Journal of Environmental Protection
Vol.4 No.1(2013), Article ID:27185,11 pages DOI:10.4236/jep.2013.41008
Heavy Metal Contaminations in Urban Soil within Baghdad City, Iraq
![]()
Environmental Research Center, University of Technology, Baghdad, Iraq.
Email: jawaddh@yahoo.co.in, athmarjaffer@yahoo.com
Received October 9th, 2012; revised November 10th, 2012; accepted December 11th, 2012
Keywords: Urban Soils; Heavy Metal; Pollution Index; Enrichment Factor, Soil Pollution
ABSTRACT
Soil samples were collected from three land use types within Baghdad urban areas. The samples analyzed for Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn indicated higher concentration of Cd, Cr, Cu, Fe, Ni, Pb and Zn in the industrial area, while higher concentration of Mn was observed in the residential areas. However, the concentration of Cd, Ni and Pb was higher than the calculated worldwide mean of unpolluted soil. For both roadside and open areas soils, industrial area exhibited high values of Cd, Ni and Pb. The highest Single Element Pollution Index (SEPI) for Cd, Ni and Pb in the industrial soils seems therefore to be that this type of soil is the most polluted in the city of Baghdad. The calculated Combined Pollution Index (CPI) for Cd, Ni and Pb ranged from 0.98 to 2.15 with a mean of 1.28 for the entire urban soil samples, with the highest values in the industrial area which suggest multi-element contamination and in some cases are recommended for treatment. Furthermore, significant to extremely high values of enrichment factors were recorded confirming an important role of anthropogenic pollution.
1. Introduction
As the urban area has high population density and intensive anthropogenic activities, it is reported that urban surfaces receive deposits issued from more or less remote sources such as vehicle emissions, industrial discharges, energy production, waste disposal, and other human anthropogenic activities [1-4].
Urban soil is an important component of the urban ecosystem [5], and can be considered both as a sink of pollutants and a source of pollution with the capacity to transfer pollutants to groundwater, into the food chain and into the human body [6-8]. Thus, urban soils were used as an indicator of urban environmental quality [9]. Moreover, in urban soil influences both the quality of life and the health of people [10]. Therefore, it is indubitable that heavy metal concentrations in urban soils are significant environmental issue.
It is reported that heavy metals are the most common contaminants in the urban soil that may come from various anthropogenic activities [11,12]. The interest in the characteristics of urban soils has increased greatly in the last two decades, and investigations of urban soils in many cities around the world have reported elevated concentrations of heavy metals in general [13-18].
Surveys on the distribution and concentration of heavy metals in the urban soils are important for planning management strategies to achieve better urban environmental quality and to control the risk associated with the excessive increase of heavy metals in the environment. Furthermore, such knowledge could be used for making accurate risk assessments concerning human health and long-term ecological effects, for setting limit values of heavy metals and for identifying priorities concerning the remediation of contaminated sites [19]. However, the available information on heavy metals in urban soil is generally insufficient in Iraq. Therefore, the present work was planned with an aim to study the level of Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn in the urban soil resulting from different land use patterns, and to assess the degree of heavy metals contamination.
2. Materials & Methods
The Capital of Iraq Baghdad City (33˚14'-33˚25'N, 44˚31'-44˚17'E), is located in the Mesopotamian alluvial plain. Tigris River divides the city into a right (Karkh) and left (Risafa) sections with a flow direction from north to south (Figure 1). It is characterized by arid to semi arid climate with dry hot summers and cold winters; the mean annual rainfall is about 151.8 mm [20]. For the purpose of collection of soil samples, the study area was divided in three main types of land use viz. residential, commercial, and industrial; and two main source areas
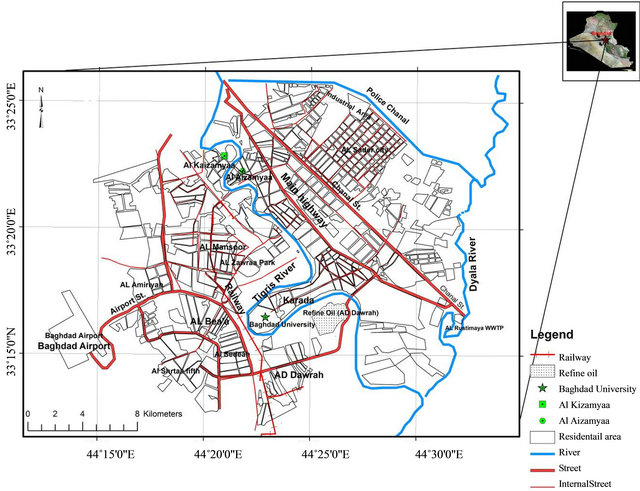
Figure 1. Map of Baghdad City.
within each land use type viz. roadside and open areas. Control soil sample was collected from a rural soil area. Soil samples were collected two times in a year during summer and winter seasons during 2009 and 2010.
Twenty soil samples (0 - 20 cm) were carefully collected from each source area in different land use types with a stainless steel spatula. They were air-dried in the laboratory, homogenized and sieved through a 2-mm polyethylene sieve to remove large debris, stones and pebbles, after they were disaggregated with a porcelain pestle and mortar. Then these samples were stored in clean selfsealing plastic bags for further analysis. pH for all samples was measured in a soil distilled water suspension in the ratio (w:v). sample (1): distilled water (2.5) by a calibrated pH meter [21]. Soil samples were wet-digested using a combination of HCIO4 and HNO3 [22]. Metal determinations were done by Atomic Absorption Spectrometry (AAS 6300, Shimadzu, Japan).
3. Results & Discussion
Table 1 summarizes the results for the heavy metals content in the soil samples. With some exceptions, all the measured parameters have mean values in excess of median values and high standard deviation reflecting positively skewed distribution and a high degree of variation. An explanation of the observed metals follows with the term concentration referring to median concentration, wherever employed.
3.1. Effect of pH
Various physico-chemical and biological factors control the mobility of metals in soils [23]. They suggested that a change in pH results in a transfer of element from one phase to another and thus permits the estimation of mobility of heavy metals in the soil. The results show that urban soil pH, ranging in a narrow interval (7.79 to 7.80), suggests that urban soil is mostly in neutral to sub-alkaline condition, which can be attributed to the high content of carbonate, ash and cinders of anthropogenic origin [24,25], and could be partly explained by extraneous materials such as bricks and construction debris included in the soil that could increase the pH [26]. The neutral to sub-alkaline condition of the urban soil may be related to the alkali components in the atmosphere which can eventually deposit on the ground and affect the soil pH [27]. Urban soil with an alkaline reaction was observed to be quite common [28,29]. The soil pH seems to have higher effect on the solubility or met als retention in soil;
Table 1. Statistical summary of soil-quality data.

ND: not detected, (pH measured with standard unit, all metals with mg/kg).
the greater retention and lower solubility of metal occurs at high soil pH [30].
3.2. Heavy Metals
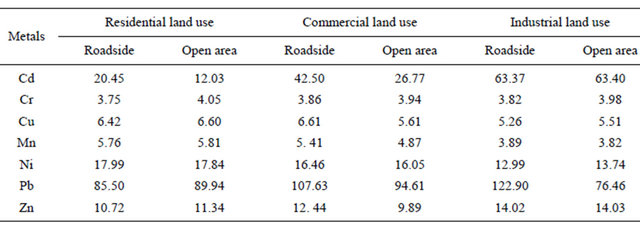
The results of occurrence of the metals indicated that Fe has emerged as the dominant metal, while Cd has the lowest concentration in soil in all types of land use and source areas. It is observed from the residential roadside, open area and commercial open area soil that the median values of the metals followed the sequence: Fe > Mn > Ni > Pb > Zn > Cr > Cu > Cd, while the median values of the metals in the commercial roadside follow the same order except Pb which has values higher than Ni. The industrial roadside soil followed the sequence: Fe > Mn > Pb >Zn > Ni > Cr > Cu > Cd, whereas the industrial open area soils followed the same order except Ni and Zn which have higher values than Pb. From all the data it can be noted that residential soils followed the order: Fe > Mn > Ni > Pb> Zn > Cr > Cu > Cd, whereas the metals in the commercial and industrial areas followed the same order except for the Pb which has a higher median values than Ni.
A comparison of metal concentration in soil among various types of land use indicated that higher concentration of Cd, Cr, Cu, Fe, Ni, Pb and Zn was observed in the industrial area, while higher concentration of Mn was observed in the residential. A look at Table 2 also indicates that roadside and open area soil in the industrial land use display higher values for most of the metals. This reflects a significant impact of industrial activities on the local environment [1,3]. A brief discussion about individual metals follows:
Cadmium: Some metals, such as Cd, accumulate in the human body over a long period of time so that negative effects may appear only after a long period of chronic exposure. Cd is highly mobile and toxic, which means that the few maxima found are critical values [31]. The Cd content varies from 0.14 to 1.05 mg/kg. The observed values in the industrial roadside and open areas soils exceed the calculated worldwide mean of non-polluted soil (0.53 mg/kg) reported after analytical surveys [32]. Concentrations above 0.5 mg/kg could reflect the influence of the human activity [33]. Human activity can contribute to increased Cd levels as a result of urbanindustrial activity and/or agricultural practices [34].
The Cd content in all soil samples was also observed to be 8.8 times higher than the values in rural soil, which contains lower amount of Cd (0.05 mg/kg). It was reported that inputs of Cd into soils maybe of different origins such as agricultural amendments, sludge and atmospheric deposition [35]. Cadmium has a wide range of uses in the industry, including paints, pigments, electroplating and plastic stabilizer [36] and many anthropogenic activities can increases soil Cd to the levels well above background levels, such as the application of solid waste from industries and home and sewage sludge, wastewater irrigation and phosphate fertilizer application had resulted in the release of significant quantities of Cd to the environment [37].
Chromium: Cr is a low mobility element, especially under moderately oxidizing and reducing conditions and near-neutral pH values. Cr6+ adsorption decreases with increasing pH, and Cr3+ adsorption increases with increaseing pH. On the other hand, Cr (VI) is toxic for biological systems [32]. Variety of small large scale industrial activities e.g. metal plating, anodizing, dyes, pigments, ceramic, glues, tanning, wood preserving and textiles are reported to contribute Cr [35].
The Cr content varies from 21.98 to 32.92 mg/kg. The observed values were below the average value from the world literature (84.0 mg /kg) [35] and also less than the reported world scale of unpolluted soils (83.0 mg/kg) [32]. However, Cr median values in the all study area soil samples were observed to be 1.6 times higher than chromium content in the rural (control) soil (17.33 mg/kg) suggesting possible sources of Cr in the urban area. The normal Cr range in calcareous soils is 5 to 16 mg/kg [32,33]. However, the clay-rich soil can increase the normal contents of Cr in soils [35].
Copper: Cu is used in numerous applications because of its physical properties. The toxicity for humans is not very high [38]. Cu normally accumulates in the surface horizons, a phenomenon explained by the bioaccumulation of the metal and recent anthropogenic sources.
The observed values of the Cu content did not exceed the normal threshold value prescribed in soil (20 - 30 mg/kg) [35], and also is within the typical world scale of non-polluted soil (24 mg/kg) reported by Kabata-Pendias & Pendias [32]. Cu concentration in the all soil samples was 1.9 times higher than the Cu concentrations in the rural soil (11.18 mg/kg). Contribution of Cu may be envisaged from dumping or accumulation of solid waste, application of fungicides, live stock manures, sludges and
Table 2. Effect of land use on metal occurrence.
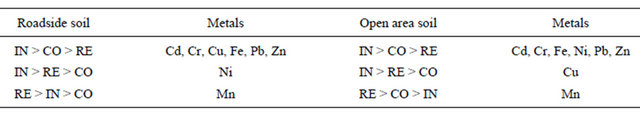
RE: Residential, CO: Commercial, IN: Industrial atmospheric deposition [35].
Iron: There is no limit to the concentration of iron in soil because it is abundant in soil. Iron was found to be in high concentrations in most of the soil samples. This can be attributed to the soil in the investigated area being rich in iron.
Manganese: The Mn content varies from 300.98 to 321.14 mg/kg. The observed values were also less than the reported average of unpolluted soil (525 mg/kg) [32]. Comparison with Mn content in the rural soil indicated that Mn concentration in the study area soil samples was observed to be 1.7 times higher than Mn content in the rural soil (182.15 mg/kg).
Nickel: Ni is widely used in electroplating and in the manufacture of batteries. Its toxicity for human beings is not very high, but it can cause respiratory diseases [38].
Ni content varies from 72.63 to 85.32 mg/kg. The observed values are higher than the world average concentration of Ni in soil which is around 20 mg/kg [35]. Results exceed the calculated world mean of unpolluted soil (34 mg/kg) [32]. Further, considering the analyzed values of rural (control) as 35.75, indicated than Ni median concentration in the all samples of the study area was observed to be 2.2 times than Ni content in the rural soil. It is evident that local solid waste and anthropogenic activities such as burning of fuel contribute to the increase in Ni content in the soil of the study area. It may be noted that many domestic cleaning products, e.g. soap, 100 - 700 mg Ni/kg; powdered detergents, 400 - 700 mg Ni/kg and powdered bleach, 800 mg Ni/kg, may prove to be important sources of Ni in the urban soils [35].
Lead: Pb is relatively low mobile element, but its mobility is restricted by tendency for adsorption to Fe-Mn oxides and insoluble organic matter. Lead is more active by formation of soluble organic complexes, or anionic complexes. It can enter the environment during numerous activities (mining, smelting, and manufacturing) and it can be very toxic for human health [38]. The species of Pb vary considerably with soil type; it is mainly associated with clay minerals, Mn oxides, Fe and Al hydroxides and organic matter. In some soil types, Pb may be highly concentrated in Ca carbonate particles or in phosphate concentrations [32].
The Pb content varies from 62.70 to 127.27 mg/kg. The observed values, although are higher than the calculated world average of unpolluted soils (44.0 mg/kg) [32], and are also higher than the observed values in the rural (control) soil (18.8 mg/kg). Deposition related to transportation sector in general (considering the long residence time of Pb) may be the major source of increase in Pb content in urban soil [39-40]. It is known that lead containing dust particles have a relatively short residence time in the atmosphere, and deposit quickly in the near vicinity of the road, hence contributing to further accumulation of lead on the roadside soil surface [41]. Pb has been shown to accumulate to high levels in urban environments from a range of sources including that derived from leaded petrol [42].
Zinc: Zn concentrations in soils are generally associated with Zn sulfide ores, Fe-Mn oxides, and mafic minerals such as hornblends and biotite, and chalcophile elements such as Cu and Pb in base metal and precious metal deposits [43]. Environmental contamination of Zn is mainly related to anthropogenic input. The anthropogenic sources of Zn are related to industries and the use of liquid manure, composted materials and agrochemicals such as fertilizers and pesticides in agriculture [44]. Zn may be derived from mechanical abrasion of vehicles, as they are used in the production of brass alloy itself and come from brake linings, oil leak sumps and cylinder head gaskets [45]. Some of the studies have also linked high Zn levels in urban soils to accumulation from garden fertilizing activities, traffic and industry input [40] and also vehicle emissions and tyre and brake abrasion [46].
The Zn content varies from 42.15 to 81.30 mg/kg. The observed values were reportedly within the common world range for total Zn concentrations in soil (10 - 300 mg/kg) [35], but are higher than the Zn content in rural (control) soil (16.15 mg/kg). Kabata-Pendias & Pendias [32] have reported the mean world Zn average for unpolluted soil as 100 mg/kg.
The investigation of soil heavy metal concentrations in roadside and open areas in the Baghdad City, Iraq, indicated that the concentrations of Cd, Ni and Pb in the soil often exceeded the calculated average mean for the world scale of unpolluted soil reported by Kabata-Pendias & Pendias [32]. Elevated concentrations of Cd, Ni and Pb in soils are commonly due to anthropogenic inputs. In fact, Cd, Ni and Pb soil pollution appears to be readily affected by anthropogenic factors [47] and have adverse effects on human health [48].
It has been stated that in general, soil pollution due to accumulation of pollutants over the years is not as severe as pollution resulting from accidental or deliberate spill of industrial wastes, but atmospheric deposition can affect large area, which depends on distribution of population and industrial activities. Hence it is important for regulatory agencies to focus their efforts on sources that alter soil composition [49]. Taking into account the paucity of available published literature on presence of heavy metals in urban soil, an attempt was made to compare the concentration of the above three metals with those from investigations conducted on soils of other urban areas (Table 3). Although this variation reflects the influence of several factors e.g. variation in parent material, soil association, traffic density and microclimatic
Table 3. Comparison of heavy metal concentration data (mg/kg) in Baghdad urban area with other data from different towns, median (mean).
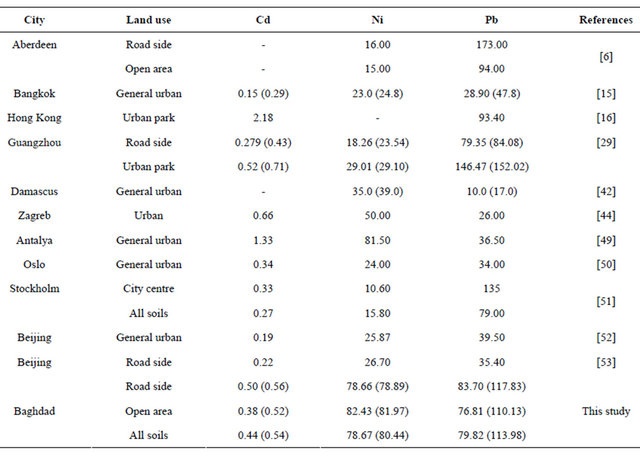
condition and nature of anthropogenic inputs etc. [46], yet it presents an interesting comparison. Noting that not much work is reported on urban soil in Iraq, urban soil in Baghdad (both roadside and open area) exhibited lower concentration of Cd than the reported values in Zagreb and Antalya urban soil and urban park of Guangzhou and Hong Kong, while urban soil in Baghdad area displays lower values of Ni than in Antalya urban soil. It can also observe that Baghdad urban soil exhibited lower values of Pb than the reported values of urban soil in Aberdeen, Stockholm and Hong Kong.
3.3. Quantification of Soil Pollution
Soil Pollution Index (SPI) may be used to quantify the degree of pollution of urban soil with respect to background. In this study, SPI was calculated in two ways as given below:
3.3.1. Single Pollution Index
An assessment index is generally applied to measure environmental quality of soil and one simple and wellknown index is the single element pollution index (SEPI) which was used as evaluation methods and to identify single-element contamination resulting in increased such metal toxicity. The formula used to calculate SEPI is as follows:
 (1)
(1)
The tolerable levels for soil suggested by KabataPendias & Pendias [32] and Kloke [54] were adopted as permissible levels and each heavy metal was classified as low contamination (SEPI ≤ 1), moderate contamination (1 < SEPI ≤ 3) or high contamination (SEPI > 3) [12].
The SEPI value of Cd in urban soils varied from 0.73 to 3.5 (Table 4) which indicated low or high contamination level with an average of 1.47 for all soil samples indicating moderate contamination level. All soil samples in the industrial land use were heavily polluted by Cd, but for roadside, open area were classified as moderate contamination level. The SEPI value of Ni ranged from 1.51 to 1.71 indicating moderate contamination with Ni. The SEPI value for Pb varied from 0.63 to 1.25 suggesting low to moderate contamination with Pb. The highest SEPI for Cd, Ni and Pb in the industrial area seems therefore to be that industrial soil is the most polluted in the study area.
3.3.2. Combined Pollution Index
It is generally agreed that most heavy metal contami-
Table 4. Single element pollution index values.

nation in the surface environment is associated with a mixture of contaminants rather than one metal contaminant [55]. Thus, the concept of a combined pollution index (CPI) which was used as another commonly evaluation methods of heavy metal accumulation and to identify multi-element contamination resulting in increased over all metal toxicity [56]. The CPI is calculated by the average ratio of metal concentrations in soil to assumed permissible level and was then classified as low (CPI ≤ 1), middle (1 < CPI ≤ 2) or high (CPI > 2) [12]. Furthermore, If CPI ≤ 1, it shows that there is no heavy metal accumulation in soil. If CPI > 1.0, it means that there is heavy metal accumulation in soil. The higher the CPI is the more serious heavy metal accumulation in soil. The tolerable levels for soil suggested by Kabata-Pendias & Pendias [32] and Kloke [54] were adopted as permissible levels and pollution index was calculated as:
 (2)
(2)
The value of CPI more than 1 indicates that metal concentrations are above the hazard criteria, the permissible level and exhibits a multi-contaminated element by anthropogenic inputs and soil recommended for treatment and continuous environmental monitoring of the area, whereas value less than 1 indicates that average levels of metals are below the selected standards but does not necessarily indicate that there are no anthropogenic sources or other enrichment over background, and suggested singular metal contamination [55].
A look at Table 5 shows that CPI is higher than 1 in all cases (with exception of residential land use) with extent of contamination being more in the case of roadside soil than the open area soil and industrial area than commercial and residential areas reflecting that roadside soil and industrial and commercial areas are more polluted by the heavy metals in the urban environment due to anthropogenic sources. Thus, it is very likely that many urban soils in Baghdad city are moderately or high-
Table 5. Combined pollution index values.

ly polluted with heavy metals.
3.3.3. Soil Enrichment Factors
In this study enrichment factor was used to assess the level of contamination and the possible anthropogenic impact in Baghdad urban soils. Thus to determine the relative degree of metal contamination, comparisons were made to background concentrations in the earth’s crust using Fe as reference element following the assumption that its content in the crust has not been disturbed by anthropogenic activity, and it has been chosen as the element of normalization because natural sources (98%) vastly dominate its input [57]. The earth’s crust data of Riley and Chester [58] were used. Deely and Fergusson [59] proposed Fe as an acceptable normalization element to be used in the calculation of the enrichment factor since they considered the Fe distribution was not related to other heavy metals. The process of standardization helps in evaluating the anthropogenic component over and above the natural component. The EFs were calculated according to the equation generalized from Zoller et al. [60]:
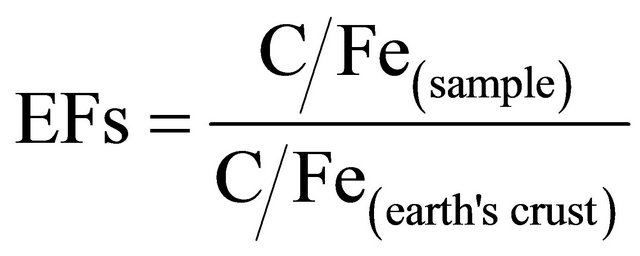 (3)
(3)
where EFs is the enrichment factor, C/Fe (sample) is the ratio of metal and Fe concentration of the sample and C/Fe(earth’s crust) is the ratio of metals and Fe concentration of the earth crust. Five contamination categories are recognized on the basis of the enrichment factor: EF < 2 states deficiency to minimal enrichment, EF = 2 - 5 moderate enrichment, EF = 5 - 20 significant enrichment, EF = 20 - 40 very high enrichment and EF > 40 extremely high enrichment [61].
It can be noted that the EF values significantly higher than 1 indicate an origin of heavy metals and may not come from the local soil background but other natural and/or anthropogenic sources in urbanized areas, includeing vehicle emissions, industrial discharges and other activities [1-3].
The EF results are presented in Table 6. Normally, as
Table 6. Enrichment factors against earth’s crust.
the EF values increase, the contributions of the anthropogenic origins also increase. Therefore, the high values of EF (3.75 - 122.90) show that the significant heavy metal pollution was likely to originate from the anthropogenic activities. Furthermore, the results of EF indicated that the urban soils of the study area were highly enriched with metals such as Pb which was observed to show extremely high enrichment, and Cd exhibit very high to extremely high enrichment, while Cu. Mn, Ni and Zn were observed to show significant enrichment.
It can be concluded from Table 7 that Pb followed by Cd was the most serious enriched heavy metals in the Baghdad urban soils. Lead is well known to be one of the less mobile elements in soils, which could explain the high values still found in urban soils. Moreover, among the metals considered for this study, it is the most toxic for humans, so the risks of its potential entry in the food chain must be carefully considered [4].
Atmospheric pollution is one of the major sources of heavy metal contamination. Heavy metals can accumulate in topsoil from atmospheric deposition by sedimenttation, impaction and interception. The persistence of heavy metals in soils is a long process [13,35].
4. Conclusions
The following conclusions have been drawn after processing the data generated from an extensive fieldwork:
1) The investigation of soil heavy metal content in roadside and open areas in the Baghdad City indicated that the concentrations of Cd, Ni and Pb often exceeded the calculated average mean for the world scale of unpolluted soil.
2) The result of SEPI indicated that all soil samples in the industrial land use were heavily polluted by Cd while, for roadside, open areas were classified as moderate contamination.
3) The SEPI values indicated moderate contamination with Ni, while SEPI values of Pb suggesting low to moderate contamination with this element. The highest SEPI for Cd, Ni and Pb in the industrial soils seems therefore to be that this type of soil is the most polluted in the
Table 7. Pattern of heavy metal contamination in decreasing order by EF methods.

study area.
4) The results of CPI suggest multi-element contamination and in some cases are recommended for treatment.
5) Pb followed by Cd was the most serious enriched heavy metals in the study area soils. However, Significant to extremely high values of enrichment factors were recorded confirming an important role of anthropogenic pollution in the soil of the study area.
6) The distribution of the metal concentration of the urban soil indicated that the industry together with the traffic were mainly responsible for metal pollution. However, these findings indicate that more attention should be paid to heavy metal pollution in the urban soils of Baghdad City. Further work is needed not only to assess the spatial distribution of metals in the urban soil but also more intensive sampling of that area needs to be carried out.
5. Acknowledgements
Many thanks to Ministry of Higher Education and Scientific Research, Research and Development Office, without their financial support the work would have not been done.
REFERENCES
- R. M. Harrison, D. P. H. Laxen and S. J. Wilson, “Chemical Associations of Lead, Cadmium, Copper and Zinc in Street Dust and Roadside Soil,” Environmental Science and Technology, Vol. 15, No. 11, 1981, pp. 1378-1383. doi:10.1021/es00093a013
- M. G. Gibson and J. G. Farmer, “Multi-Step Sequential Chemical Extraction of Heavy Metals from Urban Soils,” Environmental Pollution, Series B Chemical and Physical, Vol. 11, No. 2, 1986, pp. 117-135. doi:10.1016/0143-148X(86)90039-X
- I. Thornton “Metal Contamination of Soils in Urban Areas,” In: P. Bullock and P. J. Gregory, Eds., Soils in the Urban Environment, Blackwell Publishing, 1991. doi:10.1002/9781444310603.ch4
- M. Biasioli, R. Barberis and F. Ajmone-Marsan, “The Influence of a Large City on Some Soil Properties and Metals Content,” The Science of the Total Environment, Vol. 356, No. 1-3, 2006, pp. 154-164. doi:10.1016/j.scitotenv.2005.04.033
- X. Li and C. Huang, “Environment Impact of Heavy Metals on Urban Soil in the Vicinity of Industrial Area of Baoji City P.R. China,” Environmental Geology, Vol. 52, No. 8, 2007, pp. 1631-1637. doi:10.1007/s00254-006-0608-3
- E. Paterson, M. Sanka and L. Clark, “Urban Soils as Pollutant Sinks: A Case Study from Aberdeen, Scotland,” Applied Geochemistry, Vol. 11, No. 1-2, 1996, pp. 129- 131. doi:10.1016/0883-2927(95)00081-X
- M. Birke and U. Rauch, “Urban Geochemistry: Investigations in the Berlin Metropolitan Area,” Environmental Geochemistry and Health, Vol. 22, No. 3, 2000, pp. 233- 248. doi:10.1023/A:1026554308673
- C. S. C. Wong, X. Liand and I. Thornton “Urban Environmental Geochemistry of Trace Metals,” Environmental Pollution, Vol. 142, No. 1, 2006, pp. 1-16. doi:10.1016/j.envpol.2005.09.004
- A. Christoforidis and N. Stamatis, “Heavy Metal Contamination in Street Dust and Roadside Soil along the Major National Road in Kavala’s Region, Greece,” Geoderma, Vol. 151, No. 3-4, 2009. pp. 257-263. doi:10.1016/j.geoderma.2009.04.016
- I. van Kamp, K. Leidelmeijer, G. Marsmana and A. de Hollander, “Urban Environmental Quality and Human Well-Being: Towards a Conceptual Framework and Demarcation of Concepts; a Literature Study,” Landscape and Urban Planning, Vol. 65, No. 1-2, 2003, pp. 5-18. doi:10.1016/S0169-2046(02)00232-3
- A. D. K. Banerjee, “Heavy Metal Levels and Solid Phase Speciation in Street Dusts of Delhi, India,” Environmental Pollution, Vol. 123, No. 1, 2003, pp. 95-105. doi:10.1016/S0269-7491(02)00337-8
- T. B. Chen, Y .M. Zheng, M. Lei, Z. C. Huang, H. T. Wu, H. Chen, K. K. Fan, K. Yu, X. Wu and Q. Z. Tian, “Assessment of Heavy Metal Pollution in Surface Soils of Urban Parks in Beijing, China,” Chemosphere, Vol. 60, No. 4, 2005, pp. 542-551. doi:10.1016/j.chemosphere.2004.12.072
- J. Kelly, I. Thornton and P. R. Simpson, “Urban Geochemistry: A Study of the Influence of Anthropogenic Activity on the Heavy Metal Content of Soils in Traditionally Industrial and Non-Industrial Areas of Britain,” Applied Geochemistry, Vol. 11, No. 1-2, 1996, pp. 363- 370. doi:10.1016/0883-2927(95)00084-4
- T. B. Chen, J. W. C. Wong, H. Y. Zhou and M. H. Wong, “Assessment of Trace Metal Distribution and Contamination in Surface Soils of Hong Kong,” Environmental Pollution, Vol. 96, No. 1, 1997, pp. 61-68. doi:10.1016/S0269-7491(97)00003-1
- W. Wilcke, S. Müller, N. Kanchanakool and W. Zech, “Urban Soil Contamination in Bangkok: Heavy Metal and Aluminum Partitioning in Topsoils,” Geoderma, Vol. 86, No. 3-4, 1998, pp. 211-228. doi:10.1016/S0016-7061(98)00045-7
- X. Li, C. S. Poon and P. S. Liu, “Heavy Metal Contamination of Urban Soils and Street Dusts in Hong Kong,” Applied Geochemistry, Vol. 16, No. 11-12, 2001, pp. 1361- 1368. doi:10.1016/S0883-2927(01)00045-2
- L. Madrid, E. Dґaz-Barrientos and F. Madrid, “Distribution of Heavy Metal Contents of Urban Soils in Parks of Seville,” Chemosphere, Vol. 49, No. 10, 2002, pp. 1301- 1308. doi:10.1016/S0045-6535(02)00530-1
- Y. Lu, Z. Gong, G. Zhang and W. Burghardt, “Concentration and Chemical Speciations of Cu, Zn, Pb and Cr of Urban Soils in Nanjing, China,” Geoderma, Vol. 115, No. 1-2, 2003, pp. 101-111. doi:10.1016/S0016-7061(03)00079-X
- M. Linde, “Trace Metals in Urban Soils-Stockholm as a Case Study,” Dissertation, Swedish University of Agricultural Science, 2005.
- A. S. Al-Adili, “Geotechnical Evaluation of Baghdad Soil Subsidence and their Treatments,” Thesis, University of Baghdad, 1998.
- J. R. Okalebo, W. Gathua and P. L. Woomer, “Laboratory Methods of Soil and Plant Analysis: A Working Manual,” TSBF Programme, UNESCO-ROSTA, 1993.
- A. L. Page, R. H. Miller and D. R. Keeney, “Methods of Soil Analysis,” Part 2, 2nd Edition, Agron. Monogr, ASA and SSSA, Madison, 1982.
- S. Naim, H. K. Ozcan, G. Demir, S. Nemlioglu and C. Bayat, “Determination of Heavy Metal Concentrations in Street Dusts in Istanbul E-5 Highway,” Environmental International, Vol. 29, No. 7, 2004, pp. 979-985. doi:10.1016/S0160-4120(03)00075-8
- Y. Ge, P. Murray and W.H. Hendershot, “Trace Metal Speciation and Bioavailability in Urban Soils,” Environmental Pollution, Vol. 107, No. 1, 2000, pp. 137-144. doi:10.1016/S0269-7491(99)00119-0
- S. G. Lu and S. Q. Bai, “Contamination and Potential Mobility Assessment of Heavy Metals in Urban Soils of Hangzhou, China: Relationship with Different Land Uses,” Environmental Earth Sciences, Vol. 60, No. 7, 2010, pp. 1481-1490. doi:10.1007/s12665-009-0283-2
- C. Y. Jim, “Urban Soil Characteristics and Limitations for Landscape Planting in Hong Kong,” Landscape and Urban Planning, Vol. 40, No. 4, 1998, pp. 235-249. doi:10.1016/S0169-2046(97)00117-5
- D. Y. Kim, J. H. Ryu, J. S. Chae and S. H. Cha, “Deposition of Atmospheric Pollutants in Forest Ecosystems and Changes in Seoul Chemical Properties,” Journal of Korean Frosty Society, Vol. 85, No. 1, 1996, pp. 84-95.
- D. S. Manta, M. Angelone, A. Bellanca, R. Neri and M. Sprovieri, “Heavy Metals in Urban Soils: A Case Study from the City of Palermo (Sicily), Italy,” The Science of the Total Environment, Vol. 300, No. 1-3, 2002, pp. 229- 243. doi:10.1016/S0048-9697(02)00273-5
- Y. Lu, F. Zhu, J. Chen, H. Gan and Y. Guo, “Chemical Fractionation of Heavy Metals in Urban Soils of Guangzhou, China, Environmental Monitoring and Assessment, Vol. 134, No. 1-3, 2007, pp. 429-439. doi:10.1007/s10661-007-9634-1
- B. Škrbić and N. Miljević, “An Evaluation of Residues at an Oil Refinery Site Following Fires,” Journal of Environmental Science and Health, Vol. 37, No. 6, 2002, 1029- 1039.
- M. L. Bloemen, B. Markert and H. Lieth, “The Distribution of Cd, Cu, Pb And Zn in Topsoils of Osnabrück in Relation to Land Use,” The Science of the Total Environment, Vol. 166, No. 1-3, 1995, pp.137-148. doi:10.1016/0048-9697(95)04520-B
- A. Kabata-Pendias and H. Pendias, “Trace Element in Soils and Plants,” CRC Press, London, 2001.
- M. B. McBride, “Environmental Chemistry of Soils,” Oxford University Press, 1994.
- D. C. Adriano, “Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability and Risks of Metals,” Springer-Verlag, New York, 2001.
- B. J. Alloway, “Heavy Metals in Soils,” Blackie Academic and Professional, London, 1995. doi:10.1007/978-94-011-1344-1
- B. Volensky, “Removal and Recovery of Heavy Metals by Biosorption,” In: Biosorption of Heavy Metals, CEC Press, Boston, 1990.
- G. C. Kisku, S. C. Barman and S. K. Bhrgava, “Contamination of Soils and Plants with Potentially Toxic Elements Irrigated with Mixed Industrial Effluent and Its Impact on the Environment,” Water, Air & Soil Pollution, Vol. 120, No. 1-2, 2000, pp. 121-137. doi:10.1023/A:1005202304584
- L. Poggio, B. Vrščaj, R. Schulin, E. Hepperle and F. Ajmone-Marsan, “Metals Pollution and Human Bioaccessibility of Topsoils in Grugliasco (Italy),” Environmental Pollution, Vol. 157, No. 2, 2009, pp. 680-689. doi:10.1016/j.envpol.2008.08.009
- A. Chatterjee and R. N. Banerjee, “Determination of Lead and Other Metals in a Residential Area of Greater Calcutta,” The Science of the Total Environment, Vol. 227, No. 2-3, 1999, pp. 175-185. doi:10.1016/S0048-9697(99)00026-1
- M. Imperato, P. Adamo, D. Naimo, M. Arienzo, D. Stanzione and P. Violante, “Spatial Distribution of Heavy Metals in Urban Soils of Naples City (Italy),” Environmental Pollution, Vol. 124, No. 2, 2003, pp. 247-256. doi:10.1016/S0269-7491(02)00478-5
- A. S. Al-Chalabi. and D. Hawker, “Distribution of Vehicler Lead in Roadside Soils of Major Roads of Brisbane, Australia,” Water, Air & Soil Pollution, Vol. 118, No. 3-4, 2000, pp. 299-310. doi:10.1023/A:1005107808235
- A. Möller, H. W. Muller, A. Abdullah, G. Abdelgawad and J. Utermann, “Urban Soil Pollution in Damascus, Syria: Concentrations and Patterns of Heavy Metals in the Soils of the Damascus Ghouta. Geoderma,” Vol. 124, No. 1-2, 2005, pp. 63-71. doi:10.1016/j.geoderma.2004.04.003
- A. W. Rose, H. E. Hawkes and J. S. Webb, “Geochemistry in Mineral Exploration,” Academic Press, London, 1979.
- M. Romic and D. Romic, “Heavy Metals Distribution in Agricultural Topsoils in Urban Area,” Environmental Geology, Vol. 43, No. 7, 2003, pp. 795-805.
- A. Jiries, H. Hussein and Z. Halaseh, “The Quality of Water and Sediments of Street Runoff in Amman, Jordan,” Hydrological Processes, Vol. 15, No. 5, 2001, pp. 815-824. doi:10.1002/hyp.186
- N. I. Ward, “Multielement Contamination of British Motorway Environments,” The Science of the Total Environment, Vol. 93, No. 1, 1990, pp. 393-401. doi:10.1016/0048-9697(90)90130-M
- A. C. Martin, V. C. Rivero and M. T. L. Marin, “Contamination by Heavy Metals in Soils in the Neighbourhood of a Scrapyard of Discarded Vehicles,” The Science of the Total Environment, Vol. 212, No. 2-3, 1998, pp. 145-152. doi:10.1016/S0048-9697(98)00007-2
- S. Davydova, “Heavy Metals as Toxicants in Big Cities,” Microchemical Journal, Vol. 79, No. 1-2, 2005, pp. 133- 136. doi:10.1016/j.microc.2004.06.010
- N. Guvenç, O. Alagha and G. Tuncel, “Investigation of Soil Multi-Element Composition in Antalya, Turkey,” Environment International, Vol. 29, No. 5, 2003, pp. 631- 640. doi:10.1016/S0160-4120(03)00046-1
- L. Tijhuis, B. Brattli and O. M. Saether, “A Geochemical Survey of Topsoil in the City of Oslo, Norway,” Environmental Geochemistry and Health, Vol. 24, No. 1, 2002, pp. 67-94. doi:10.1023/A:1013979700212
- M. Linde, H. Bengtsson and I. Öborn, “Concentrations and Pools of Heavy Metals in Urban Soils in Stockholm, Sweden,” Water, Air & Soil Pollution, Vol. 1, No. 3-4, 2001, pp. 83-101.
- X. Xia, X. Chen, R. Liu and H. Liu, “Heavy Metals in Urban Soils with Various Types of Land Use in Beijing, China,” Journal of Hazardous Materials, Vol. 186, No. 2-3, 2011, pp. 2043-2050. doi:10.1016/j.jhazmat.2010.12.104
- X. Chen, X. Xia, Y. Zhao and P. Zhang, “Heavy Metal Concentrations in Roadside Soils and Correlation with Urban Traffic in Beijing, China,” Journal of Hazardous Materials, Vol. 181, No. 1-3, 2010, pp. 640-646. doi:10.1016/j.jhazmat.2010.05.060
- A. Kloke, “Content of Arsenic, Cadmium, Chromium, Fluorite, Lead, Mercury and Nickel in Plants Grown on Contaminated Soil. Paper Presented at United Nations ECE Sump, on Effects of Airborne Pollution on Vegetation, Warsaw, 1979.
- M. C. Jung, “Heavy Metal Contamination of Soils and Waters in and Around the Imcheon Au-Ag Mine, Korea,” Applied Geochemistry, Vol. 16, No. 11, 2001, pp. 1369- 1375. doi:10.1016/S0883-2927(01)00040-3
- C. H. Lee, “Assessment of Contamination Load on Water, Soil and Sediment Affected by the Kongjujeil Mine Drainage, Republic of Korea,” Environmental Geology, Vol. 44. No. 5, 2003, pp. 501-515. doi:10.1007/s00254-003-0786-1
- V. K. Tippie, “An Environmental Characterization of Chesapeake Bay and a Frame Work for Action,” In: V. Kennedy, Ed., The Estuary as a Filter, Academic Press, New York, 1984.
- J. P. Riley and R. Chester, “Introduction to Marine Chemistry,” Academic Press, London, 1971.
- J. M. Deely and J. E. Fergusson, “Heavy Metal and Organic Matter Concentration and Distributions in Dated Sediments of a Small Estuary Adjacent to a Small Urban Area,” The Science of the Total Environment, Vol. 153, No. 1-2, 1994, pp. 97-111. doi:10.1016/0048-9697(94)90106-6
- [61] W. H. Zoller, E. S. Gladney and R. A. Duce, “Atmospheric Concentrations and Sources of Trace Metals at the South Pole,” Science, Vol. 183, No. 4121, 1974, pp. 198- 200. doi:10.1126/science.183.4121.198
- [62] R. A. Sutherland, “Bed Sediment-Associated Trace Metals in an Urban Stream, Oahu, Hawaii,” Environmental Geology, Vol. 39, No. 6, 2000, pp. 611-627. doi:10.1007/s002540050473

