Advances in Chemical Engineering and Science
Vol.3 No.1(2013), Article ID:26912,4 pages DOI:10.4236/aces.2013.31008
Relationship between Structure and Thermodynamic Activity of Carbon Black
1Ural State Mining University, Yekaterinburg, Russia
2Institute of Chemistry and Chemical Technology, Mongolian Academy of Sciences, Ulaanbaatar, Mongolia
Email: *bpurevsuren.icct@gmail.com
Received November 1, 2012; revised December 4, 2012; accepted December 13, 2012
Keywords: Carbon; Carbon Black; Reduction Reaction; Globular Amorphous Structure
ABSTRACT
The role of carbon black is especially important in cokeless metallurgy. Carbon black can be isolated at less hot zones (less than 720˚C) in metallurgical ovens according to equation of Buduara: 2CO = C + CO2. The particles of carbon black obtained by the reaction of Buduara are characterizing with complicated open-work structure including globular amorphous parts and graphitized crystalline elements connected by crosspieses with size in nanometric range (0.1 μm - 3 μm). The carbon black is characterizing with increased Gibbs’s energy and high kinetical activity because of it’s dispersed and amorphous structure.
1. Introduction
Carbon black is a more active reductor in metallurgical process in comparison with other solid fuels. It can be isolated at less hot zones (less than 720˚C) in metallurgical ovens according to equation of Buduara [1].
 (1)
(1)
The role of carbon black is especially important in cokeless metallurgy. The content of carbon in a metalized pellets (briquette) should correspond to the mark of smelting steel. It can be regulated by saturation of spongy iron carbon with carbon black, being a product of methane decomposition in a cooler zone of shaft furnace.
The important advantage of carbon black in comparison with other solid fuels consists in the fact that it is isolated or formed from the gas phase and therefore does not contain ash and other admixtures of ordinary fuel.
Increased thermodynamic and kinetic activity of carbon black is based on its high dispersion, highly developed reaction surface and also by unstable internal structure of the atomic packing, often having not crystalline but amorphous structure.
Let’s consider the increase of thermodynamic activity after dispersing.
If we a mole of the substance with a volume V = M/d (M-molecular weight, d-density) pound onto particles with radius-r after which a new surface SV will be created, a work A = σSV will be done, and the thermodynamic potential (G) will be increased by the same value ΔG = A = σSV. If we accept that the particles have spherical form, then specific surface S is equal to their surface 4 рr2, divided onto the volume V = (4рr3)/3, S = (4рr2)/(4рr3)/3 = 3/r, then
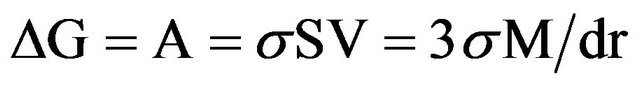 (2)
(2)
The pressure P of steam, thermodynamic activity and constant K of reaction equilibrium, in which participate dispersed substance depends from the potential G by exponent. Therefore increasing the potential G of liquid by the value (2) brings to growth of the pressure P of steam and constant K in  once in comparison with the value for weight of substances P0, K0:
once in comparison with the value for weight of substances P0, K0:
 (3)
(3)
Therefore for example the pressure of water steam increases when the size of water drops decrease. Lets to see a mist consists of nano-drops of water with radius 1 nanometer. The tension of water σ =73 mJ/m2, molecular weight M = 18 × 10−3 kg/mole, density d = 1000 kg/m3. So for such drops with radius r = 1 nm at the temperature T = 273 К we can write following:
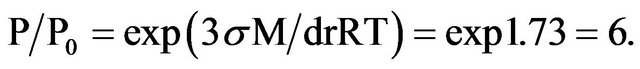
Water’s nano-drops give pressure and activity of water by 6 times higher than ordinary water.
It is also possible to consider similarly crystalline substances. In this case radius-r is for smallest elements in the molecules of the substance. For example the snowflakes can have a size of several millimeter, but they consist very fine needles with radius of 1 nm. Also in this case the pressure of their steam will be for example 6 times higher than ordinary like with the water’s nanodrops.
Carbon is characterized by high value of surface tension. Chemical bonds are very strong in crystals and the heat of evaporation is 720 kJ/mole. Atoms of carbon are strongly packed and the mole volume V is small, it equals to 3.42 × 10−6 m3/mole at the density d = 3500 kg/m3. Experimental determination of the value of liquid carbon tension is not reliable and rather difficult. Probably the carbon has the highest surface tension of all other elements. The value of surface tension of carbon estimated by formula of Stefan and by another analogous equation [2] give σ = 6 J/m2 which is approximately by 3 times higher than σ of iron.
2. Experimental
For the investigation the carbon black obtained by reaction (1) of CO2 on the surface of graphite at temperature 700˚C - 1000˚C. As it was mentioned above the carbon black forms in the zone of reactor with lower temperature.
For structural investigation of carbon black particles by electron microscope EVO 40 have been prepared a suspension in ethanol and spreaded on a polished metal surface. The size of fine carbon black particles is in range of 50 - 100 μm.
3. Results and Discussion
The particles of carbon black like snowflakes have very open-work structure and size of their smaller elements in the formula (1) and it is possible much more fine than total size of particles which showed in the investigation of their structure (Figure 1).
The equilibrium constant of metallization reaction of FeO + C = Fe + CO type, gasification (1) and other reactions with participation of carbon black will be increased exponentially (3) with the decreasing size of r.
According to (3) the equilibrium constant of carbon nano-particles with radius 1 nm at T = 1000 K will be increased for example 1000 times.
Some increase of thermodynamic activity (double) will be obtained by estimation of particles with size bigger than 10 nm and a little increase of activity (3%) is observed for particles with radius 0.1 μm.
If the equilibrium curve of gasification reaction (1) of carbon with its doubled activity increase according to the Equation (3), to plot onto the classical diagram of iron oxide reduction by carbon oxide, then the starting reduction temperature of iron reduction will be decreased approximately by 35˚C in comparison with the activity of carbon characterized for macrosizes. Figure 2 shows that the reduction temperature decreases to 260˚C, when the activity is increased by order. Therefore carbon black is capable to reduce iron at lower temperatures than ordinary carbon-containing fuels.
Such changes of properties in practical conditions depending on the size of samples often became at much bigger size of particles than in the case of calculations. For example according to the calculations the changes of properties should start at order of nanometers, but in real experimental case the changes of properties start at order of micrometers.
The changes of Gibbs energy is a motive force for chemical reactions similar for example running of electricity at difference of electrical potentials. Therefore the rate of chemical reactions V is V = kΔG. High thermodynamic activity of carbon black helps for increasing of experimentally determined reduction rate of metal’s oxides and gasification (1) [1].
For the carbon black particles it is very important not only increasing the thermodynamic activity, but also increasing of reaction rate because of the growth of surface reaction connected with smallest size and their branched structures as it shown in Figure 1.
The reactions of metallization and gasification can be limited by adsorption-chemical actions on the frontier interphase. In such kinetical regime of the process the rate of reaction does not depend from the intensity of mixing in gas phase. It is inversely proportional dependence with the size r of smaller elements of these particles. The kinetic regime of the process starts at sufficiently mixing in gas phase especially in the turbulent gas stream.
At the less intensive mixing of gas the reaction surface forms as thin film of practical unmoving gas in which the mass transfer is performing by molecular diffusion and in such regime the rate is proportional with the reaction surface. However the more intensive mixing of gas can be accelerated and thickness of the unmoving gas film decrease on the surface. In such case can talk about regime of internal diffusion.
Let’s consider the obtained experimental results of investigation of the carbon black structure.
As it is mentioned above the carbon black obtained by reaction (1) of CO2 on the surface of graphite at temperature 700˚C - 1000˚C and the carbon black forms in the zone of reactor with lower temperature.
For structural investigation of carbon black particles by electron microscope EVO 40 have been prepared a suspension in ethanol and spreaded on a polished metal surface. The size of fine carbon black particles is in range of 50 - 100 μm. The particles themselves are characterizing with complicated structures as are shown in Fig-


Figure 1. The structure of carbon black at different magnification.
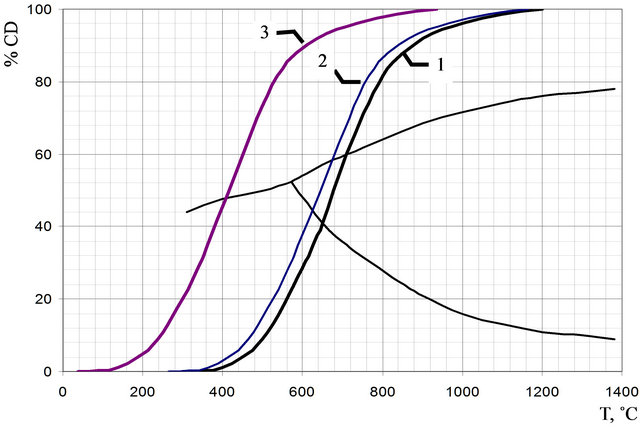
Figure 2. Combined diagram of iron oxide reduction by carbon oxide at pressure 1 atm. 1: for bigger size particles of carbon; 2: for particles of carbon with radius 10 nm; 3: for particles of carbon with radius 1 nm.
ure 1. In addition as experimental tests showed that their structures also depend from the condition of experiments and it is due to variety of atomic structure of carbon atom. For example graphite has a hexagonal structure with characteristic hexangular shape, diamond is crystallized in tetragonal structure, and carbon black has an amorphous structure. The hydrocarbons have characteristic carbon structures. The carbon atoms in the molecule of benzene ring form a hexagonal shape of structure and also carbon atoms in the molecule of hydrocarbons form a chain structure of carbon atoms. Probably, because of this reason the forming of carbon black particles depends from the initial substance in which they consist of [3].
For the last decade have been discovered the structure of fullerenes and graffenes. According to [3], (2000) the nucleus or fist stage carbon black is monolayer fullerenes and the structure of carbon black particles is similar that shown in left-hand part of the photography (Figure 2).
From the photography can be seen that the carbon black forms also globules of nonright sphere and the size is in range of 0.1 - 3.0 μm. In most of work the carbon black particles are considered as globules. Also from the photography can be seen that it is possible to assume the globules of carbon consist of internal cavities and the carbons often form porosity structures.
As it is known that the globular form of particles characterize with amorphous phase and globules are not isolated. They form bridges and crosspieces between them and their thickness is in nanometer range. As it was shown early the increasing of thermodynamical activity of dispersed substances can be determined on the basis of smallest particles and in our case the size of globul and crosspieces.
Amorphous substances are characterizing by nonequilibrium phase with increased thermodynamical activity. The increasing of energy U and thermodynamical potential G reach the value of melting heat ΔHm this substance or value RTm: ΔG = ΔHm = RTm in relationship with the structural amorphization. The carbon is most refractory element in comparison with all known substances for example Tm = 4000 K and therefore ΔG is very higher. This is an other factor leading to increasing of thermodynamical activity carbon black.
According to the Ya. I. Frenkel’s theory for embryo of new phase first embryo forms by the fluctuation of density. The probability W for forming of such fluctuation of embryo is determined from the exponent value of ΔG at it’s forming: W = Kexp (ΔG/RT). The critical size for embryo of new phase rcr can be determined at the condition d(ΔG)/dr = 0 and equal around 8 nm by our calculation. As it shown in photography the globules of carbon black have much bigger sizes. Of course the sizes of all they considerably increased in comparison with size of starting embryo.
4. Conclusion
Therefore it was shown that the particles carbon black obtained by the reaction of Buduara are characterizing with complicated open-work structure including globular amorphous parts and graphitized crystalline elements connected by crosspieces with size in nanometric range. The carbon black is characterizing with increased Gibbs’s energy and high kinetical activity because of it’s dispersed and amorphous structure.
5. Acknowledgements
This work is performed by the financial support of Russian Fund of Fundamental Research and Science-Technology Fund of Mongolia.
REFERENCES
- O. A. Esin and P. V. Geld, “Physical Chemistry of Pyrometallurgical Processes,” 2nd Edition, GNTIL on Ferrous and Nonferrous Metallurgy, Sverdlovsk, 1962, p. 671.
- V. V. Pavlov, “About the Crisis of Kinetic Theory of Liquid and Hardening,” Publisher of USMU (Russian Abbreviation UGGU), Yekaterinburg, 1997, p. 392.
- V. I. Berezkin, “Fullerene as an Nucleus of Carbon Black,” Physics of Solid Body, Vol. 4, No. 3, 2000, pp. 567-572.
NOTES
*Corresponding author.

