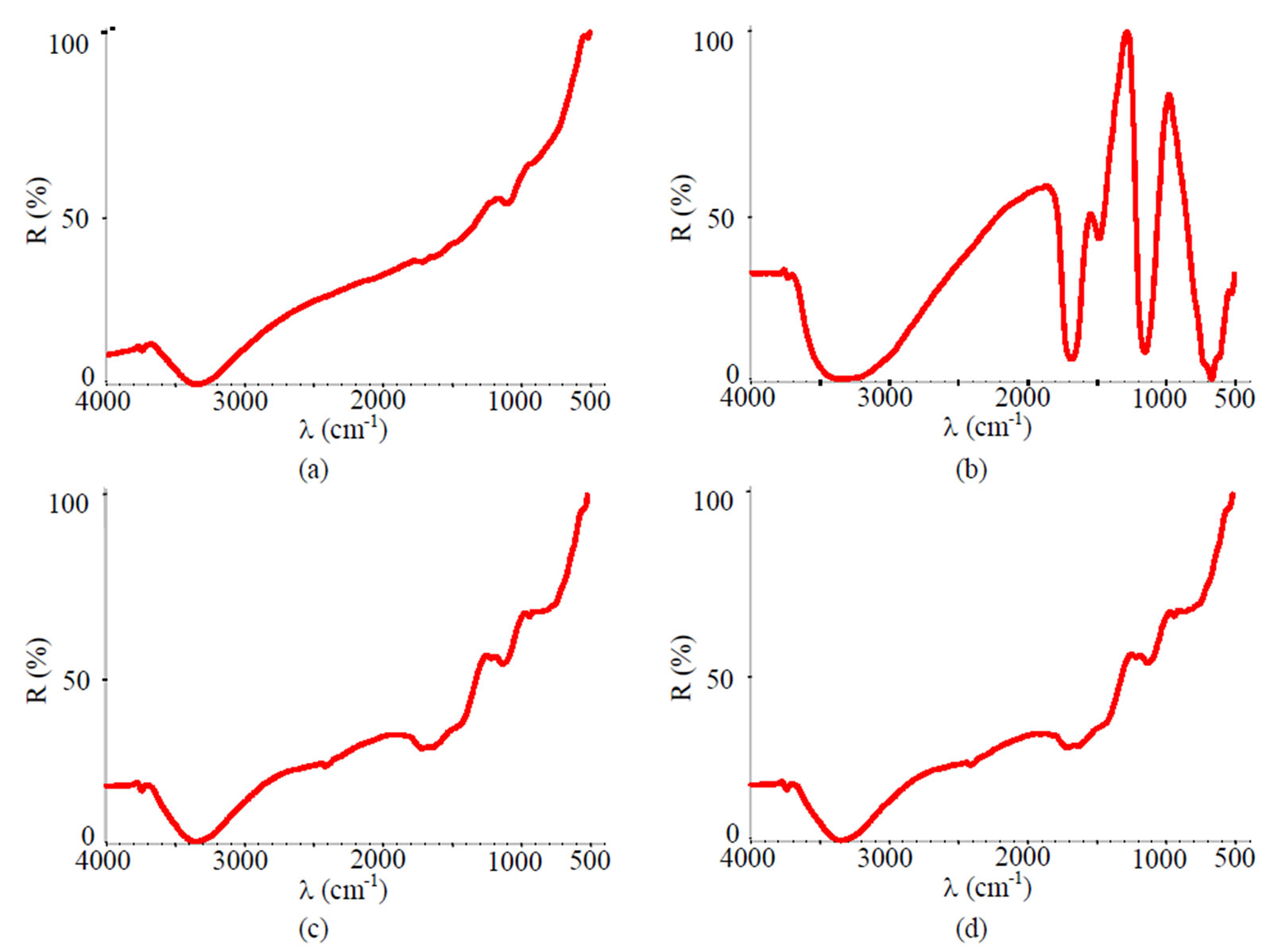
Figure 15. FTIR spectra of samples chromated in the solution Likonda 3Cr5 after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Fusarium proliferatum + Paecilomyces lilacinus + Penicillum stoloniferum; (b) Alternaria alternata + Geomyces pannorum + Cladosporium herbarum + Penicillium carneum; (c) Arthrinium phaeospermum + Aspergillus spp. + Cladosporium cladosporioides + Chaetomium elatum; (d) A reference sample without contact with fungi.
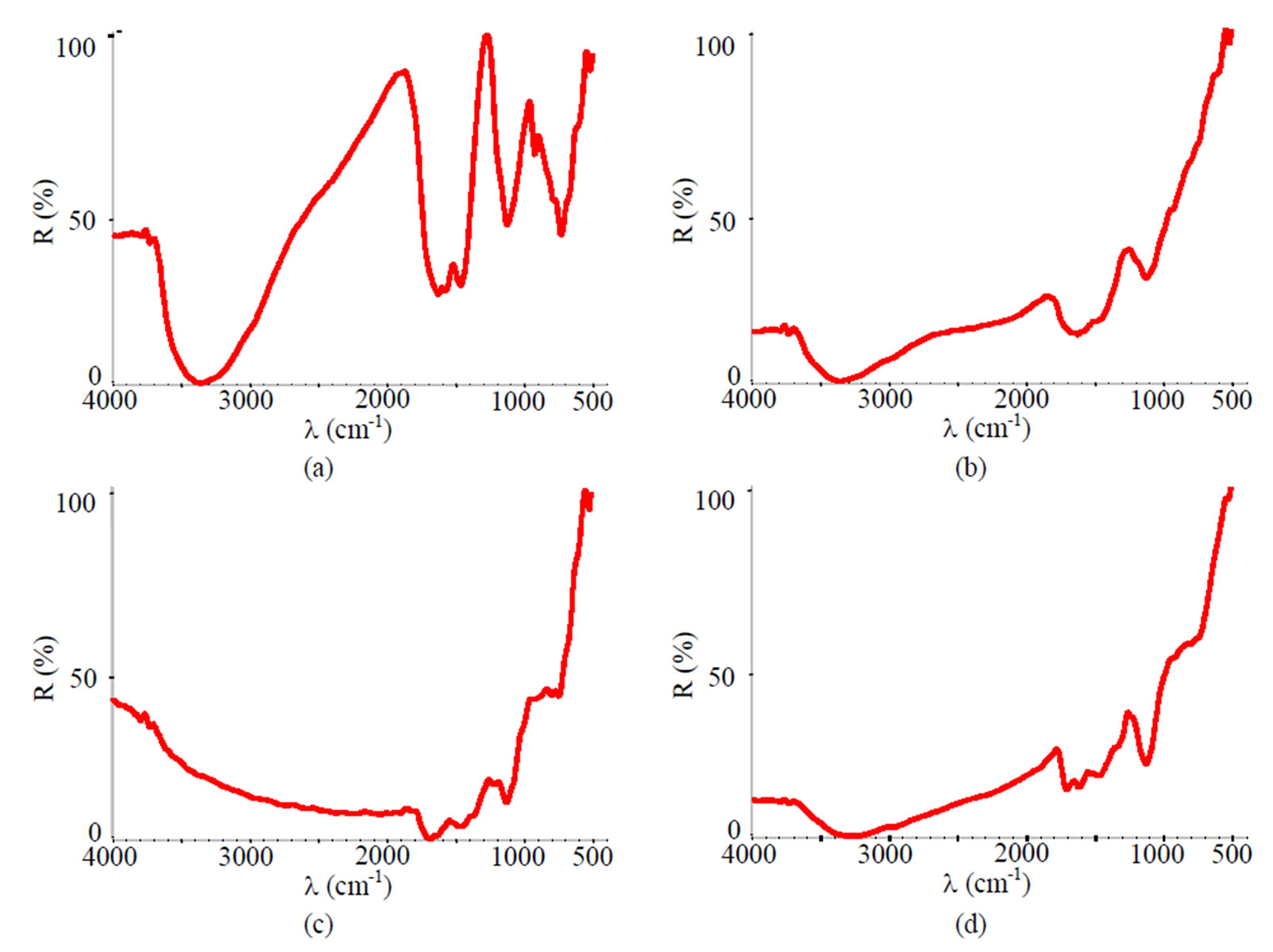
Figure 16. FTIR spectra of samples chromated in the solution Likonda 3CrMC after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Fusarium proliferatum + Paecilomyces lilacinus; (b) Alternaria alternata + Aspergillus penicilloides + Cladosporium cladosporioides + Penicillium carneum; (c) Arthrinium phaeospermum + Chaetomium elatum + Phoma exigua; (d) A reference sample without contact with fungi.
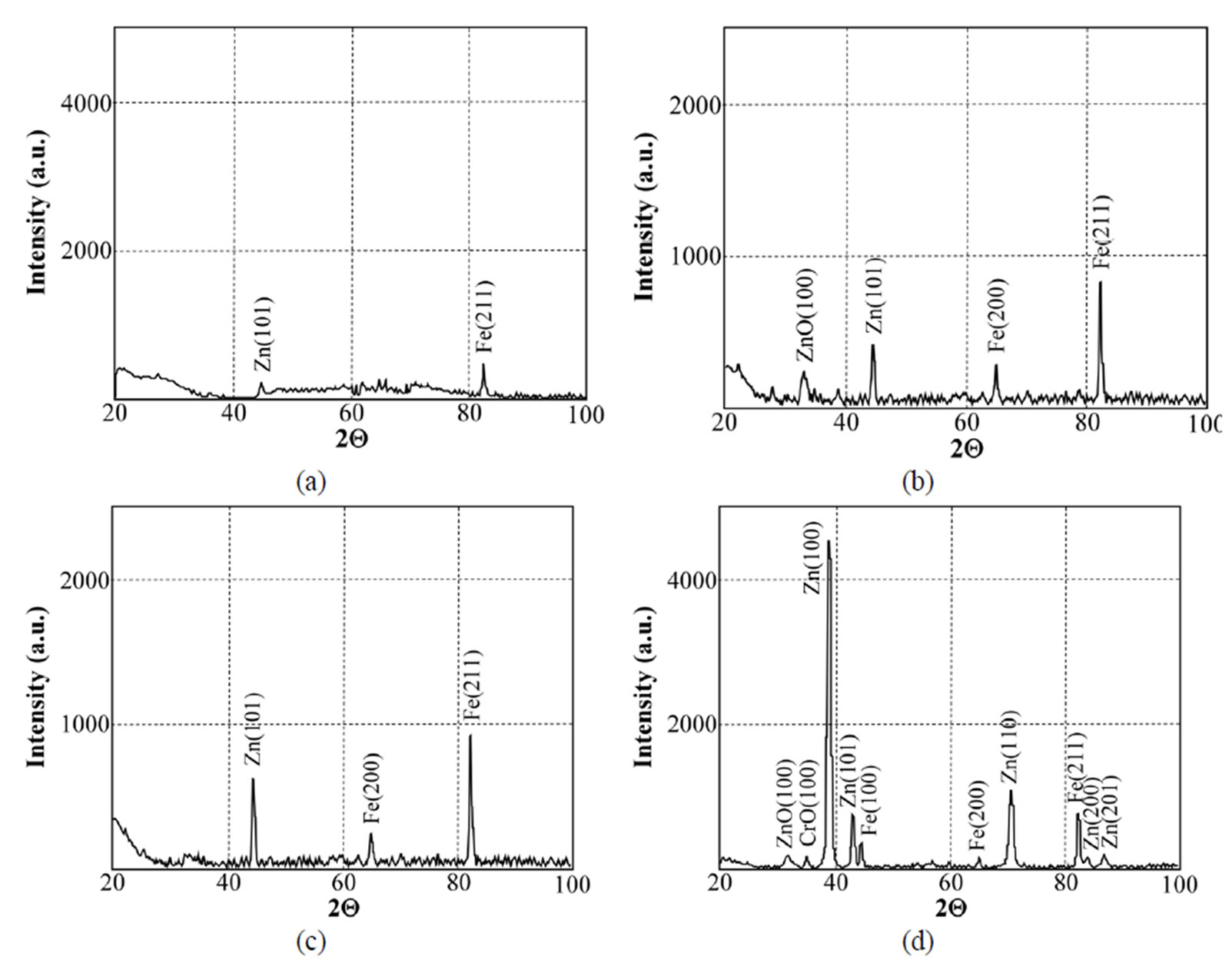
Figure 17. X-ray diffraction patterns of samples chromated in the solution Likonda 2AT after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Aspergillus penicilloides; (b) Alternaria alternata + Geomyces pannorum + Penicillium carneum + Tilachlidiopsis gracilis; (c) Arthrinium phaeospermum + Aspergillus spp. + Chaetomium elatum + Phoma exigua; (d) A reference sample without contact with fungi.
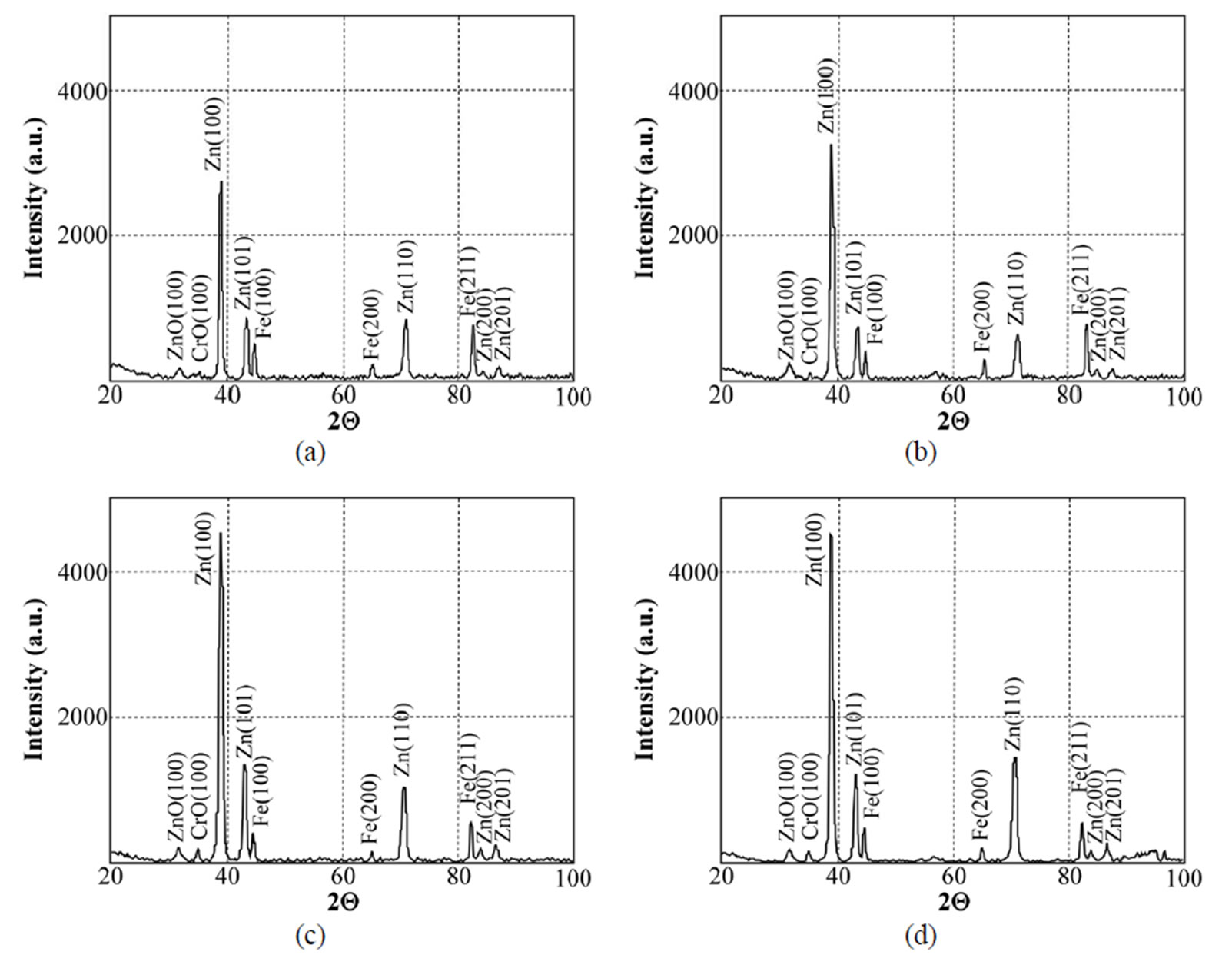
Figure 18. X-ray diffraction patterns of samples chromated in the solution Cr(NO3)3·9H2O+malonic acid after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Aspergillus penicilloides; (b) Alternaria alternata + Aspergillus penicillloides + Geotrichum candidum + Penicillium carneum + Aureobasidium pullulans; (c) Arthrinium phaeospermum + Aspergillus spp. + Cladosporium herbarum + Phoma exigua + Penicillium carneum; (d) A reference sample without contact with fungi.
give very similar results for the Likonda 3Cr5 solution after 60 days of contact of steel plates with micromycetes of different species (variants a, c). However in variant c, where the plates were in contact with Alternaria alternate + Geomyces pannorum + Cladosporium herbarium + Penicillium carneum fungi species, the XRD pattern was markedly changed and these changes were also recorded by the FTIR method, but unfortunately we failed to find the reason of the changes.
The spectra of specimens chromated in the Likonda 3CrMC solution after 60 days of contact with different species of microscopic fungi, as compared to the specimens free from fungi, were changed only slightly in variants a and c, only in variant b these change were more pronounced (Figure 20).
The results of XRD studies imply that the Zn coating chromated in the Cr(NO3)3·9H2O + malonic acid solution holds the greatest promise of all the studied measures to protect metals.
From the data presented in Figure 21 it is seen that steel plates coated with passyvated zinc coatings in different solutions possess different resistance to certain fungi species and their mixtures. A different impact of fungi on different coatings which is expressed by changes in their weight should be related to the specific metabolism characteristic of certain fungi species, their ability to produce organic acids and other metabolites capable of neutralizing the negative impact of the components of zinc plating solution, as well as produce compounds promoting or impeding the formation of crystallites on the surface of the subject studied and determine the changes in coating weight. Further studies are needed to establish more explicit regularities.
4. Conclusions
1) Atmospheric corrosion of metals is determined by the multitude of external factors, among which microscopic fungi occupy a prominent place and which are widely spread in the places of metal exploitation. They often are the cause of initial corrosion and determine its behaviour.
2) The activity of microscopic fungi on the surface of steel plates coated with the Zn coating chromated in different solutions is promoted by organic contaminants which find their way on the surface together with dust containing fungi propagules.
3) Microscopic fungi produce various metabolites, which damage the surface of steel plates coated with the Zn coating chromated in different chromium solutions. It should be noted that the steel plates coated with zinc coatings chromated in different solutions possess different sensitivity to the impact of fungi, and vice versa,
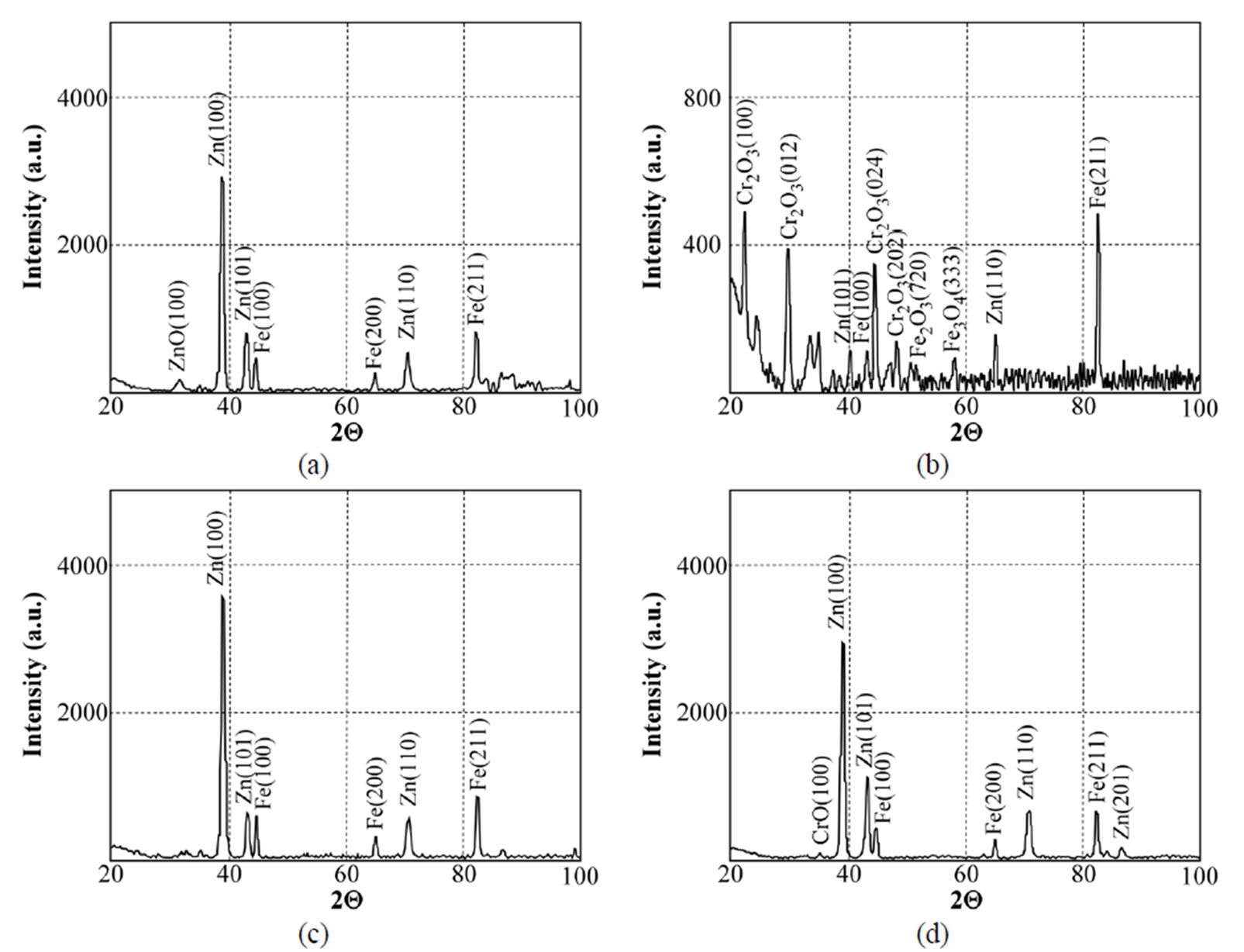
Figure 19. X-ray diffraction patterns of samples chromated in the solution Likonda 3Cr5 after 60 days of contact with different species of microscopic fungi: (a) Chrysosporium merdarium + Fusarium proliferatum + Paecilomyces lilacinus + Penicillum stoloniferum; (b) Alternaria alternata + Geomyces pannorum + Cladosporium herbarum + Penicillium carneum; (c) Arthrinium phaeospermum + Aspergillus spp. + Cladosporium cladosporioides + Chaetomium elatum; (d) A reference sample without contact with fungi.
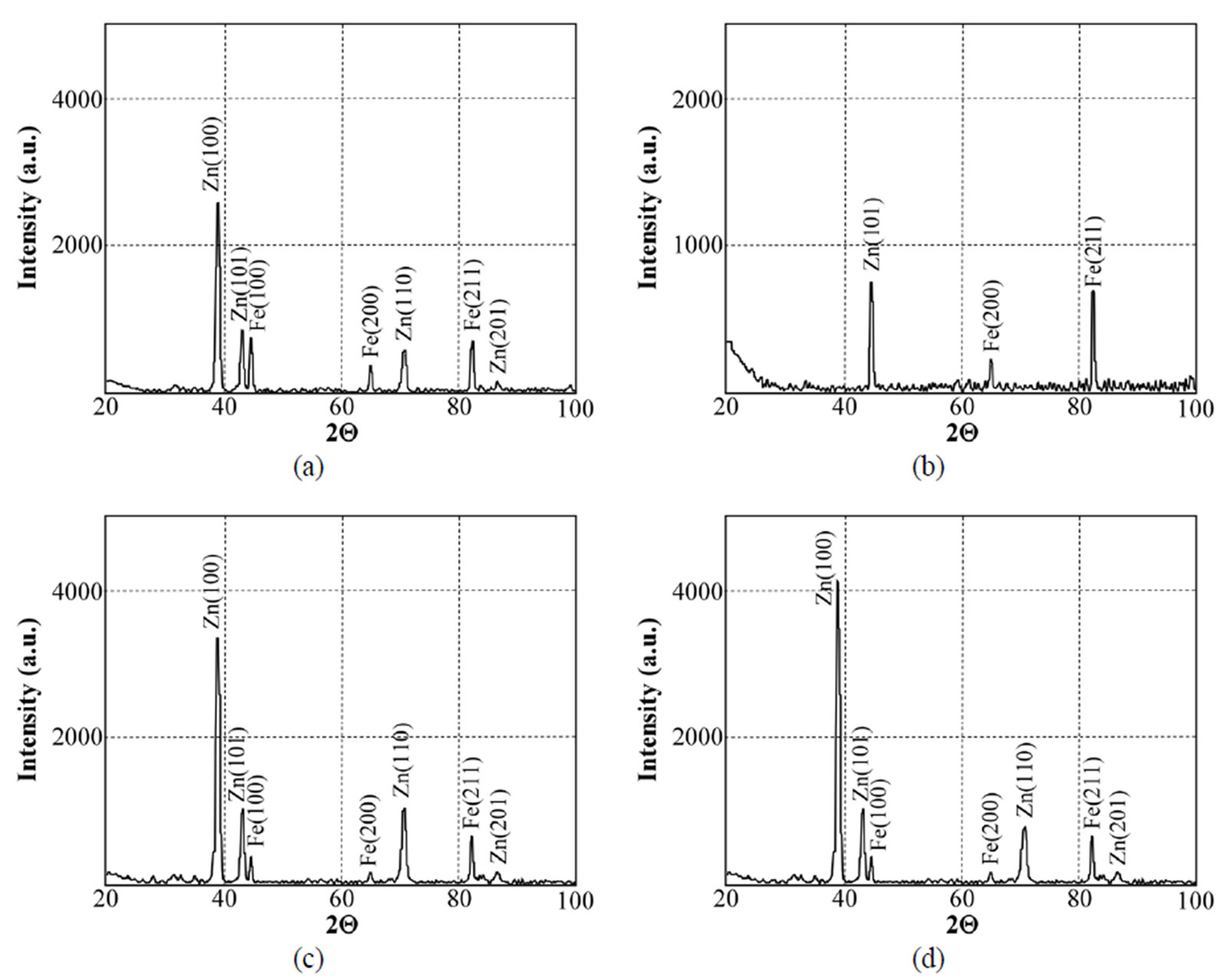
Figure 20. X-ray diffraction patterns of samples chromated in the solution Likonda 3CrMC after 60 days of contact with different species of microscopic fungi: fungi (a) Chrysosporium merdarium + Fusarium proliferatum + Paecilomyces lilacinus; (b) Alternaria alternata + Aspergillus penicilloides + Cladosporium cladosporioides + Penicillium carneum; (c) Arthrinium phaeospermum + Chaetomium elatum + Phoma exigua; (d) A reference sample without contact with fungi.
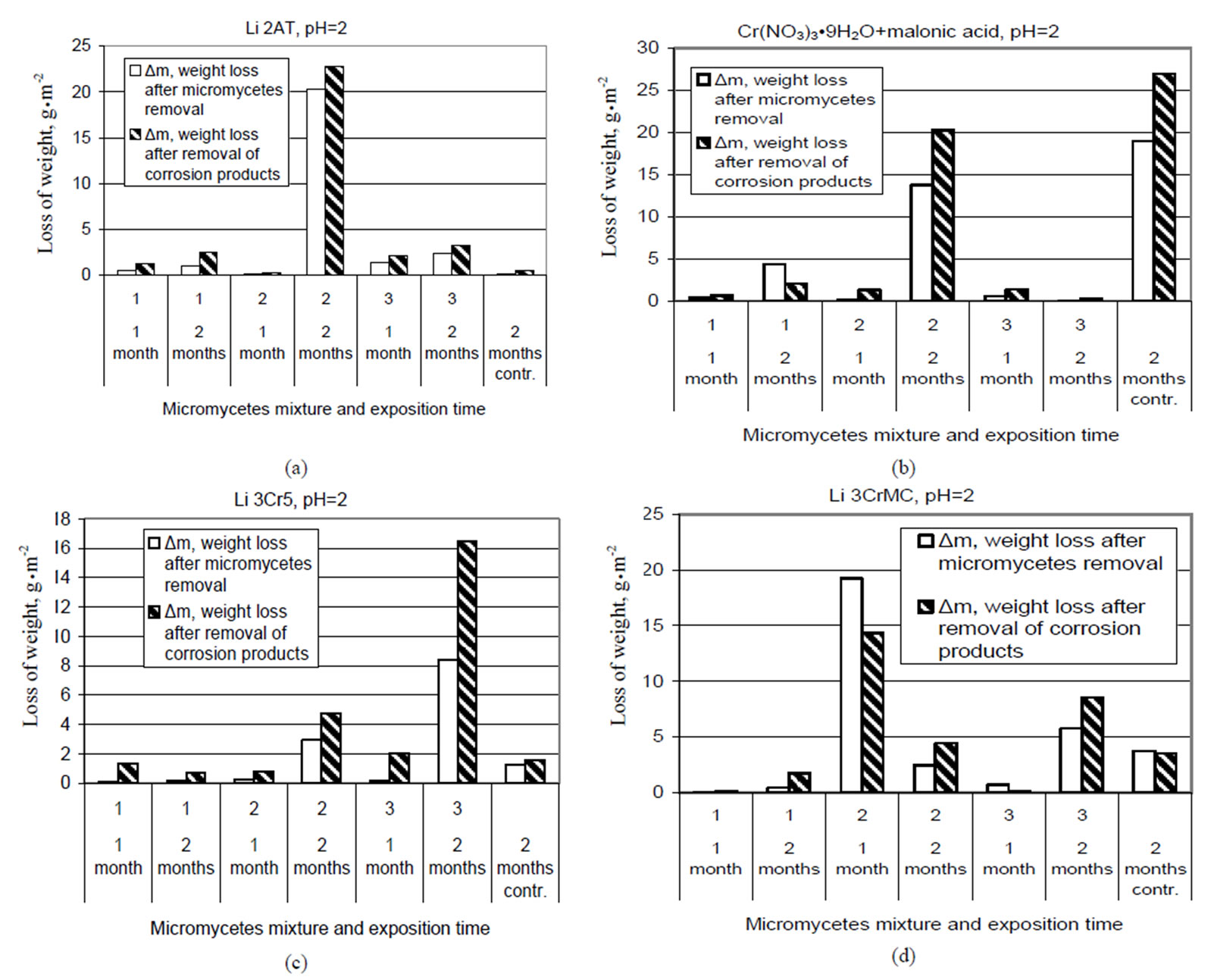
Figure 21. The influence of microscopic fungi on the surface of plates passivated in different solutions after 1 and 2 months in contact with different species of fungi. Test solution: (a) Likonda 2AT; (b) Cr(NO3)3·9H2O+malonic acid; (c) Likonda 3Cr5; (d) Likonda 3CrMC.
some fungi species are capable of developing under such environmental conditions that are unfavourable for other species development.
4) With the help of various methods it has been determined that application of the chromium compound Cr(NO3)·9H2O + malonic acid can be a promising corrosion resistant measure which together with certain mixtures of microscopic fungi species, for example Alternaria alternate + Aspergillus penicilloides + Geomyces pannorum + Cladosporium herbarum + Penicillium carneum can impede corrosion damage of the metal surface and this fact will promote further studies in order to find a practical implementation of the phenomena.
5. REFERENCES
- C. R. Tomachuk and O. B. Ferraz, “Investigation by EIS of Trivalent Chromate Conversion Coating on Galvanized Steel,” 16th International Corrosion Congress, Bejing, 22 September 2005, pp. 19-24.
- F. J. Chen, N.-T. Wen, M.-D. Ger, Y. N. Pan and C.-S. Lin, “Characterization of Hexavalent and Trivalent Chromium Conversion Coatings on Electrogalvanized Steel,” The 213 Electrochemical Society Meeting, Phoenix, 18-22 May 2008.
- M. Kending and R. G. Buchheit, “Corrosion Inhibition of Aluminium and Aluminium Alloys by Soluble Chromates, Chromate Coatings and Chromate-Free Coatings,” Corrosion, Vol. 59, No. 5, 2003, pp. 379-400.
- X. Zhang, C. van den Bos, W. G. Sloof, A. Hovestad, H. Terryn and J. H. W. de Wit, “Comparison of the Morphology and Corrosion Perfomance of Cr(VI) and Cr(III) Based Conversion Coatings on Zinc,” Surface & Coatings Technology, Vol. 199, No. 1, 2005, pp. 92-104.
- H. Asqari, M. R. Toroghejod and M. A. Golozaz, “On Texture, Corrosion Resistance and Morphology of Hot-Dip Galvanized Zinc Coatings,” Applied Surface Science, Vol. 253, No. 16, 2007, pp. 6719-6777.
- V. Rėzaitė, M. Samulevičienė, I. Demčenko, V. Dikinis and R. Šarmaitis, “Kai Kurių Organinių Medžiagų Inhibicinis Poveikis Plieno Korozijai Neorganinių RŪGščių, Jų Mišinių Ir Cr(III) Pasyvacijos RŪG-ščiuosiuose Tirpaluose/Inhibition of Corrosion of Steel in Solutions of Some Inorganic Acids Their Mixtures and Acidic Solution of Cr(III) Passivation of Zn,” Cheminė Technologija, Lithuanian, Vol. 51, No. 2, 2009, pp. 59-69.
- S. Katz and H. Salem, “The Biological and Environmental Chemistry of chromium,” VCH Publishers, New York, 1994.
- N.-T. Wen, C.-S. Lin, C.-Y. Bai and M.-D. Ger, “Structures and Characteristics of Cr(III)-Based Conversion Coatings on Elektrogalvanized Steels,” Surface and Coatings Technology, Vol. 203, No. 3-4, 2008, pp. 317-323.
- S. S. Abd El Rehim, M. A. M. Ibrahim and M. M. Dankeria, “Thin Films of Chromium Electrodeposition from Trivalent Chromium Electrolyte,” Transaction of the Institute of Metal Finishing(UK), Vol. 80, No. 1, 2002, pp. 29-33.
- R. Cook, J. Elliott and S. Sapp, “Electrochemical and Computational Screening of Non-Toxic Organic Replacements for Hexavalent Chromate Corrosion Inhibitors,” Tri-service Corrosion Conference (Army·Navy·Air Force·Starines·Coost Guard·Nasa), Denver, 3-7 December 2007, pp. 1-5.
- V. Dikinis, V. Rėzaitė, I. Demčenko, A. Selskis, T. Bernatavičius and R. Šarmaitis, “Regularities of Zinc Corrosion and Formation of Conversion Films on the Zinc Surface in Acidic Solution of Cr(III) Compounds,” Transactions of the Institute of Metal Finishing, Vol. 82, No. 4, 2004, pp. 1-7.
- V. Dikinis, V. Rėzaitė, J. Vaičiūnienė, A. Sudavičius, I. Demčenko and R.Šarmaitis, “Geležies Ir Cinko Korozija Kai Kurių Neorganinių Ir Organinių Rugsčių Tirpaluose/Corrosion of Iron and Zinc in Solution of Inorganic and Organic Acids,” Cheminė Technologija, Vol. 1, No. 47, 2008, pp. 13-16.
- V. Dikinis, V. Rėzaitė, J. Vaičiūnienė, A. Sudavičius, I. Demčenko and R.Šarmaitis, “Cr3+ Ir Co2+ Jonų Įtaka Geležies Ir Cinko Korozijai Kai Kurių Neorganinių Ir Oraganinių Rūgsčių Tirpaluose/Influence of Cr3+ and Co2+ ions and Zinc Corrosion in Solution of Some Inorganic and Organic Acids,” Cheminė Technologija, Vol. 2, No. 48, 2008, pp. 15-29.
- Pérez Néstor, “Electrochemistry and Corrosion Science,” Springer, Massachusetts, 2004.
- K. H. Cheung and J. D. Gu, “Mechanizm of Hexavalent Chromium Detoxification by Microorganism and Bioremedation Application Potential: A Review,” International Biodeterioration & Biodegradation, Vol. 59, No. 1, 2007, pp. 8-15.
- D. Park, Y. S. Yun and J. M. Park, “Use of Dead Fungal Biomass for the Detoxification of Hexavalent Chromium: Screening and Kinetics,” Process Biochemistry, Vol. 40, No. 7, 2008, pp. 2559-2565.
- A. Lugauskas, D. Pečiulytė, R. Ramanauskas, D. Bu- činskienė, A. Narkevičius and V. Ulevičius, “Mikromicetai Metalų Korozijos Procesuose Atmosferos Sąlygomis,” Ekologija, in Lithuanian, Nr. 1, 2005, pp. 11-26.
- A. Lugauskas, K. Leinartas, A. Grigucevičienė, A. Selskienė and E. Binkauskienė, “Possibility of Micromycetes Detected in Dust to Grow on Metal (AL,Fe,Cu,Zn) and Polyaniline-Modified Ni,” Ecology, Vol. 54, No. 3, 2008, pp. 149-157.
- H. A. Videla and L. K. Herrera, “Microbiologically Influenced Corrosion: Looking to the Future,” International Microbiology, Vol. 8, No. 3, 2005, pp. 169-180.
- H. A. Videla and L. K. Herrera, “Understanding Microbial Inhibition of Corrosion. A Comprehensive Overview,” International Biodeterioiration & Biodegradation, Vol. 63, No. 7, 2009, pp. 896-900.
- B. Poljsak, I. Pocsi, P. Raspor and M. Pesti, “Interference of Chromium with Biological Systems in Yeasts and Fungi: A Review,” Journal of Basic Microbiology, Vol. 50, No. 1, 2010, pp. 21-36.
- M. A. Dominguez-Crespo, E. Onofre-Bustamante, A. M. Torres-Huerta and F. J. Rodriguez Gomez, “Morphology and Corrosion Performance of Chromate Conversion Coatings on Different Substrates,”Journal of the Mexican Chemical Society, Vol. 52, No. 4, 2008, pp. 235-240.
- S. Maruthamuthu, N. Muthukumar, M. Natesan and N. Palaniswamy, “Role of Air Microbes on Atmospheric Corrosion,” Current Science, Vol. 94, No. 3, 2008, pp. 359-363.
- I. Beech, A. Bergel, A. Mollica, H.-C. Flemming (Leader), V. Scotto and W. Sand, “Simple Methods for the Investigation of the Role of Biofilms in Corrosion,” Brite Euram Thematic Network on Mic of Industrial Materials, Biofilm Publication, 2000, pp. 1-27.
- M. V. da Costa, C. T. Oliveira, T. L. Menezes, I. L. Muller and C. F. Malfatti, “Electrochemical Study of Silane Films and Chromate Conversion Coatings Applied on Zinc Coatings,” 11th International Coference on Advanced Materials, Brasil, 20-25 September 2009, pp. 20-25.
- M. Pesti, Z. Gazdag and J. Belagyi, “In Vivo Interaction of Trivalent Chromium with Yeast Plasma Membrane, as Revealed by EPR Spectroscopy,” FEMS Microbiology Letters, Vol. 182, No. 2, 2000, pp. 375-380.
- G. Gunasekaran, S. Chongdar, S. N. Gaonkar and P. Kumar, “Influence of Bacteria on Film Formation Inhibiting Corrosion,” Corrosion Science, Vol. 46, No. 8, 2004, pp. 1953-1967.
- T. Fukuda, Y. Ishino, A. Ogawa, K. Tsutsumi and H. Morita, “Cr(VI) Reduction from Contaminated Soils by Aspergllus sp N2 and Penicillium sp N3 Isolated from Chromium Deposits,” Journal of General and Applied Microbiology, Vol. 54, No. 5, 2008, pp. 295-303.
- G. Wang, L. Huang and Y. Zhang, “Cathodic Reduction of Hexavalent Chromium [Cr(VI)] Coupled with Electricity Generation in Microbial Fuel Cells,” Biotechnology Letters, Vol. 30, No.11, 2008, pp. 1959-1966.
- D. G. Zvyagintsev, “Methods of Soil Microbiology and Biochemistry,” Publishing House of Moscow University, Moscow, 1991.
- V. Mohoček-Grošev, R. Božač and G. J. Puppels, “Vibrational Spectroscopic Characterization of Wild Growing Mushrooms and Toadstools,” Spectrochimica Acta, Vol. 57, No.14, 2001, pp. 2815-2829.

