World Journal of Vaccines
Vol. 3 No. 2 (2013) , Article ID: 31526 , 11 pages DOI:10.4236/wjv.2013.32012
Proteoliposome and Polysaccharide-Based Meningococcal Vaccine Are Immunogenic in Infants and Toddlers and Primes for Memory against Serogroup C Polysaccharide
![]()
Immunology Department, Basic Research Vice Direction, Finlay Institute, Havana, Cuba.
Email: *oliverp@finlay.edu.cu
Copyright © 2013 Oliver Pérez et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 29th, 2013; revised April 30th, 2013; accepted May 8th, 2013
Keywords: Proteoliposome; Meningococcal Vaccine; Infants; Memory Response; Polysaccharide
ABSTRACT
Neisseria meningitidis capsular polysaccharides are the main target of the protective immune response against bacterial meningitis. They are thymus-independent type 2 (TI-2) antigens that are poorly immunogenic and not protective in young children, and their administration may impair subsequent challenge with the same polysaccharide. These problems have been addressed using three different vaccines consisting of 1) polysaccharide alone, 2) polysaccharide covalently conjugated to a carrier protein, and 3) polysaccharide with Proteoliposome (PL) adsorbed onto Al(OH)3. VA-MENGOC-BC® is one of the third types of vaccine. It contains PL (detergent-extracted external membrane proteins forming vesicles) and polysaccharide (Ps) from N. meningitidis serogroups B and C (PsC), respectively. Nevertheless, there is a concern that to overcome the TI-2 nature of Ps the covalently conjugation to a carrier is mandatory. Therefore, we evaluated the immune response induced by VA-MENGOC-BC® in infants and toddlers in order to determine whether it stimulates the response against the PsC. High IgG anti PsC and anti PL responses were seen following the administration of two doses in infants and toddlers, after a 3rd dose in pre-teenagers, and after confirmed carrier stages in young adults. The anti PL IgG response persisted longer than anti PsC IgG response and IgM response against both antigens was maintained. An IgG1 anti PL response predominated, as well as IgG4 > IgG3 > IgG1 anti PsC responses. These results suggest that non-covalent incorporation of PsC onto Al(OH)3 containing PL as adjuvant is immunogenic, primes for memory, and induces long-lasting specific antibody response. The improved PsC immunogenicity of this vaccine may be due to the preferential and potent Th1 response induced in mice and human by the PL as adjuvant.
1. Introduction
Meningococcal capsular polysaccharides (Ps) are thymus-independent type 2 (TI-2) antigens, which induce IgM and IgG antibodies in adults; they neither induce immunological memory, nor boost the response after primary immunisation and do not contribute to herd immunity. Furthermore, Ps can induce the phenomenon of hyporesponsiveness, which more of the knowledge comes from the experiments conducted with meningococcal Ps vaccine serogroup C [1-3]. In addition, a rapid decline of serum antibodies, especially in children less than 4 years old, was reported [4,5]. These features contrast with the fact that Ps are the main target of protection in many bacterial infections. For these reasons, three different strategies have been used for immune prophylaxis against these bacteria. The first, Ps-based vaccines against Neisseria meningitidis serogroup A and C, Streptococcus pneumoniae, and Haemophilus influenzae b were developed [1] and they proved unsuccessful in infants. Subsequently, Ps vaccines were introduced against groups W135 and Y; a meningococcal quadrivalent A, C, W135, and Y Ps vaccine, which has been licensed in the United States since 1981. As a second approach, Ps vaccines were covalently conjugated to T cell-dependent (TD) antigens [6,7]. This procedure transformed Ps TI-2 into TD antigens, which stimulated T cell help. This allowed the induction of higher specific antibody responses, increased antibody affinity, and priming for immunological memory. Furthermore, because of their ability to recruit T cell help, these conjugate antigens also stimulated re- sponses in infants and toddlers whose B cells were unresponsive to the unconjugated form of the Ps [3,8,9]. The third alternative is the non-covalent incorporation of Ps either on the surface of or within liposome structures.
Native serogroup B Ps can not be used to vaccinate against N. meningitidis serogroup B due to its low immunogenicity and more importantly the concern that it use could lead to an autoimmune response due to cross reactivity with human tissue [10]. The wild-type outer membrane vesicle (wtOMV) vaccines are the only formulations that have shown efficacy against serogroup B meningococcal disease [11]. The Cuban meningococcal vaccine VA-MENGOC-BC® contains outer membrane vesicles (Proteoliposome, PL) from serogroup B and Ps from serogroup C (PsC) adsorbed onto Al(OH)3 gel [12- 14]. VA-MENGOC-BC® induced a good response against the PL, when it was evaluated in nursing babies [15]. However, little is known about the response induced against the second main antigen, PsC, although but epidemiological studies have shown protection also against serogroup C.
Nevertheless, Ps vaccines have been used mainly in mass campaigns vaccinating children during outbreaks, and concerns have been expressed about the induction of immunological hypo-responsiveness [16,17] against PsC. This is assuming as the reduced induction of specific antibodies and its functional bactericidal activity, but not the absence of them. This problem is overcome by the conjugate PsC vaccine [18].
In Cuba since 1991, VA-MENGOC-BC® has been included in the National Immunization Program. The first dose is administered to infants of 3 months of age and the second one 6 to 8 weeks later. Cuban vaccination coverage is over 99% with all children vaccinated against meningococcal meningitidis (BC) [19]. However, the possibility of the induction of hypo-responsiveness to PsC to subsequent challenge must be investigated but unfortunately the PsC conjugated vaccine in 1997-1998, when this work was performed, was not available yet. Therefore, we determined serum anti PL and anti PsC IgG responses from VA-MENGOC-BC® vaccinees. The response against a challenge (either natural or a 3rd dose of the vaccine) was also evaluated. High immune responses at specific and bactericidal levels against both antigens were induced.
2. Material and Methods
Antigen preparation. PL of the epidemic meningococcal strain Cu385 (B:4:P1.19,15, L3,7,9 (Cu 385-83)) from Neisseria meningitidis serogroup B was grown until early stationary phase and PL was extracted by 0.1 M tris-HCl, pH 8.6, 10 mM EDTA, and 0.5% (w/v) Na deoxycholate. This was purified by sequential centrifugation steps at 20,000 x g for 30 min. Following ultracentrifugation at 125,000 x g for 2 h, the pelleted PL was homogenized in phosphate-buffered saline (PBS), pH 7.2, purified by chromatography and further purified by column chromatography [13,14]. PsC was produced from N. meningitisdis strain C11 (ATCC) at the Finlay Institute as described in [20].
Subjects and immunization. The population under study consisted of 408 subjects under 18 years old from Havana and Holguin Cuban provinces during 1997-1998. Two cross-sectional studies were carried out. One to determine the induction of meningococcal immune response in 63 infants (<3.5 - 8 months of age) and the other to evaluate duration of the specific immune response that include 88 toddler (9 - 12 months of age), 136 children (from 2 to 10 years old), and 35 pre-teenagers and teenagers (from 11 - 15 years old) were performed. All subjects were vaccinated with two 0.5 mL doses of VA-MENGOC-BC® (Finlay Institute, Havana, Cuba) at 3.5 months and the second one at 6 to 8 weeks later during infant life. Each dose contains 50 µg of purified outer membrane vesicles of serogroup B and 50 µg of purified PsC adsorbed onto Aluminium Hydroxidegel.
The vaccination was performed by family doctors and was verified by us during sample collection. For ethical reasons, the sera from infants, toddlers, and children came from subjects that were admitted for blood test, with the agreement of their parents, at the Juan Manuel Marquez Paediatric Hospital, Havana, Cuba. No samples from immunedeficiencies, malignant disorders, immunosuppressive treatments, severe/chronic illness, or history of previous meningococcal disease were included.
Also, three interventional studies were conducted. The first one was to determine the memory response induced. Twenty vaccinated teenagers from Holguin province were challenged with a third dose (a booster dose) of the vaccine and blood samples were taken before and 21 days after the booster. Second one was to evaluate the influence of natural challenge over PsC specific immune response in internal student accommodation facility in Havana. Forty six healthy young adults (17 and 18 years old) where the risk of natural transmission was high were included. Carrier state was evaluated by cultures of nasopharyngeal exudates immediately before taking the blood sample. The third was to determine the specific IgG subclass response induced and the PsC specific immune response. Fifteen healthy young adults (17 and 18 years old) from the same student facility were immunized with one dose 0.5 mL of group A-C meningococcal Ps vaccine containing 50 µg each of meningococcal groups A and C Ps (Mengivac® A-C, Pasteur Merieux Serums and Vaccines, Lyon, France).
All individuals were requested to donate blood sample and signed informed consent were obtained from each participant, his/her parent or legal guardian. Vaccination status and age of vaccination were recorded. The study proposals were approved by the Ethical Testing Committee at the Finlay Institute.
Measurement of anti Proteoliposome antibody classes by ELISA. A standardized ELISA was performed in duplicate in microtiter plates (Maxisorp, Nunc, Denmark) as described [21]. Briefly, plates were coated with 100 mL per well of a suspension of 20 mg/mL PL in Na2CO3- NaHCO3 (0.1 M and pH 9.6) buffer at 4˚C overnight. After washing with 0.05% Tween-20 in distilled water (used in all washes), plates with samples or controls diluted in 3% skimmed milk in PBS were incubated for 1 h at 37˚C. After three washes in 0.05% Tween-20, the plates were incubated with 100 µL per well of horseradish peroxidase-labelled goat anti-human IgG antibody (Sigma, USA) for 1 h at 37˚C. After washing as described above, 100 µL per well of the substrate-chromogen mixture (O-phenylendiamine and H2O2 citratephosphate buffer, pH 5) were added. The reaction was stopped by adding 2 M H2SO4 and the absorbance measured in a micro-plate reader (Titertek, Multiskan Plus) at 492 nm. All ELISA results were expressed as arbitrary units per mL, referred to an in-house standard. Titres higher than 367 units were considered as positive. The same methodology for IgM and IgA was used with the exception of anti IgM and IgA conjugates and in the absence of a standard the results were expressed in OD and considered positive if it was ³ 0.4.
Anti polysaccharide C antibody classes measured by ELISA. A standardized ELISA was performed in duplicate in microtiter plates (Maxisorp, Nunc, Denmark) as previously described [22]. Briefly, anti PC antibodies were detected by using sensitized plates with 0.5 mg of poly-L-lysine (Sigma, USA) per well for 1 h at 37˚C. Plates were washed five time with PBS, coated overnight at 4˚C with 2.5 mg/mL of PC per well, and blocked with 3% skimmed milk for 1 h at 37˚C. After washing in PBS with 0.05% Tween-20, plates with samples or controls diluted in PBS containing 3% skimmed milk were incubated for 1 h at 37˚C. After three washes, the plates were incubated with 100 µL per well of goat anti human IgG horseradish peroxidase-labelled antibody (Sigma, USA) for 1 h at 37˚C. After washing as above, the substratechromogen mixture (O-phenylendiamine and H2O2 citrate-phosphate buffer, pH 5) were added, 100 µL per well. The reaction was stopped by adding 2 M H2SO4 and the absorbance measured in a micro-plate reader (Titertek, Multiskan Plus) at 492 nm. All ELISA results were expressed as arbitrary units per mL, referred to an in-house standard. Titres higher than 367 units were considered as positive. The same methodology for IgM and IgG was used with the exception of anti IgM and IgA conjugated and in the absence of a standard the results were expressed in optical density (OD) and were considered positive if it was ³ 0.4.
Determination of IgG subclasses. ELISA tests were performed according to standard protocols. Briefly, the plates were coated with 20 mg/mL of PL in Na2CO3- NaHCO3 (0.1 M and pH 9.6) buffer at 4°C overnight. After saturation with 1% of bovine serum albumin (Sigma, USA), plates were incubated with test sera for 2 h followed by anti IgG1, IgG2, IgG3, or IgG4 biotinylated antibodies for 2 h and streptavidin peroxidase complex for 1 h. One hundred µL per well of the substrate-chromogen mixture (O-phenylendiamine and H2O2 citratephosphate buffer, pH 5) were added. The reaction was stopped by adding 2 M H2SO4 and the absorbance measured in a micro-plate reader (Titertek, Multiskan Plus) at 492 nm. Tests were considered positive if the OD was ³0.4.
Colorimetric Bactericidal Activity (cSBA). cSBA was performed as previously described [23]. Briefly, microtitre plates with a bacterial inoculums of 100 colony forming units of Cu 385-83 serogroup B N. meningitidis strain (37˚C with a 30 minutes incubation) or C11 ATCC serogroup C N. meningitidis strain (37˚C with a 60 min. incubation) were used. The assay for serogroup B was performed in the presence of external human plasma and for serogroup C in the presence of baby rabbit serum as complement sources. Glucose and bromocresol purple pH indicator were added to the medium to estimate growth of cSBA target cell survivors through colour change. The reciprocal of the highest serum dilution causing complete inhibition of bacterial grown marked by the colour invariability of pH indicator was recorded as the bactericidal titer. cSBA was performed only in sera from subjects less than 12 months.
Statistical Analysis
Geometric mean titres (gmt) and their 95% confidence intervals were calculated using the graph pad prism 4 software (CA, USA) differences in pre-booster vaccination values and post-booster vaccination values to evaluate the PsC specific response were analysed using t-test of graph pad prism 4 software (CA, USA).
3. Results
3.1. VA-MENGOC-BC® Induces Good Immune Response against Both Proteoliposome and Polysaccharide Components in Infants and Toddlers
In this retrospective evaluation, using sera from VAMENGOC-BC® vaccinees taken at different ages, anti PL serum IgG responses were detectable in children under one year old (infants and toddlers).
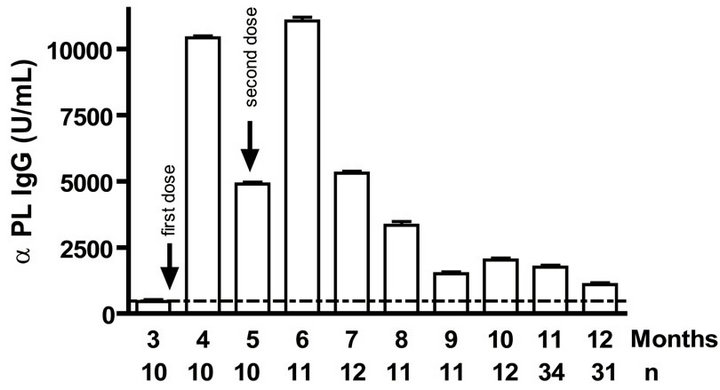
Levels of anti PL specific IgG increased significantly (p < 0.001) after the primary and secondary immunizations of infants (Figure 1(a)). Remarkably, similar behaviour but with lower titers of anti PsC specific IgG response was observed in serum (Figure 1(b)). Anti PL IgM was detectable after the first vaccination dose and it is maintained in all groups at least in the first year. However, anti PsC IgM was detected after the primary immunization but it remains near of the cut off value until 11 months (Figures 1(c) and (d)). We did not detect any levels of anti PL or anti PsC serum IgG or IgM before the vaccine first dose (Figure 1).
Serum bactericidal activities against both B and C serogroups were over 25% in almost all age groups except before the first dose and at 5 and 7 months (Figure 1(e)). No significant level in IgA antibody against serogroups B and C was detected in either one of the age groups (data not shown). Overall VA-MENGOC-BC® induces strong immune response against both PL and PsC components in the first year of life. This is especially relevant for the Ps antigen, because it is known that unconjugated Ps antigens are not immunogenic in infants.
3.2. VA-MENGOC-BC® Induces a Long-Lasting Response
Twelve months after the 2 doses of VA-MENGOC-BC® applied at 3 and 6 - 7 months respectively, the anti PL
 (a)
(a)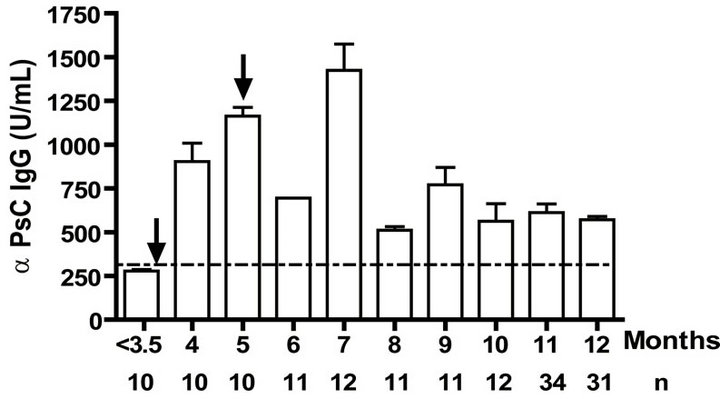 (b)
(b) (c)
(c) (d)
(d)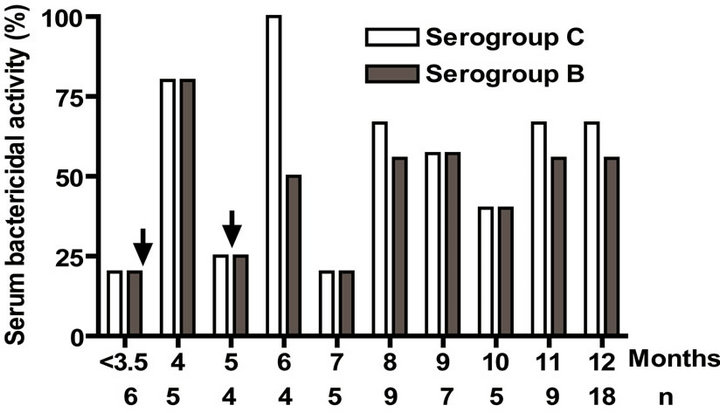 (e)
(e)
Figure 1. Serum IgG, IgM, and bactericidal specific responses to vaccination with meningococcal vaccine in the first year of life. Each age group of n sera came from 151 vaccinated infants (63) and toddlers (88) which were vaccinated during the national immunization schedule: (a) Anti Proteoliposome (PL from serogroup B N. meningitidis) IgG response; (b) Anti Polysaccharide C (PsC from serogroup C N. meningitidis) IgG; (c) Anti PL IgM; (d) Anti PsC IgM; and (e) Serum bactericidal activity. Arrows represent first (3.5 months) and second (5 months) vaccination doses of the bivalent (serogroups (b) and (c)) meningococcal vaccine, VA-MENGOC-BC®. The broken line means cut off value.
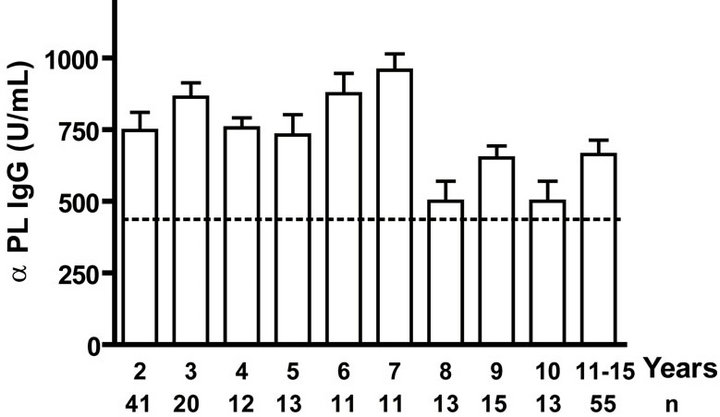
IgG as well as anti PL IgM response was positive to all infants. Even when we evaluated anti PL IgG and IgM response until 15 years old, all children and teenagers showed positive values for both classes of antibodies (Figures 2(a) and (c)). However, anti PsC IgG was transitory and it was negative or near to cut off value in children up to age 15 (Figure 2(b)). Nevertheless, the presence of anti PsC IgM was observed in these age groups (Figure 2(c)). Overall, a long-lasting immune response was induced which is more potent against the PL vaccine component.
3.3. VA-MENGOC-BC® Changes the Subclasses Pattern Induced by a Plain Polysaccharide Vaccine
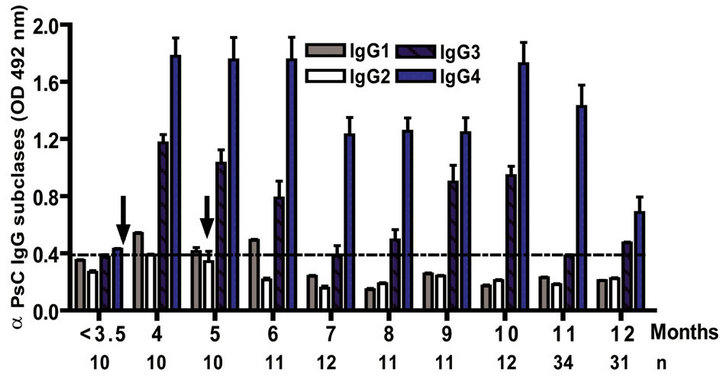
The IgG subclass distribution of antibodies against PL and PsC from N. meningitidis in child vaccinees was evaluated. Anti PL IgG1 predominated after immunization followed by IgG3 and it was observed in all groups in the first year of life after the first dose (Figure 3(a)). Vaccinated infants maintained the same anti PL IgG1 specific response from 2 to 15 years. Also, the presence of anti PL IgG3 was observed in children older than 3 years old (Figure 3(c)). In contrast, anti PsC IgG4 and IgG3 subclasses predominated in the response to PsC and anti PsC IgG1 were only induced until 2 years after vaccination (Figures 3(b) and (d)). The plain Ps A + C vaccine mainly induced antibodies of IgG2 subclass against PsC (classic subclass response against plain Ps) which was never observed against PL or PsC after VA-MENGOCBC® vaccination (Figure 3(e)). Overall, anti PL and PsC subclasses responses were dominated by IgG1 and IgG4/ IgG3, respectively. The later pattern is completely different to that induce by plain Ps vaccines.
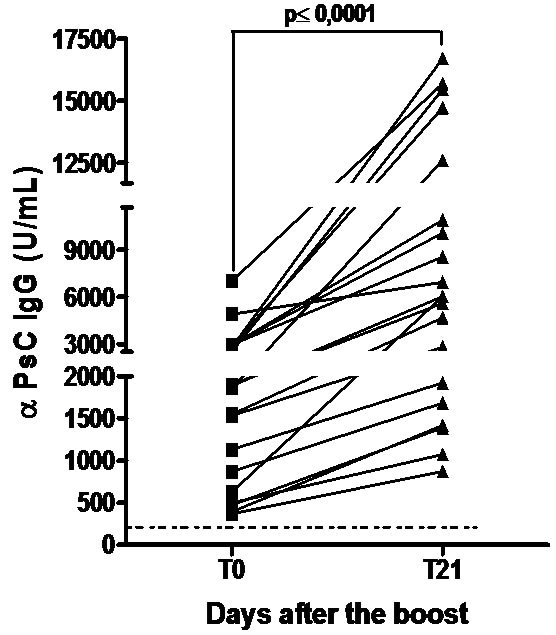
3.4. VA-MENGOC-BC® Induces Polysaccharide Immunological Response after a 3rd Dose or to Natural Neisseria Challenge
Teenagers were boosted with a 3rd dose of VA-MENGOC-BC® which contain non covalent conjugated PsC from N. meningitidis serogroup C. All subjects showed a significant increase in anti PsC IgG response (P < 0.0001) and even those without a response before boost seroconverted in the anti PsC IgG response (Figure 4(a)). The effect of a natural challenge on the anti PsC response was assessed by comparing anti PsC IgG responses in carriers of N. meningitidis serogroup C versus non-carriers. No significant difference in anti PsC IgG response was observed between carriers and non-carriers. (Figure 4(b)). Overall, neither the third vaccine dose nor natural Neisseria challenge impaired the PsC IgG response.
 (a)
(a) 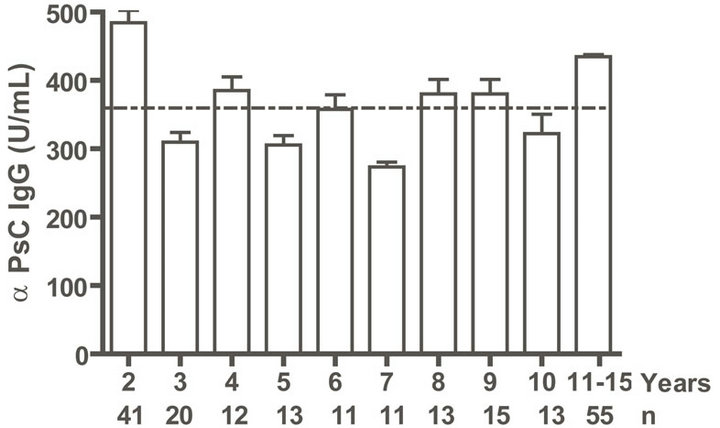 (b)
(b)  (c)
(c) 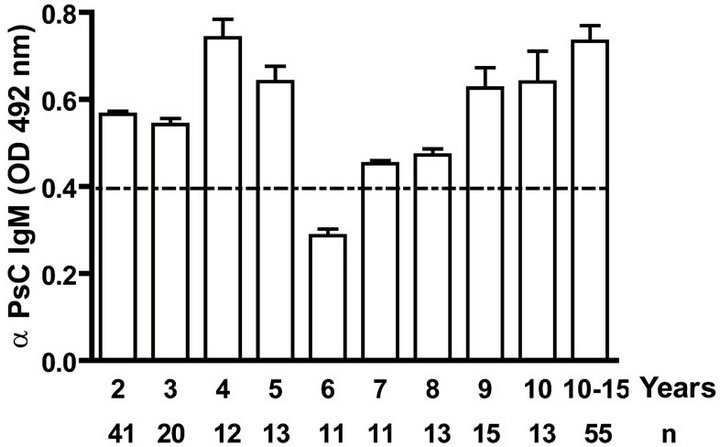 (d)
(d)
Figure. 2.. Serum IgG and IgM specific responses to vaccination with meningococcal vaccine from 2 to 15 years old. Each age group of n sera came from 191 vaccinated children (136) and pre-teenagers and teenagers (55) who were vaccinated during the national immunization schedule at 3.5 and 5 months of age with anti-Neisseria meningitidis BC vaccine, VA-MENGOC-BC®. (a) Anti Proteoliposome (PL from serogroup B N. meningitidis) IgG; (b) Anti Polysaccharide C (PsC from serogroup C N. meningitidis) IgG; (c) Anti PL IgM; and (d) Anti PsC IgM. The broken line means cut off value.
4. Discussion
4.1. Immunization of Infants with VA-MENGOC-BC® Induces Immune Responses against the Proteins from Serogroup B and the Polysaccharide from Serogroup C
The Cuban National Immunization Program is aimed at total coverage of children and at risk populations. The current vaccine schedule targets all children against 13 diseases for immunization with 11 vaccines. Consequently, in the particular case of N. meningitidis B and C all the population less than 22 years old are vaccinated with VA-MENGOC-BC® because they were born after 1991 when this vaccine was introduced in the national program of vaccination. In addition, several million subjects were vaccinated during phase III clinical trial or in national campaigns before 1991. For those reasons, by taking serum samples from babies attending a paediatric hospital laboratory, we have the certainty that those samples came from a vaccinated person; also the vaccination schedule of each subject was recorded. With this study,
 (a)
(a)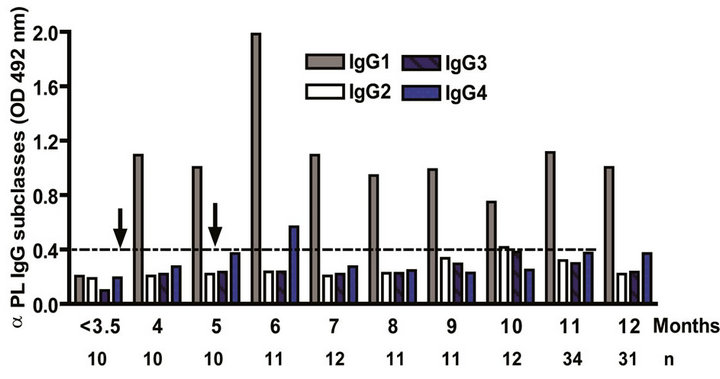 (b)
(b)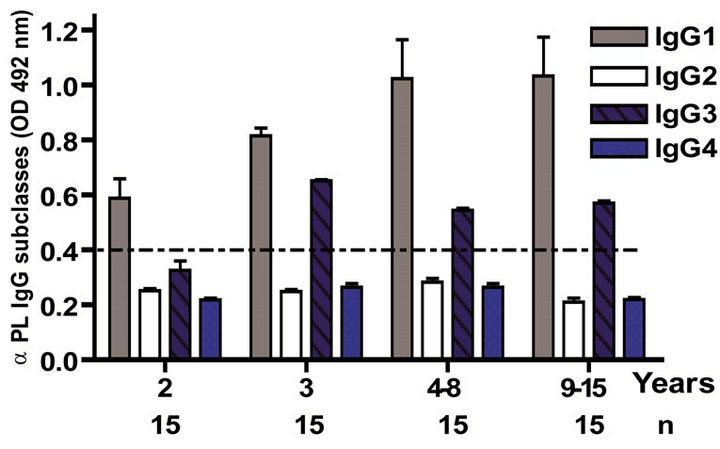 (c)
(c) (d)
(d) (e)
(e)
Figure 3. Serum IgG specific subclasses responses to vaccination with meningococcal vaccine. Each age group of n sera came from 388 vaccinated infants (63), toddlers (88), children (136), pre-teenagers and teenager (55), and young adults (46) who were vaccinated during the national immunization schedule when they were 3.5 months of age with anti-Neisseria meningitidis BC vaccine, VA-MENGOC-BC®. Fifteen additional young adults were vaccinated with one dose of the A + C plain polysaccharide vaccine (Pasteur Merieux Connaught): (a) Anti Proteoliposome (PL from serogroup B N. meningitidis) IgG subclasses under the age of one; (b) Anti Polysaccharide C (PsC from serogroup C N. meningitidis) IgG subclasses under the age of one; (c) Anti PL IgG subclasses from 2 to 15 years old; (d) Anti PsC IgG subclasses from 2 to 15 years old; and (e) Anti PsC IgG subclasses of 15 subjects vaccinated with A + C vaccine.
 (a)
(a) (b)
(b)
Figure 4. Serum Anti Polysaccharide C (PsC) IgG antibodyies. Each sera came from teenagers or young adults who were vaccinated during the national immunization schedule when they were 3.5 and 5 months of age with anti-Neisseria meningitidis BC vaccine, VA-MENGOC-BC®: (a) Anti Polysaccharide C (PsC from serogroup C N. meningitidis) IgG of sera from 15 teenager evaluated before (T0) and 21 (T21) days after a third dose of meningococcal vaccine, VAMENGOC-BC®; and (b) Anti PsC IgG titers of sera from 46 young adults samples and their relationship with Neisseria carrier stage.
we are also indirectly evaluating the efficiency of this vaccination program. Unfortunate, at that time the PsC conjugated vaccine was not available for this study and direct comparison with infant vaccinated with covalently conjugated vaccines can not be performed.
In order to know, if the two main antigens (PL and PsC) of VA-MENGOC-BC® were immunogenic when the vaccination is started very early in life (3.5 months of age), we measured the serum specific antibody responses (IgG, IgM, and IgG subclasses) from infants before (<3.5 months of age), during (3.5 - 5 months of age) and immediately after vaccination (8 months), and in toddlers from 8 to 12 months of age. Anti PL IgG responses were positive after the first dose with a substantial increase in titers and were positive in all the age groups evaluated.
An early immune response to PsC was also seen, which was positive in all age groups evaluated. Transplacental passage of IgG from vaccinated mothers may explain the presence of low titres of anti PL antibodies prior to vaccination. For this reason, the primary specific IgM antibody response was also measured and it was negative excluding the possibility of natural contact before vaccination at least in the evaluated infant sera.
4.2. VA-MENGOC-BC® Induces a Long-Lasting Response against Both Vaccine Components
In order to determine whether VA-MENGOC-BC® primes for immunological memory after the vaccination when they were infants, we measured the serum specific IgG and IgM responses from children (from 2 to 15 years old). Specific antibodies after infant immunization against PL persisted until at least 15 years of age, although anti-PsC antibody response in this age range was lower. However, anti PsC antibody response persistence was sustained until 2 years old.
The most common way of evaluating memory is to challenge the population with a booster dose. Nevertheless, in areas where natural exposure is frequent, we can assume that this acts as a booster dose. However, a natural boosting, either by the homologous organisms or by cross-reactive organisms, is difficult to document. To this end, the anti PL IgM response was tested, because this response is low and transitory as a consequence of the short antibody isotype half-life. The presence of anti PL and anti PsC IgM antibodies would therefore indicate a recent natural contact, rather than a more long-lasting response induced by vaccination. In addition, the absence of IgG anti PsC antibodies in children over 2 years old which have anti PsC IgM antibodies suggests possibility of natural contact with cross-reactive organisms rather than contact with N. meningitidis serogroup C.
4.3. VA-MENGOC-BC® Changes the Subclasses Pattern of Plain Polysaccharide Vaccine
The induction of anti PL IgG1 antibodies by VA-MENGOC-BC® has been previously described [21]; we now further report the induction of specific IgG3 antibodies later in life which may be stimulated by natural contact. Any way, both IgG1 and IgG3 in human are coming form a cellular Th1 pattern which was postulated by our group in 2001 [16]. Changes in the IgG1/IgG2 ratio following vaccination can indicate the activation of cellular control mechanisms typical of a T-cell-dependent immune response. The rise in IgG2 may indicate a T-cell-independent response [24]. Anti PsC IgG4, IgG3, and IgG1 antibodies were also induced by this vaccination, however IgG1 response detected was transitory. As expected, plain Ps vaccine preferentially induced the IgG2 antibody subclass, a fact that has been documented in previous reports [25-28]. IgG4 does not activate the classical complement pathway but could be important in vivo in phagocytosis or alternative pathway activation [29]. The differences observed in subclasses anti PsC responses indicate that VA-MENGOC-BC® can modulate the response to the non covalently incorporated PsC. Also, it is important to remember that this vaccine is adsorbed onto Al(OH)3 and that PL-containing LPS is the most important Th1 driver present in this vaccine [23] and could certainly influence the response against the PsC incorporated in it.
4.4. VA-MENGOC-BC® Induces Polysaccharide Immunological Responses after a Third Dose or to Natural Neisseria Challenge
In order to determine the priming immune response by VA-MENGOC-BC® we determined the response to a 3rd dose or to natural N. meningitidis challenge. In the former, the anti PsC IgG responses were observed in 100% of the 20 vaccinated teenagers. Previous studies have concluded that the administration of plain meningococcal Ps vaccine to young children induces a hyporesponsive state to meningococcal Ps [30]. Keyserling et al. showed that the response for serogroup C of subjects vaccinated 3 years earlier with a tetravalent Ps vaccine was lower than those who previously received a tetravalent conjugate vaccine [31]. Interestingly, we obtained a significantly higher serogroup C response after a third dose of VA-MENGOBCTM in teenagers vaccinated during infant life. Unfortunately, we could not include a covalent conjugated vaccine to determine the induction or not of hyporesponsiveness.
In the latter group, consisting of 46 young adults with 32.6% being Neisseria carriers, the anti PsC response was observed in 84.8% of the cases and 11/15 carriers had significant anti PsC IgG antibodies. Unfortunately, we only detected the genera and neither the species nor the serogroup. Before natural challenge, anti PsC IgM antibodies were presented in many cases (data not shown). These results are consistent with the fact that we have predominance of N. meningitidis serogroup B carriers. Indeed, carrier studies performed showed that circulation of serogroup C was only rarely detected in Cuba (I Martínez, personal communication). Nevertheless, the anti PsC IgG responses suggest that some serogroup C N. meningitidis strains are circulating, and the presence of circulating antibodies play a key role in protection against the disease [31].
It is not clear whether plain PsC vaccines which induce hyporesponsiveness would perhaps affect susceptibility to meningococcal C disease in the long term [19]. It is therefore important to note that the incidence of N. meningitidis serogroup C disease did not increase in Cuba following VA-MENGOC-BC® vaccination. It is necessary to point out that the Cuban population under 40 years of age is vaccinated with this vaccine, and all those under 22 years old were vaccinated when they were infants. Therefore, susceptibility to serogroup C has not increased, thereby excluding the possibility of immunological hyporesponsiveness induced by VA-MENGOC-BCÒ as was reported for other non-conjugated vaccines [19]. Certainly, the induction of hyporesponsiveness mean the lower specific IgG and bactericidal responses but not that these responses are under the level of protection.
Theses results suggest that the formulation of PsC and PL adsorbed onto AL(OH)3, as a non covalent combination, primes the immune system in a different way to the plain Ps. The other immunopotentiator (adjuvant) present in the vaccine is the lipopolysaccharide (LPS). The small amounts presented are incorporated into the vesicles and not free. The LPS that remained was strongly associated with membrane, probably accounting for its lower toxicity. A previous work showed that membrane associated LPS after detergent treatment reduced endotoxicity and induced IFNg production [32] which is in agreement with the preferential cellular, Th1 pattern induced by VAMENGOC-BC® [15].
There are reasons why the inclusion of PsC in the vaccine may be beneficial. On the one hand, B cells can produce Th1 cytokines, including IFNg, IL-2, and IL-12 [33-36] and NK cells produce IFNg. Both B cells and NK cells are recruited by TI-2 antigens; the latter can in turn activate B cells by direct interaction with them [37]. Therefore, PsC may be contributing to the Th1 pattern induced by VA-MENGOC-BC®. In addition, IFNg and IL-2 could enhance the antibody response to TI antigens [38-40] by acting as B-cell growth and differentiation factors regulating B-cell maturation [41] and Ig class and subclass switching [42]. On the other hand, it has been reported that the time at which IFNg exerts its effect may be critical, since the stimulatory effect of IFNg on Ig secretion in vitro is maximal when its addition is delayed, even by as little as 24 h [9]. We have previously reported that when peripheral mononuclear cells from VAMENGOC-BC® immunised subjects were re-stimulated in vitro with PL, a delayed IFNg response was observed [16]. This may explain the good results of the combination of PL and PsC and the other immunopetentiators present in VA-MENGOC-BC®.
Furthermore it must be observed that the bactericidal activity in sera of infants and toddlers less than one year old was only observed after vaccination, with the highest percentage after the first and second doses. These bactericidal activities are similar for both vaccine components. It is important to note that this bactericidal activity does not mean seroconversion because only one serum sample was evaluated. These bactericidal activities are not as high as that induce by Ps covalently conjugated vaccines [43]; but that a formulation of non covalently Th-2 PsC induced long-lasting immune response and memory, changed the subclass pattern, and induced bactericidal activity similar than against the protein component of this vaccine is a relevant fact.
In conclusion, these studies provide evidences that a non covalently conjugated vaccine can be immunogenic and protective in infants against the TI antigen, induces detectable levels of bactericidal activity, primes for memory, and might not induce hyporesponsiveness after a repeated administration aspect that need to be deeper explored.
REFERENCES
- P. Richmond, E. Kaczmarski, R. Borrow, J. Findlow, S. Clark, R. McCann, et al., “Meningococcal C Polysaccharide Vaccine Induces Hyporesponsiveness in Adults That Is Overcome by Conjugate Vaccine,” Journal of Infectious Diseases, Vol. 181, No. 2, 2000, pp. 761-764. doi:10.1086/315284
- J. Southern, S. Deane, L. Ashton, R. Borrow, D. Goldblatt, N. Andrews, et al., “Effects of Prior Polysaccharide Vaccination on Magnitude, Duration, and Quality of Immune Responses to and Safety Profile of a Meningococcal Serogroup C Tetanus Toxoid Conjugate Vaccination in Adults,” Clinical and Diagnostic Laboratory Immunology, Vol. 11, No. 6, 2004, pp. 1100-1104.
- A. Leach, P. A. Twumasi, S. Kunah, W. S. Banya, S. Jaffar, B. D. Forrest, et al., “Induction of Immunologic Memory in Gambian Children by Vaccination in Infancy with a Group A Oplus Group C Meningococcal Polysaccharide-Protein Conjugate Vaccine,” Journal of Infectious Diseases, Vol. 175, No. 1, 1997, pp. 200-204. doi:10.1093/infdis/175.1.200
- A. L. Reingold, C. V. Broome, A. W. Hightower, G. W. Ajello, G. A. Bolan, C. Adamsbaum, et al., “Age-Specific Differences in Duration of Clinical Protection after Vaccination with Meningococcal Polysaccharide A Vaccine,” Lancet, Vol. 2, No. 8447, 1985, pp. 114-118. doi:10.1016/S0140-6736(85)90224-7
- S. J. Cessay, S. J. Allen, A. Menon, J. E. Todd, K. Cham, G. M. Carlone, et al., “Decline in Meningococcal Antibody Levels in African Children 5 Years after Vaccination and the Lack of an Effect of Booster Immunization,” Journal of Infectious Diseases, Vol. 167, No. 5, 1993, pp. 1212-1216. doi:10.1093/infdis/167.5.1212
- J. Eskola and H. Kayhty, “Ten Years’ Experience with Haemophilus influenzae Type b (Hib) Conjugate Vaccines in Finland,” Reviews in Medical Microbiology, Vol. 7, No. 4, 1996, pp. 179-231. doi:10.1097/00013542-199610000-00005
- H. J. Jennings and C. Lugowski, “Immunochemestry of Groups A, B, and C Neningococcal Polysaccharide-Tetanous Toxoid Conjugates,” The Journal of Immunology, Vol. 127, 1981, pp. 1011-1018.
- P. C. Richmond, R. Borrow, E. Miller, S. Clark, F. Sadler, A. Fox, et al., “Meningococcal Serogroup C Conjugate Vaccine Is Immunogenic in Infancy and Primes for Memory,” Journal of Infectious Diseases, Vol. 179, No. 6, 1999, pp. 1569-1572. doi:10.1086/314753
- J. J. Mond, A. Lees and C. M. Snapper, “T Cell-Independent Antigens Type 2,” Annual Review of Immunology, Vol. 13, 1995, pp. 655-692. doi:10.1146/annurev.iy.13.040195.003255
- F. E.Wyle, M. S. Artenslein, R. L. Branck, E. C. Tramont, D. L. Kasper, P. L. Altieri, et al., “Immunological Response in Man to Group B Meningococcal Polysaccharide Vaccines,” Journal of Infectious Diseases, Vol. 126, No. 5, 1972, pp. 514-522. doi:10.1093/infdis/126.5.514
- J. Holst, D. Martin, R. Arnold, C. C. Huergo, P. Oster, J. O’Hallahan, et al., “Properties and Clinical Performance of Vaccines Containing Outer Membrane Vesicles from Neisseria meningitides,” Vaccine, Vol. 27, No. 2, 2009, pp. B3-B12. doi:10.1016/j.vaccine.2009.04.071
- C. Huergo, G. Sierra, M. M. Gutiérrez, G. Bisset, L. G. Garcia, G. Puentes, et al., “Method of Producing Neisseria meningitides B Vaccine, and Vaccine Produced by Method,” European Patent 885900077.8, 1988.
- C. C. Huergo, V. G. Sierra, M. M. Gutiérrez, G. Bisset, L. G. Garcia, G. Puentes, et al., “Method of Producing Neisseria meningitides B Vaccine, and Vaccine Produced by Method,” United States Patent N. 5597572, 1997.
- G. V. Sierra, C. H. Campa and M. Varcárcel, “Vaccine against Group B Neisseria meningitidis: Protection Trial and Mass Vaccination Results in Cuba,” NIPH Annals, Vol. 14, No. 2, 1991, pp. 195-210.
- O. Pérez, M. Lastre, J. Lapinet, G. Bracho, M. Díaz, C. Zayas, et al., “Immune Response Induction and New Effector Mechanisms Possibly Involved in Protection Conferred by the Cuban Anti-Meningococcal BC Vaccine,” Infection and Immunity, Vol. 69, No. 7, 2001, pp. 4502- 4508. doi:10.1128/IAI.69.7.4502-4508.2001
- “Meningococcal Disease in Universitary Students,” CDR Wkly, Vol. 8, No. 1, 1998, pp. 49-52.
- N. MacDonald, S. A. Halperin, B. J. Law, B. Forrest, L. E. Danzig, and D. M. Granoff, “Induction of Immunologic Memory by Conjugated vs Plain Meningococcal C Polysaccharide Vaccine in Toddlers,” JAMA, Vol. 280, No. 19, 1998, pp. 1685-1689. doi:10.1001/jama.280.19.1685
- N. E. MacDonald, S. A. Heparin, B. J. Law, L. E. Danzig and D. M. Granoff, “Can Meningococcal C Conjugate Vaccine Overcome Immune Hyporesponsiveness Induced by Previous Administration of Plain Polysaccharide Vaccine?” JAMA, Vol. 283, No. 14, 2000, pp. 1826-1827. doi:10.1001/jama.283.14.1826
- G. Reed and M. A. Galindo, “Cuba’s National Immunization Program,” MEDICC Review, Vol. 9, No. 1, 2007, pp. 5-7.
- “Normas para la Vacuna Polisacáridas Meningococcicas,” Informe OMS, 27.
- X. Ferriol, A. G. García, R. Ochoa, I. Bravo, R. Blanco, E. Estrada et al., “Validación de un ELISA para la Cuantificación de IgG Humana Anti Proteína de Neisseria meningitidis Serogrupo B,” Revista Cubana de Medicina Tropical, Vol. 5, No. 2, 1999, pp. 99-105.
- M. Nerey, R. Ochoa, J. C. Martínez, T. Licea, X. Ferriol, A. García, et al., “Validación de un ELISA para la Cuantificación de IgG Humana Anti-Polisacárido Capsular de Neisseria meningitidis Serogrupo C,” Biotecnología Aplicada, Vol. 16, No. 2, 1999, pp. 113-115.
- T. Rodríguez, M. Lastre, B. Cedré, J. del Campo, G. Bracho, C. Zayas et al., “Standardization of Neisseria meningitidis Serogroup B Colorimetric Serum Bactericidal Assay,” Clinical and Diagnostic Laboratory Immunology, Vol. 9, No. 1, 2002, pp. 109-114.
- H. Findlow, J. Southern, L. Mabey, P. Balmer, R. S. Heyderman, C. Auckland, et al., “Immunoglobulin G Subclass Response to a Meningococcal Quadrivalent Polysaccharide-Diphtheria Toxoid Conjugate Vaccine,” Clinical and Vaccine Immunology, Vol. 13, No. 4, 2006, pp. 507-510. doi:10.1128/CVI.13.4.507-510.2006
- W. F. Reisen, F. Skvaril and D. G. Braun, “Natural Infection of Man with Group A Streptococci. Levels, Restriction in Class, Subclass and Type; and Clonal Appearance of Polysaccharide Group-Specific Antibodies,” Scandinavian Journal of Immunology, Vol. 5, No. 4, 1976, pp. 383-390.
- W. J. Yount, M. M. Dorner, H. G. Kunkel and E. A. Kabat, “Studies on Human Antibodies. VI. Selected Variations in Subgroup Composition and Genetic Markers,” The Journal of Experimental Medicine, Vol. 127, No. 3, 1968, pp. 633-646. doi:10.1084/jem.127.3.633
- D. J. Barrett and E, M. Ayoub, “IgG2 Subclass Restriction of Antibody to Pneumococcal Polysaccharides,” Clinical & Experimental Immunology, Vol. 63, No. 1, 1986, pp. 127-134.
- M. G. Scott, D. E. Briles, P. G. Shackelford, D. S. Smith and M. H. Nahm, “Human Antibodies to Phosphocholine. IgG Anti-PC Antibodies Express Restricted Numbers of V and C Regions,” The Journal of Immunology, Vol. 138, No. 10, 1987, pp. 3325-3331.
- A. Pollard, R. Galassini, E. Van der Voort, R. Booy, P. Langford, S. Nadel, et al., “Humoral Immune Responses to Neisseria meningitides in Children,” Infection and Immunity, Vol. 67, No. 5, 1999, pp. 2441-2451.
- M. Broker and K. Veitch, “Quadrivalent Meningococcal Vaccines: Hyporesponsiveness as an Important Consideration When Choosing between the Use of Conjugate Vaccine or Polysaccharide Vaccine,” Travel Medicine and Infectious Disease, Vol. 8, No. 1, 2010, pp. 47-50. doi:10.1016/j.tmaid.2009.12.001
- H. Keyserling, T. Papa, K. Koranyi, R. Ryall, E. Bassily, M. J. Bybel, et al., “Safety, Immunogenicity, and Immune Memory of a Novel Meningococcal (Groups A, C, Y and W-135) Polysaccharide Diphtheria Toxoid Conjugate Vaccine (MCV-4) in Healthy Adolescents,” Archives of Pediatrics & Adolescent Medicine, Vol. 159, No. 10, 2005, pp. 907-913. doi:10.1001/archpedi.159.10.907
- K. E. Quakyi, C. E. Frasch, N. Buller and C. M. Tsai, “Immunization with Meningococcal Outer-Membrane Protein Vesicles Containing Lipooligosaccharide Protects Mice against Lethal Experimental Group B Neisseria meningitidis Infection and Septic Shock,” Journal of Infectious Diseases, Vol. 180, No. 3, 1999, pp. 747-754. doi:10.1086/314927
- D. Benjamin, D. P. Hartmann, L. S. Bazar, R. J. Jacobson and M. S. Filmore, “Human B Cell Line Can Be Triggered to Secrete an Interleukin-2-Like Molecule,” Cellular Immunology, Vol. 121, No. 1, 1989, pp. 30-48. doi:10.1016/0008-8749(89)90003-8
- M. Kobayashi, L. Fitz, M. Ryan, R. M. Hewick, S. C. Clark, S. Chan, et al., “Identification and Purification of Natural Killer Cell Stimulatory Factor (NSKD), a Cytokine with Multiple Biologic Effects on Human Lymphocytes,” The Journal of Experimental Medicine, Vol. 170, No. 3, 1989, pp. 827-845. doi:10.1084/jem.170.3.827
- J. Mengel, L. Dare, G. M. Dare, M. Delgado, A. Nomizo, J. S. Silva, et al., “An Activated Murine B Cell Lymphoma Line Produces a Factor Like Activity Which Is Functionally Related to Human NK Cell Stimulatory Factor,” European Journal of Immunology, Vol. 22, No. 12, 1992, pp. 3137-3178. doi:10.1002/eji.1830221222
- Y. Pang, Y. Norihisha, S. Benjamin, R. R. S. Kantor and H. A. Young, “IFNg Gene Expression in Human B Cell Lines: Induction by IL-2 PKC Activators and Possible Effect of Hyporesponsiveness on Gene Regulation,” Blood, Vol. 80, No. 3, 1992, pp. 724-729.
- K. Kuwano, S. Arai, T. Munakata, Y. Tomita, Y. Uoshitake and K. Kumagai, “Suppressive Effects of Human NK Cells or EBV Induced Immunoglobulin Synthesis,” The Journal of Immunology, Vol. 137, No. 5, 1980, pp. 1462- 1467.
- R. O. Enders, E. Kuchmir, J. W. Kappler, P. Marrack and S. C. Kinsky, “A Requirement for Nonspecific T Cell Factors in Antibody Responses to ‘T Cell Independent’ Antigens,” The Journal of Immunology, Vol. 130, No. 2, 1983, pp. 781-89.
- B. L. Pike and G. J. V. Nossal, “A Reappraisal of ‘TIndependent’ Antigens,” The Journal of Immunology, Vol. 132, No. 4, 1984, pp. 1687-1695.
- B. L. Pike, A. Raubitschek and G. J. V. Nossal, “Human Interlekin 2 Can Promote the Growth and Differentiation of Single Hapten-Specific B Cells in the Presence of Specific Antigen,” Proceedings of the National of Academy of Sciences of the United States of America, Vol. 81, No. 24, 1984, pp. 7917-21. doi:10.1073/pnas.81.24.7917
- P. M. Anderson, C. Caliguiri, T. O. Brien, T. Manley, J. Ritz and J. F. Schlossman, “Fcg Receptor Type III (CD16) Is Included in the V NK Receptor Complex Expressed by Human NK Cells,” Proceedings of the National of Academy of Sciences of the United States of America, Vol. 87, No. 6, 1990, pp. 2274-2279. doi:10.1073/pnas.87.6.2274
- C. M. Snapper, T. M. McIntyre, R. Mandler, L. M. T. Pacanha, F. D. Finkelman, A. Lees, et al., “Induction of IgG3 Secretion by IFNg: A Model for T Cell-Independent Class Switching in Response to T Cell-Independent Type 2 Antigens,” The Journal of Experimental Medicine, Vol. 175, No. 5, 1992, pp. 1367-1371. doi:10.1084/jem.175.5.1367
- M. D. Snape, K. P. Perrett, K. J. Ford, T. M. John, D. Pace, L. M. Yu, J. M. Langley, S. McNeil, P. M. Dull, F. Ceddia, A. Anemona, S. A. Halperin, S. Dobson and A. J. Pollard, “Immunogenicity of a Tetravalent Meningococcal Glycoconjugate Vaccine in Infants: A Randomized Controlled Trial,” JAMA, Vol. 299, No. 2, 2008, pp. 173-84. doi:10.1001/jama.2007.29-c
NOTES
*Corresponding author.

