Advances in Bioscience and Biotechnology
Vol.3 No.8(2012), Article ID:25825,11 pages DOI:10.4236/abb.2012.38139
N-acetylcyteine and flavonoid rich diet: The protective effect of 15 different antioxidants on cigarette smoke-damaged primary human osteoblasts
![]()
1Department of Traumatology, Eberhard Karls Universität Tübingen, Tübingen, Germany
2Department of Traumatology, MRI, Technische Universität München, München, Germany
3Department of Plastic Surgery and Hand Surgery, Technische Universität München, München, Germany
4FONDAP Center for Genome Regulation, Facultad de Ciencias, Universidad de Chile, Santiago, Chile
Email: sehnert@bgu-tuebingen.de
Received 17 September 2012; revised 19 October 2012; accepted 30 November 2012
Keywords: Cigarette Smoke; Osteoblasts; Oxidative Stress; N-Acetylcysteine; Flavonoids
ABSTRACT
Cigarette consumption increases oxidative stress in many organs. Increased oxidative stress harms bone cells, which negatively affects bone-matter and -stability. This leads to an increased fracture risk and delayed fracture healing in smokers. A supporting therapy with antioxidants could be of great benefit for surgeons dealing with delayed fracture healing due to increased oxidative stress. In this article we complement and compare our published data with hitherto unpublished data and show the protective effect of 15 different antioxidants on cigarette smoke induced damage in primary human osteoblasts. Exposure to cigarette smoke medium (CSM) rapidly induces formation of ROS in osteoblasts in a concentrationand time-dependent manner. Massive cell damage is seen already after 4 h (EC50 ≈ 0.75 OD320). Pre-, coand post-incubation with the different antioxidants reduces the formation of ROS and consequently improves the viability of the CSM exposed osteoblasts. Small compounds, e.g. N-acetylcysteine, proved highly effective if preor co-incubated before exposure to the CSM. Thus, they are good candidates for acute therapy support as they can be administered in high doses. However, our data suggest that a balanced daily diet could lead to an accumulation of various natural antioxidants (flavonoids) that effectively protect osteoblasts from oxidative stress-induced damage in all three settings investigated. Together with their partly phytoestrogenic properties this may even abate alterations in bone and thus reduce fracture risk on the long run.
1. INTRODUCTION
Bone is one of the most metabolically active tissues of the body which undergoes a constant and complex process of remodeling [1]. In this cycle osteoblasts play an essential role. Their main task is to produce new bone matrix and, as osteocytes, to support the bone structure itself. Impairment of osteoblasts thus can lead to several dysfunctions, e.g. osteopenia, osteoporosis, impaired fracture healing or an increased rate of pseudarthrosis.
Tobacco smoking contributes to the pathogenesis of a variety of diseases, e.g. cancer, cardiovascular and pulmonary diseases [2]. Recently, more and more studies have described the negative effects of tobacco smoking on bone, e.g. increased fracture risk, delayed fracture healing, reduced bone density, alterations in bone mineral content and osteoporosis [3-9]. The underlying mechanisms, however, are diverse. Over 150 of the more than 6000 molecular species in cigarette smoke are proven toxins. These toxins not only initiate and aggravate tissue injury, but also impair reparative processes via the initiation of inflammatory responses [10,11]. Hence tissue destruction develops either through direct toxic effects (e.g. DNA damage), altered gene regulation or indirectly through increased oxidative stress [12-16]. Thus, a supporting therapy with antioxidants could be of great benefit for surgeons, revolutionizing initial fracture treatment in patients with increased oxidative stress.
Reactive oxygen species (ROS) are widely believed to cause or aggravate several human pathologies, e.g. neurodegenerative diseases, cancer or stroke. Also bone metabolism is strongly affected by oxidative stress. Particularly, the cigarette smoke induced formation and accumulation of ROS has been shown to damage osteoblasts in vitro [17-19]. This may hinder fracture healing, reducing the patients’ quality of life by prolonged hospital stays and reduced mobility, overall leading to higher expenditures in health care. Antioxidants are emerging as novel therapeutic drugs for free radical mediated diseases. Antioxidants are assumed to counteract the harmful effects of ROS and therefore prevent or treat oxidative stress-related diseases.
In addition to a number of potentially bioactive components such as fiber, folate and potassium, fruits, vegetables, herbs and spices are important sources of dietary antioxidants (often flavonoids) [20]. There is increasing evidence that a high dietary intake of flavonoids from fruits and vegetables as well as from tea and red wine may reduce coronary heart disease mortality [21]. The individual intake of flavonoids, however, varies considerably depending on the type of diet consumed. Thus, there is currently an extensive range of flavonoid supplements available on the market [22]. Suppliers of such supplements recommend daily flavonoid intakes in amounts that are many times higher than those doses which can normally be achieved from a flavonoid-rich diet [23]. For instance, the flavonol quercetin has been marketed as a dietary supplement with recommended daily doses of up to 1 g and more [24], whereas the daily quercetin intake from foods has been estimated to be 10 - 100 mg [25].
On the contrary, aspirin or N-acetylcystein are drugs which have a great ability to scavenge reactive oxygen species. These small chemical substances have the great advantage that they can be taken up in high doses and that they are approved for clinical use.
With this regard we wanted to compare (hitherto unpublished data in comparison to already published data) the effect of 15 commonly used antioxidants on cigarette smoke medium (CSM)-damaged primary human osteoblasts. The antioxidants (partly flavonoids) investigated are naturally occurring substances in fruits and vegetables, e.g. garlic [18] (garlic oil blend (GOB), diallyl-disulfide (DADS) and diallyl-sulfide (DAS)), grapes (quercetin [17] and resveratrol) or citrus fruits (hesperidine, naringenin, naringin and vitamin c), spices (anisole, cinnamic acid and vanillic acid), cacao and tea (catechin [19]) as well as the clinically used acetylsalicylic acid (ASA) and N-acetylcysteine (NAC). We here compare the effect of the antioxidants on ROS formation and viability of CSM treated primary human osteoblasts in a pre-, coand post-stimulation setting as previously described [17-19].
2. MATERIALS AND METHODS
Cell Culture Medium and supplements were obtained from PAA Laboratories (Cölbe, Germany); Primaryand secondary antibodies were purchased from Santa Cruz Biotechnology (Heidelberg, Germany); Chemicals if not stated differently from Sigma (Munich, Germany).
2.1. Isolation and Culture of Primary Human Osteoblasts
Osteoblasts were isolated from femur heads of patients undergoing total hip replacement, in accordance to the ethical vote of the “Klinikum rechts der Isar” (MRI, Technische Universität München, Germany) and the BG Trauma Center Tübingen (Eberhard Karls Universität Tübingen, Germany) and the patients’ written consent. Briefly, cancellous bone was removed mechanically from the femur head and washed 3 - 5 times with Dulbecco’s Phosphate Buffered Saline (DPBS). Afterwards bone pieces were incubated with an equal volume of digestion buffer (DPBS, 0.07% collagenase II—Biochrom AG, Berlin, Germany) for 1 h at 37˚C. After digestion, bone pieces were washed with culture medium (MEM/- Ham’s F12, 10% FCS, 100 U/ml penicillin, 100 µg/ml streptomycin, 50 µM L-ascorbate-2-phosphate, 50 µM bglycerol-phosphate) and wash fraction was transferred to cell culture flasks to allow adherence of cells. For expansion of cells the medium was changed every 4 - 5 days. In passage 3 cells were plated for the experiments (20,000 cells/cm2) [26].
2.2. Generation of CSM
CSM was prepared, from commercially available cigarettes (Marlboro, Philip Morris, Munich, Germany), as suggested by the International Organisation for Standardisation (ISO 10362-2) and the Federal Trade Commission [27]. Briefly, the filter-free cigarettes were placed in a standard gas washing bottle (Lenz Laborglas GmbH & Co.KG, Wertheim, Germany) which was subjected to negative pressure by using a peristaltic pump. In total 3 cigarettes were bubbled through 25 ml of DMEM over a time-frame of 15 minutes at a rate of 1 puff (2 sec) per min (Figure 1(a)). The freshly prepared CSM was supplemented with 10% FCS, 100 U/ml penicillin and 100 µg/ml streptomycin and filtered (0.22 µm-filter, Sarstedt, Nürnberg, Germany) before use. The concentration of CSM was determined and normalized by its optical density at 320 nm (OD320) in a plate reader (BMG Labtech GmbH, Offenburg, Germany) as described [17-19].
2.3. Determination of ROS (DCFH-DA Assay)
Cells were incubated with 10 µM 2’,7’-dichlorofluorescein-diacetate (DCFH-DA) in serum free culture medium for 30 min at 37˚C. After washing cells 3 times with DPBS cells were stimulated with the smoke extract according to the experimental setup. After 15 min the
 (a)
(a) (b)
(b)
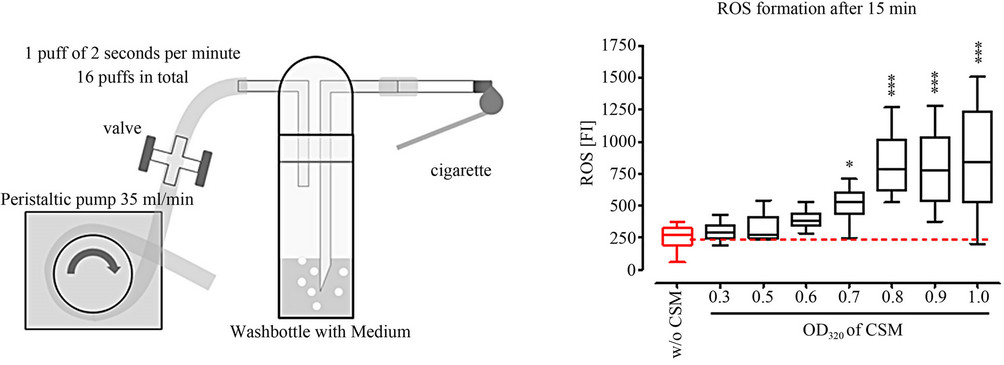
Figure 1. Experimental setup. (a) Schematic overview on the generation of cigarette smoke medium (CSM) with a standard gas washing bottle; (b) ROS measurement (DCFH-DA assay) in primary human osteoblasts (N = 3; n = 4) after 15 min treatment with different concentrations of CSM (OD320 = 0 (co), 0.3, 0.5, 0.6, 0.7, 0.8, 0.9 and 1); (c) Viability measurement (resazurin conversion) of primary human osteoblasts (N = 3, n = 6) after 4 h treatment with different concentrations of CSM (OD320 = 0 (co), 0.3, 0.5, 0.6, 0.7, 0.8, 0.9 and 1); (d) Schematic overview on the experimental setup. *p < 0.05, ***p < 0.001 as compared to untreated cells.
fluorescence (ex/em = 485/520 nm) was measured [17- 19].
2.4. Resazurin Conversion
Resazurin conversion was used to determine cells’ viability. Briefly, 1/10 volume of a 0.025% (w/v) resazurin solution (in DPBS) was added to the cells. After 1 h incubation at 37˚C fluorescence was measured (ex/em = 544/590 nm) and corrected to background control (no cells). Viability is given as % of untreated control [17,18].
2.5. Statistics
Results are expressed as mean ± SEM of at least 3 independent experiments (N ≥ 3) measured as triplicates or more (n ≥ 3). Data sets were compared by one-way analysis of variance followed by Bonferroni’s multiple comparison test (GraphPad Prism Software, El Camino Real, USA). p < 0.05 was taken as minimum level of significance.
3. RESULTS
3.1. Definition of the Experimental Setup
To determine formation of ROS primary human osteoblasts (N = 3, n = 4) loaded with DCFH-DA were exposed to different concentrations of CSM. After 15 min ROS formation was measured by an increase in fluorescence intensity. A significant increase in ROS formation was observed starting at an OD320 of 0.5 (p < 0.01). For better comparison to the viability assay cells were stimulated with CSM with an OD320 of 0.8 for all further experiments (Figure 1(b)).
As described before, primary human osteoblasts were stimulated with concentrated smoke extract for 0, 1.5, 3, 6, 12 and 24 h (N = 3, n = 6). After 3 h viability was significantly reduced in the presence of CSM (72.5% ± 5.8% fractional survival; p < 0.05). 100% toxicity was seen after 12 h exposure to CSM [19]. To establish a standardized experimental protocol we determined the EC50 after 4 h treatment with CSM (N = 3, n = 6, Figure 1(c)). The concentration range of CSM, determined photometrically by its optical density at 320 nm (OD320), was between 0.3 and 1. After 4 h the EC50 was reached by a CSM with an OD320 of 0.75 ± 0.03 [17-19]. Thus, we defined the experimental setup to be 1) 4 h pre-incubation with sub-toxic concentrations of the target substances followed by 4 h incubation with CSM (OD320 = 0.8); 2) 4 h co-incubation with sub-toxic concentrations of the target substances with CSM (OD320 = 0.8) and 3) 4 h incubation with CSM (OD320 = 0.8) followed by 4 h post-incubation with the same concentrations of the target substances (Figure 1(d)).
3.2. Definition of the Assay Concentrations of the Investigated Antioxidants
In order to determine assay concentrations of the different antioxidants that are not toxic for the osteoblasts we determined the EC50 for each substance after 24 h treatment (N = 3, n = 4). The resulting assay concentrations were defined to be the highest concentration of the substance that didn’t show toxicity. The individual EC50 values, resulting assay concentrations, natural source and chemical structures of the antioxidants are summarized in Table 1. Noteworthy, the clinically used NAC is highly compatible with osteoblasts, represented by a high EC50 value of 69.77 mM. The osteoblasts reacted more sensitive towards the fruit flavonoids hesperidin, quercetin and resveratrol, where the individual EC50 values were between 200 µM and 300 µM.
3.3. Pre-Incubation with NAC, ASA and Garlic Components Most Effectively Protect Osteoblasts from CSM-Induced Damage
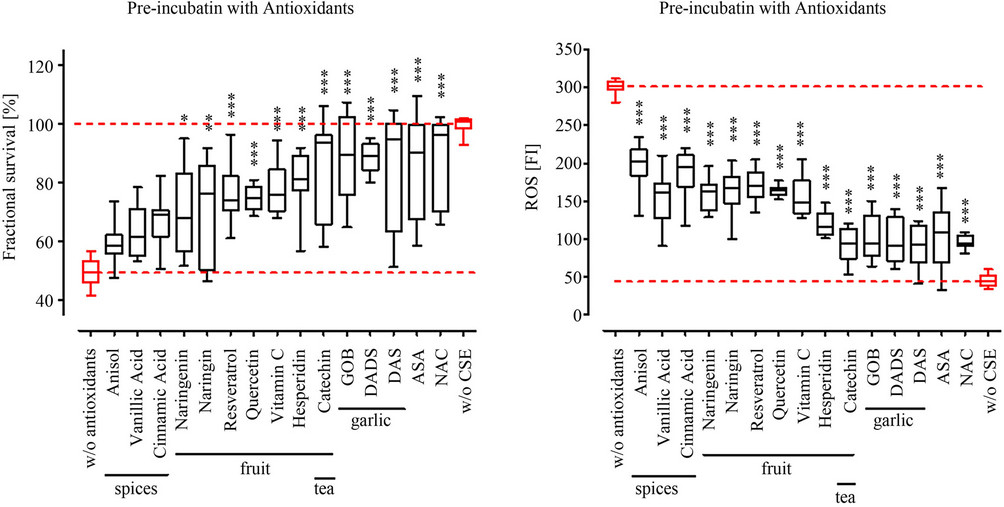
By pre-incubation with the different antioxidants we wanted to identify their potential as prophylactic agent for CSM-induced damage in osteoblasts, e.g. for supporting a functional bone metabolism. Primary human osteoblasts (N = 3, n = 4) were pre-treated with the different antioxidants for 4 h, then stimulation with CSM for another 4 h before measuring viability. In the here used assay concentrations NAC, ASA and the garlic components DADS and DAS [18] most effectively protected the primary human osteoblasts from CSM-induced damage. NAC, ASA, DADS and DAS reached viabilites comparable to untreated cells. These substances are directly followed by catechin [19], the group of grape [17] and citrus fruit components (catechin, hesperidin, vitamin C, quercetin, resveratrol, naringin and naringenin). These substances reach viabilities between 70% and 90% of untreated cells. The least effect in the pre-incubation setting showed the group of antioxidants from spices, vannilic acid, cinnamic acid and anisole. Where the viability was only between 60% and 70% of untreated cells (Figure 2(a)).
Pre-treatment with the different substances led to a reduction (40% to 80%) of ROS formation in primary human osteoblasts treated with CSM (N = 3, n = 4). The order in which the substances reduced ROS formation most effectively resembled the viability data of the preincubation setting. NAC, ASA and the garlic components most effectively prevented ROS formation in CSMtreated osteoblasts (~80%). The spice flavonoids anisole, cinnamic acid and vannilic acid showed least effect on ROS formation (~40% reduction) when pre-incubated before stimulation with CSM (Figure 2(b)).
3.4. Co-Incubation with NAC, Fruit and Tea Antioxidants Most Effectively Protect Osteoblasts from CSM-Induced Damage
With the co-incubation of the different antioxidants and CSM we wanted to identify their potential as acute therapeutic, e.g. to support fracture healing. Primary human osteoblasts (N = 3, n = 4) were co-stimulated with the different antioxidants and CSM for 4 h before measuring viability. Again NAC proved to most effectively protect the primary human osteoblasts from CSM-induced damage. Reaching viability levels comparable to untreated cells. In the co-incubation setting however, quercetin [17], catechin [19] as well as the citrus fruit components hesperidin, naringenin, naringin and vitamin C, more effectively protected the osteoblasts from CSM-induced damage (80% to 100% viabitlity) than the garlic components DADS and DAS (~80% viability) [18]. The spice flavonoids vannilic acid, cinnamic acid and anisole received comparable results (70% to 80% viability) to the garlic components DAS and DADS. Interestingly, ASA and Resveratrol, vith about 60% to 70% viability, showed the least effect in the co-incubation setting (Figure 3(a)).
Comparable to the pre-treatment with the different substances the co-incubation also reduced the formation of ROS in CSM-treated primary human osteoblasts (N = 3, n = 4). The order in which the substances reduced ROS formation most effectively resembled the viability data of the co-incubation setting. NAC and quercetin most effectively reduced ROS formation (~85%). The Resveratrol and ASA showed least effect on ROS formation (~20% reduction) when co-incubated with CSM (Figure 3(b)).
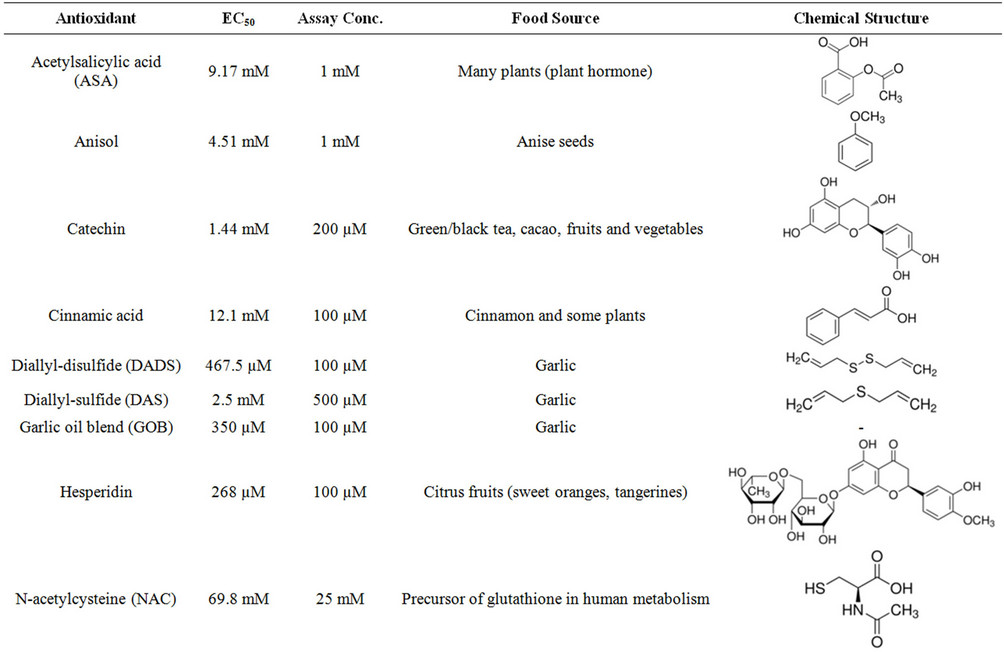
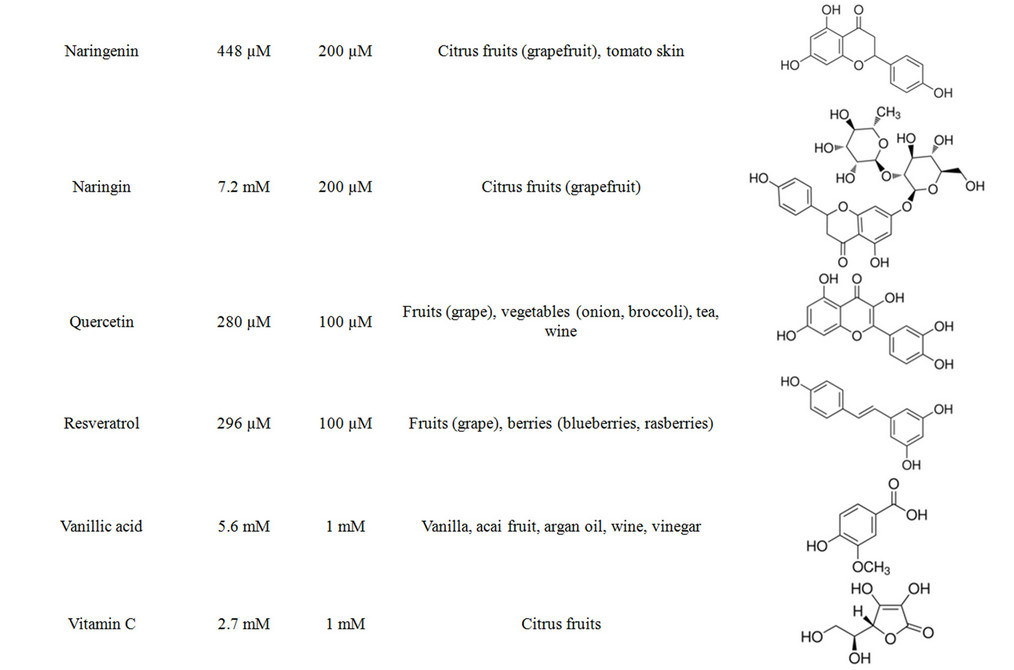
Table 1. Summary on the investigated antioxidants.
 (a) (b)
(a) (b)
Figure 2. Pre-incubation with antioxidants reduced ROS formation and increases viability in CSM treated primary human osteoblasts. Primary human osteoblasts (N = 3, n = 4) were pre-treated with 15 different antioxidants at defined concentrations (see Table 1) for 4 h before stimulation with CSM (OD320 = 0.8). (a) After 4 h exposure to CSM viability was determined by resazurin conversion; (b) After 15 min ROS formation was measured using the DCFH-DA assay. All substances investigated reduced ROS formation and consequently improved viability of CSM-treated osteoblasts. Overall, the more the ROS formation was reduced the better the viability of the CSM-treated osteoblasts. *p < 0.05, **p < 0.01, ***p < 0.001 as compared to cells not pre-treated with antioxidants.
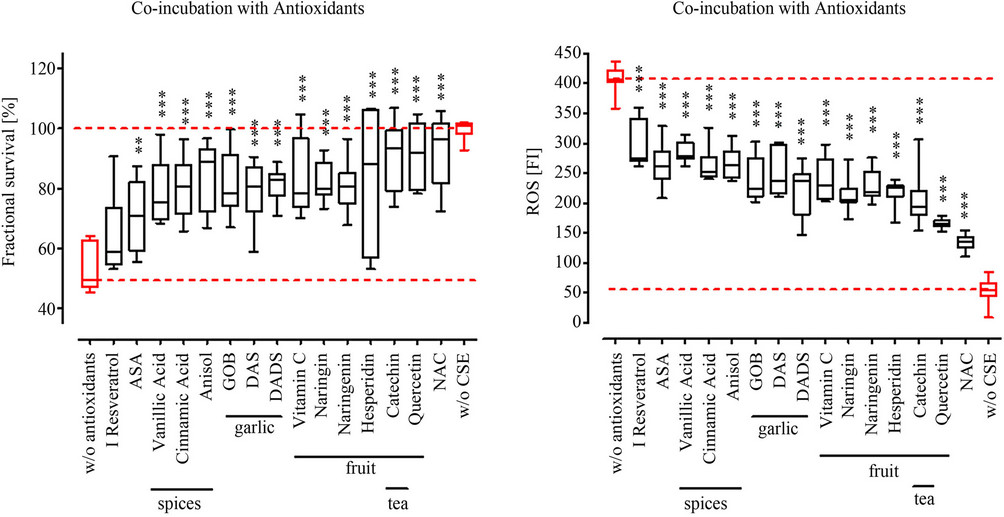
Figure 3. Co-incubation with antioxidants reduced ROS formation and increases viability in CSM treated primary human osteoblasts. Primary human osteoblasts (N = 3, n = 4) were co-incubated with 15 different antioxidants at defined concentrations (see Table 1) and CSM (OD320 = 0.8). (a) After 4 h viability was determined by resazurin conversion; (b) After 15 min ROS formation was measured using the DCFH-DA assay. All substances investigated reduced ROS formation and consequently improved viability of CSM-treated osteoblasts. Again there is a relation between ROS reduction and viability observable. **p < 0.01, ***p < 0.001 as compared to cells not treated with antioxidants.
3.5. Grape and Tea Flavonoids Most Effectively Protect Osteoblasts from CSM-Induced Damage in a Post-Incubation Setting
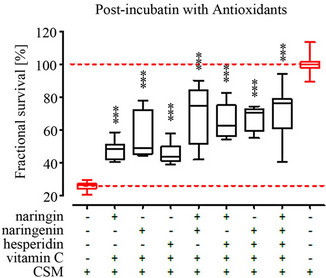
By post-incubation with the different antioxidants we wanted to identify their potential as therapeutic agent, e.g. to support fracture healing or treat osteoporosis. Primary human osteoblasts (N = 3, n = 4) were stimulated with CSM for 4 h, then treated with the different antioxidants for 4 h before measuring viability. Due to the elongated culture time after CSM-treatment in the post-incubation setting the viability was further reduced to an average of 28.6% without antioxidant treatment as compared to the preor co-incubation setting in which the viability was 49.1% without antioxidant treatment. In the post-incubation setting all substances investigated significantly improved viability of CSM-treated primary human osteoblasts. The grape flavonoids quercetin [17] and resveratrol as well as catechin [19] most effectively rescued osteoblasts’ viability from CSM-treatment, reaching a viability of approximately 80% of untreated cells. Directly followed by the spice flavonoids (~70% viability), NAC, garlic [18] and citrus fruit components, which showed all a comparable protective effect in the postincubation setting. Post-incubation with these substances result in approximately 60% viability compared to untreated cells. Again ASA had the least protective effect as observed in the co-incubation setting with about 50% of viability (Figure 4).
3.6. Additive Effect of Citrus Fruit Antioxidants in Protecting Osteoblasts from CSM-Damage
As a diet rich in fruits and vegetables will provide a great variety of antioxidants we investigated the combined effect of vitamin C, naringenin, naringin and/or hesperidin on CSM-induced damage in primary human osteoblasts (N = 3, n = 4). In all three settings the combined treatment with the antioxidants further increased the protective effect. Especially the combination of vitamin C and naringin and/or naringenin strongly improved survival of CSM-treated osteoblasts in all three settings. In the preand co-incubation setting a combination of all 4 antioxidants reached the best effect, with over 70% viability. Interestingly, in the post-incubation setting the combination of vitamin C, naringenin and naringin showed best protection, with approximately 80% viability. Further addition of hesperidin could not further improve the outcome (Figure 5).
4. DISCUSSION
In the here presented work we complemented our already published data with hitherto unpublished data, such that we could compare the protective effect of 15 antioxi-

Figure 4. Post-incubation with antioxidants increases viability in CSM treated primary human osteoblasts. Primary human osteoblasts (N = 3, n = 4) were pre-treated CSM (OD320 = 0.8) for 4 h before stimulation with 15 different antioxidants at defined concentrations (see Table 1). After 4 h incubation with the different antioxidants viability was determined by resazurin conversion. All substances investigated improved viability of CSM-treated osteoblasts. ***p < 0.001 as compared to cells not treated with antioxidants.
dants in CSM-treated primary human osteoblasts. Our results clearly demonstrate that the toxic effect of CSM on primary human osteoblasts is related to an increased production of ROS. According to recent experimental findings this plays a key role in clinical observations such as delayed fracture healing, increased rate of osteoporosis, osteopenia or reduced bone mass [3-6,8,28, 29]. In our experiments all the investigated antioxidants were able to protect primary human osteoblasts from CSM-induced damage. Thus a therapy based on antioxidants can support fracture healing by increasing survival of osteoblasts under oxidative stress. Following clinical approval this might revolutionize the initial treatment of fractures in smokers. Latest data clearly support this result, showing that cellular stress defense is positively modulated by antioxidant-rich food in human cigarette smokers [30]. The effectiveness in our experiments, however, was strongly dependent on the experimental setting. Overall the protective effect of the investigated substances inversely correlated with their ability to reduce formation of ROS.
While the small chemical molecule NAC was most effective in the preand co-incubation setting, fruit and vegetable flavonoids proofed to be more effective in the post-incubation setting. This might be due to the fact,
 (a)
(a) (b)
(b)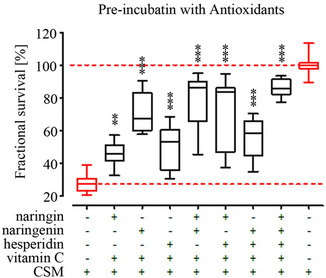 (c)
(c)
Figure 5. Combination of citrus fruit flavonoids further improves survival of CSM treated primary human osteoblasts in the pre-, coand post-incubation setting. Primary human osteoblasts (N = 3, n = 4) were treated with the citrus fruit ingredients vitamin C, hesperidin, naringenin and naringin (concentrations see Table 1) at different combinations for 4 h (a) before; (b) with or (c) after stimulation with CSM (OD320 = 0.8). A combination of the different substances strongly improved survival of the CSM-treates osteoblasts in all three settings. **p < 0.01, ***p < 0.001 as compared to cells not treated with antioxidants.
that NAC is precursor of glutathione with high direct radical scavenging properties, which can be easily taken up by the cells due to its small chemical structure. This is supported by clinical report showing a beneficial effect of NAC on organs, e.g. heart, liver, kidneys and pancreas, after abdominal surgery, mainly mediated by a reduction in ROS production [31-35]. Similar is observed with the two main active ingredients of garlic oil, DAS and DADS. They had a great potential to protect primary human osteoblasts from CSM-induced damage in the preand coincubation setting. Thus application of NAC, DAS or DADS is more effective in prophylactic or acute therapy. DAS and DADS have been shown to also increase the anti-oxidative enzymes heme-oxygenase (HO)-1 and superoxide-dismutase (SOD)-1 in primary human osteoblasts [18], thus protecting the cells not only by direct radical scavenging but also by up-regulating the cellular antioxidative defense mechanisms [36]. This is supported by the finding of Chae and co-workers that show that HO-1 is necessary to protect osteoblasts from tumor necrosis factor (TNF)-alpha-induced apoptosis [37]. Although, the work from Lin and colleagues suggests an inhibitory effect of HO-1 on osteoblasts maturation and mineralization [38], Bargallo and co-workers were able to show improvement of osteogenic stem cell differentiation by HO-1 [39]. Furthermore, garlic has been ascribed antiosteoporotic properties [40,41] mainly by its function as phytoestrogen. This is in accordance with recent epidemiological and experimental findings demonstrating positive effects of garlic on bone, e.g. increased BMD in ovariectomized rats either by improved calcium uptake, estrogenic functions, inhibiting osteoclasts [40-43] or by reducing oxidative stress [44]. So the garlic components DAS and DADS are not only suitable for acute therapy but also for long term prophylaxis. Similar is reported for the antioxidants with flavonoid structure, e.g. quercetin [17], resveratrol or catechin [19]. These substances had already good protective effects in our pre-incubation setting, but especially in the coand post-incubation setting they almost completely recovered the osteoblasts from CSM-induced oxidative stress. This might be due to their potential to induce the cellular anti-oxidative defense mechanisms, comparable to DAS and DADS [17,19]. The protective effect of quercetin is supported by the findings of Wattel et al. showing inhibitory effects of Quercetin on bone resorption by inhibiting osteoclast activity [45]. Similarly, for catechin, there are several in vivo studies reporting a positive effect on green tea consumption on osteoporosis [46-50]. Interestingly, the spice ingredients anisole, cinnamic acid and vanillic acid had a better affectivity in the post-incubation setting compared to the preor co-incubation setting. Due to their relatively small chemical structure these substances might be easily taken up by the cells. As they do not have as many reactive groups as the other substances investigated, they are limited in their use as direct radical scavenger. Adversely, antioxidants from citrus fruit well protected the osteoblasts from CSM in the preand co-incubation setting. However, similar to NAC this effect was reduced in the post-incubation setting. Suggesting, that these substances due to their structure are great radical scavengers but not necessarily modulate cellular anti-oxidative defense mechanisms. Noteworthy, a combinatory treatment of the citrus fruit flavonoids further improved the protective effect on CSM-treated osteoblasts. This is of special importance as a diet rich in fruits and vegetables will provide a great variety of antioxidants (partly flavonoids) that accumulate in the body. The flavonoid antioxidants can than protect the cells by inducing the cellular anti-oxidative defense mechanisms, e.g. HO-1 and SOD-1 [37,39]. This is supported by the finding that several vegetables and rutin, a quercetin glycoside, effectively inhibit bone resorption in vivo in ovariectomized rats [51,52].
Besides these positive effects on CSM-treated osteoblasts one has to take into account that we used the maximum dose of antioxidants that is non-toxic to the cells. These concentrations might not be reached in patients by a simple diet rich in fruits and vegetables. Thus, today the market for flavonoid supplements is booming [22] with suppliers recommending daily flavonoid intakes in amounts that are many times higher than those doses which can normally be achieved from a flavonoid-rich diet [23]. As an example, the recommended daily uptake of Quercetin is up to 1 g and more [24], but the regular quercetin intake from daily diet has been estimated to be below 100 mg [25].
In summary our data suggest that a balanced diet rich in fruits, vegetables, spices and herbs in the long run can protect the skeleton from bone mineral loss due to their phytoestrogenic properties and, especially in smokers, due to a decreased oxidative stress. The observed effect is mainly due to a combination and accumulation of active substances. However, this might not be sufficient for clinical practice, protecting the already damaged bone from further damage due to oxidative stress, as the dietary flavonoids often exert their activity by up-regulating the cellular anti-oxidative defense mechanisms. For use as acute therapeutic in clinical applications small molecules, e.g. NAC, are superior to these natural flavonoids as they are already established drugs which can be administered directly in higher doses. In abdominal surgery NAC is more and more used to protect the inner organs from increased oxidative stress due to surgery [31-35]. Thus, we propose NAC as possible treatment agent to reduce bone damage after orthopedic or trauma surgery in order to support recovery of the patients. This during rehab might be complemented and finally substituted with a balanced diet of fruits, vegetables, herbs and spices, rich in flavonoids and antioxidants.
REFERENCES
- Clarke, B. (2008) Normal bone anatomy and physiology. Clinical Journal of the American Society of Nephrology, 3, S131-S139. doi:10.2215/CJN.04151206
- Mokdad, A.H., Marks, J.S., Stroup, D.F. and Gerberding, J.L. (2005) Correction: Actual causes of death in the United States, 2000. The Journal of the American Medical Association, 293, 293-294. doi:10.1001/jama.293.3.293
- Akhter, M.P., Lund, A.D. and Gairola, C.G. (2005) Bone biomechanical property deterioration due to tobacco smoke exposure. Calcified Tissue International, 77, 319-326. doi:10.1007/s00223-005-0072-1
- Cesar-Neto, J.B., Benatti, B.B., Manzi, F.R., Sallum, E.A., Sallum, A.W. and Nociti, F.H. (2005) The influence of cigarette smoke inhalation on bone density. A radiographic study in rats. Brazilian Oral Research, 19, 47-51. doi:10.1590/S1806-83242005000100009
- Cesar-Neto, J.B., Duarte, P.M., Sallum, E.A., Barbieri, D., Moreno, H., Jr. and Nociti, F.H., Jr. (2003) A comparative study on the effect of nicotine administration and cigarette smoke inhalation on bone healing around titanium implants. Journal of Periodontology, 74, 1454-1459. doi:10.1902/jop.2003.74.10.1454
- Rapuri, P.B., Gallagher, J.C., Balhorn, K.E. and Ryschon, K.L. (2000) Smoking and bone metabolism in elderly women. Bone, 27, 429-436. doi:10.1016/S8756-3282(00)00341-0
- Schmitz, M.A., Finnegan, M., Natarajan, R. and Champine, J. (1999) Effect of smoking on tibial shaft fracture healing. Clinical Orthopaedics and Related Research, 184-200. doi:10.1097/00003086-199908000-00024
- Ueng, S.W., Lin, S.S., Wang, C.R., Liu, S.J., Tai, C.L. and Shih, C.H. (1999) Bone healing of tibial lengthening is delayed by cigarette smoking: Study of bone mineral density and torsional strength on rabbits. The Journal of Trauma, 46, 110-115. doi:10.1097/00005373-199901000-00018
- Ward, K.D. and Klesges, R.C. (2001) A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcified Tissue International, 68, 259-270. doi:10.1007/BF02390832
- Nakamura, Y., Romberger, D.J., Tate, L., Ertl, R.F., Kawamoto, M. and Adachi, Y. (1995) Cigarette smoke inhibits lung fibroblast proliferation and chemotaxis. American Journal of Respiratory and Critical Care Medicine, 151, 1497-1503.
- Wang, H., Liu, X., Umino, T., Skold, C.M., Zhu, Y. and Kohyama, T. (2001) Cigarette smoke inhibits human bronchial epithelial cell repair processes. American Journal of Respiratory Cell and Molecular Biology, 25, 772-779.
- Cesar-Neto, J.B., Duarte, P.M., de Oliveira, M.C., Tambeli, C.H., Sallum, E.A. and Nociti, F.H., Jr. (2007) Smoking modulates interleukin-6:interleukin-10 and RANKL:osteoprotegerin ratios in the periodontal tissues. Journal of Periodontal Research, 42, 184-191. doi:10.1111/j.1600-0765.2006.00934.x
- Giorgetti, A.P., Cesar Neto, J.B., Ruiz, K.G., Casati, M.Z., Sallum, E.A. and Nociti, F.H., Jr. (2011) Cigarette smoke inhalation modulates gene expression in sites of bone healing: A study in rats. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology, 110, 447-452.
- Kim, H., Liu, X., Kobayashi, T., Conner, H., Kohyama, T. and Wen, F.Q. (2004) Reversible cigarette smoke extractinduced DNA damage in human lung fibroblasts. American Journal of Respiratory Cell and Molecular Biology, 31, 483-490. doi:10.1165/rcmb.2002-0300OC
- Mortaz, E., Kraneveld, A.D., Smit, J.J., Kool, M., Lambrecht, B.N. and Kunkel, S.L. (2009) Effect of cigarette smoke extract on dendritic cells and their impact on Tcell proliferation. PLoS One, 4, e4946. doi:10.1371/journal.pone.0004946
- Rothem, D.E., Rothem, L., Soudry, M., Dahan, A. and Eliakim, R. (2009) Nicotine modulates bone metabolismassociated gene expression in osteoblast cells. Journal of Bone and Mineral Metabolism, 27, 555-561. doi:10.1007/s00774-009-0075-5
- Braun, K.F., Ehnert, S., Freude, T., Egana, J.T., Schenck, T.L. and Buchholz, A. (2011) Quercetin protects primary human osteoblasts exposed to cigarette smoke through activation of the antioxidative enzymes HO-1 and SOD-1. Scientific World Journal, 11, 2348-2357. doi:10.1100/2011/471426
- Ehnert, S., Braun, K.F., Buchholz, A., Freude, T., Egana, J.T. and Schenck, T.L. (2012) Diallyl-disulfide is the effective ingredient of garlic oil that protects primary human osteoblasts from damage due to cigarette smoke. Food Chemistry, 132, 724-729. doi:10.1016/j.foodchem.2011.11.008
- Holzer, N., Braun, K.F., Ehnert, S., Egana, J.T., Schenck, T.L. and Buchholz, A. (2012) Green tea protects human osteoblasts from cigarette smoke-induced injury: Possible clinical implication. Langenbeck’s Archives of Surgery, 397, 467-474. doi:10.1007/s00423-011-0882-8
- Christen, P. and Cuendet, M. (2012) Plants as a source of therapeutic and health products. Chimia (Aarau), 66, 320- 323. doi:10.2533/chimia.2012.320
- Huxley, R.R. and Neil, H.A. (2003) The relation between dietary flavonol intake and coronary heart disease mortality: A meta-analysis of prospective cohort studies. European Journal of Clinical Nutrition, 57, 904-908. doi:10.1038/sj.ejcn.1601624
- Espin, J.C., Garcia-Conesa, M.T. and Tomas-Barberan, F.A. (2007) Nutraceuticals: Facts and fiction. Phytochemistry, 68, 2986-3008. doi:10.1016/j.phytochem.2007.09.014
- Manach, C., Williamson, G., Morand, C., Scalbert, A. and Remesy, C. (2005) Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. The American Journal of Clinical Nutrition, 81, 230S-242S.
- Harwood, M., Danielewska-Nikiel, B., Borzelleca, J.F., Flamm, G.W., Williams, G.M. and Lines, T.C. (2007) A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food and Chemical Toxicology, 45, 2179-2205. doi:10.1016/j.fct.2007.05.015
- Scalbert, A. and Williamson, G. (2000) Dietary intake and bioavailability of polyphenols. Journal of Nutrition, 130, 2073S-2085S.
- Ehnert, S., Baur, J., Schmitt, A., Neumaier, M., Lucke, M. and Dooley, S. (2010) TGF-beta1 as possible link between loss of bone mineral density and chronic inflammation. PLoS One, 5, e14073. doi:10.1371/journal.pone.0014073
- Roemer, E., Stabbert, R., Rustemeier, K., Veltel, D.J., Meisgen, T.J. and Reininghaus, W. (2004) Chemical composition, cytotoxicity and mutagenicity of smoke from US commercial and reference cigarettes smoked under two sets of machine smoking conditions. Toxicology, 195, 31-52. doi:10.1016/j.tox.2003.08.006
- Iwaniec, U.T., Fung, Y.K., Akhter, M.P., Haven, M.C., Nespor, S. and Haynatzki, G.R. (2001) Effects of nicotine on bone mass, turnover, and strength in adult female rats. Calcified Tissue International, 68, 358-364. doi:10.1007/s00223-001-0011-8
- Lee, L.L., Lee, J.S., Waldman, S.D., Casper, R.F. and Grynpas, M.D. (2002) Polycyclic aromatic hydrocarbons present in cigarette smoke cause bone loss in an ovariectomized rat model. Bone, 30, 917-923. doi:10.1016/S8756-3282(02)00726-3
- Bohn, S.K., Myhrstad, M.C., Thoresen, M., Holden, M., Karlsen, A. and Tunheim, S.H. (2011) Blood cell gene expression associated with cellular stress defense is modulated by antioxidant-rich food in a randomised controlled clinical trial of male smokers. BMC Medicine, 8, 54. doi:10.1186/1741-7015-8-54
- Hynninen, M.S., Niemi, T.T., Poyhia, R., Raininko, E.I., Salmenpera, M.T. and Lepantalo, M.J. (2006) N-acetylcysteine for the prevention of kidney injury in abdominal aortic surgery: A randomized, double-blind, placebo-controlled trial. Anesthesia & Analgesia, 102, 1638-1645. doi:10.1213/01.ANE.0000219590.79796.66
- Mahmoud, K.M. and Ammar, A.S. (2011) Effect of Nacetylcysteine on cardiac injury and oxidative stress after abdominal aortic aneurysm repair: A randomized controlled trial. Acta Anaesthesiologica Scandinavica, 55, 1015-1021.
- Moore, N.N., Lapsley, M., Norden, A.G., Firth, J.D., Gaunt, M.E. and Varty, K. (2006) Does N-acetylcysteine prevent contrast-induced nephropathy during endovascular AAA repair? A randomized controlled pilot study. Journal of Endovascular Therapy, 13, 660-666. doi:10.1583/06-1833.1
- Narasimhan, S., Khwaja, H.A., Dutta, S. and Mitchenere, P. (2008) Use of N-acetylcysteine for postnecrosectomy peripancreatic collections in a patient with severe, acute pancreatitis. Canadian Journal of Surgery, 51, E133-134.
- Ozdil, B., Kece, C., Cosar, A., Akkiz, H. and Sandikci, M. (2011) Potential benefits of combined N-acetylcysteine and ciprofloxacin therapy in partial biliary obstruction. The Journal of Clinical Pharmacology, 50, 1414-1419. doi:10.1177/0091270010361257
- Williams, R.J., Spencer, J.P. and Rice-Evans, C. (2004) Flavonoids: Antioxidants or signalling molecules? Free Radical Biology & Medicine, 36, 838-849. doi:10.1016/j.freeradbiomed.2004.01.001
- Chae, H.J., Chin, H.Y., Lee, G.Y., Park, H.R., Yang, S.K. and Chung, H.T. (2006) Carbon monoxide and nitric oxide protect against tumor necrosis factor-alpha-induced apoptosis in osteoblasts: HO-1 is necessary to mediate the protection. Clinica Chimica Acta, 365, 270-278. doi:10.1016/j.cca.2005.09.011
- Lin, T.H., Tang, C.H., Hung, S.Y., Liu, S.H., Lin, Y.M. and Fu, W.M. (2011) Upregulation of heme oxygenase-1 inhibits the maturation and mineralization of osteoblasts. Journal of Cellular Physiology, 222, 757-768.
- Barbagallo, I., Vanella, A., Peterson, S.J., Kim, D.H., Tibullo, D. and Giallongo, C. (2011) Overexpression of heme oxygenase-1 increases human osteoblast stem cell differentiation. Journal of Bone and Miner Metabolism, 28, 276-288. doi:10.1007/s00774-009-0134-y
- Mukherjee, M., Das, A.S., Das, D., Mukherjee, S., Mitra, S. and Mitra, C. (2007) Role of peritoneal macrophages and lymphocytes in the development of hypogonadal osteoporosis in an ovariectomized rat model: Possible phytoestrogenic efficacy of oil extract of garlic to preserve skeletal health. Phytotherapy Research, 21, 1045-1054. doi:10.1002/ptr.2209
- Mukherjee, M., Das, A.S., Mitra, S. and Mitra, C. (2004) Prevention of bone loss by oil extract of garlic (Allium sativum Linn.) in an ovariectomized rat model of osteoporosis. Phytotherapy Research, 18, 389-394. doi:10.1002/ptr.1448
- Mukherjee, M., Das, A.S., Das, D., Mukherjee, S., Mitra, S. and Mitra, C. (2006) Role of oil extract of garlic (Allium sativum Linn.) on intestinal transference of calcium and its possible correlation with preservation of skeletal health in an ovariectomized rat model of osteoporosis. Phytotherapy Research, 20, 408-415. doi:10.1002/ptr.1888
- Mukherjee, M., Das, A.S., Das, D., Mukherjee, S., Mitra, S. and Mitra, C. (2006) Effects of garlic oil on postmenopausal osteoporosis using ovariectomized rats: Comparison with the effects of lovastatin and 17beta-estradiol. Phytotherapy Research, 20, 21-27. doi:10.1002/ptr.1795
- Assayed, M.E., Khalaf, A.A. and Salem, H.A. (2010) Protective effects of garlic extract and vitamin C against in vivo cypermethrin-induced cytogenetic damage in rat bone-marrow. Mutation Research, 702, 1-7. doi:10.1016/j.mrgentox.2010.02.020
- Wattel, A., Kamel, S., Prouillet, C., Petit, J.P., Lorget, F. and Offord, E. (2004) Flavonoid quercetin decreases osteoclastic differentiation induced by RANKL via a mechanism involving NF kappa B and AP-1. Journal of Cellecular Biochemistry, 92, 285-295. doi:10.1002/jcb.20071
- Devine, A., Hodgson, J.M., Dick, I.M. and Prince, R.L. (2007) Tea drinking is associated with benefits on bone density in older women. The American Journal of Clinical Nutrition, 86, 1243-1247.
- Hamdi Kara, I., Aydin, S., Gemalmaz, A., Akturk, Z., Yaman, H. and Bozdemir, N. (2007) Habitual tea drinking and bone mineral density in postmenopausal Turkish women: Investigation of prevalence of postmenopausal osteoporosis in Turkey (IPPOT Study). International Journal for Vitamin and Nutrition Research, 77, 389-397. doi:10.1024/0300-9831.77.6.389
- Hegarty, V.M., May, H.M. and Khaw, K.T. (2000) Tea drinking and bone mineral density in older women. The American Journal of Clinical Nutrition, 71, 1003-1007.
- Shen, C.L., Chyu, M.C., Yeh, J.K., Felton, C.K., Xu, K.T. and Pence, B.C. (2009) Green tea polyphenols and Tai Chi for bone health: Designing a placebo-controlled randomized trial. BMC Musculoskeletal Disorders, 10, 110. doi:10.1186/1471-2474-10-110
- Shen, C.L., Yeh, J.K., Cao, J.J. and Wang, J.S. (2009) Green tea and bone metabolism. Nutrition Research, 29, 437-456. doi:10.1016/j.nutres.2009.06.008
- Muhlbauer, R.C., Lozano, A., Reinli, A. and Wetli, H. (2003) Various selected vegetables, fruits, mushrooms and red wine residue inhibit bone resorption in rats. Journal of Nutrition, 133, 3592-3597.
- Horcajada-Molteni, M.N., Crespy, V., Coxam, V., Davicco, M.J., Remesy, C. and Barlet, J.P. (2000) Rutin inhibits ovariectomy-induced osteopenia in rats. Journal of Bone and Mineral Research, 15, 2251-2258. doi:10.1359/jbmr.2000.15.11.2251
NOTES
*Contributed equally.

