Food and Nutrition Sciences
Vol. 2 No. 10 (2011) , Article ID: 16361 , 9 pages DOI:10.4236/fns.2011.210146
Vitamin E Bioavailability: Past and Present Insights
![]()
Department of Veterinary Sciences and Technology for Food Safety, Università di Milano, Milan, Italy.
Email: antonella.baldi@unimi.it
Received August 3rd, 2011; revised September 19th, 2011; accepted September 27th, 2011.
Keywords: Vitamin E, Dairy Cows, Bioavailability, Milk
ABSTRACT
Over the past decades several studies have investigated the importance of an adequate vitamin E status to sustain both animal health and production in dairy cows. Cow milk is considered as a remarkable source of bioactive components promoting human health, which has renewed interest in the effects of vitamin E supplementation on its nutritional value, sensory quality and shelf life. Thus, defining relative bioavailability, utilisation and transfer into milk of different vitamin E formulations is particularly important to assess the adequate levels of supplementation for animal health and milk quality. In nature vitamin E is present under one isomeric form, RRR α-tocopherol; when α-tocopherol is synthesized chemically, a racemic mixture of 8 possible isomers of α-tocopherol in equimolar concen-trations is produced (all-rac α-tocopherol). The different stereoisomers have different biopotencies in humans and livestock; the conversion factor between RRR and all-rac vitamin E was estimated by early studies on the basis of the rat foetal resorption bioassay, and then extended to other species. Recent advances on the distribution of vitamin E stereoisomers in plasma and tissues have highlighted the need to formulate new conversion factors in dairy cows as well as in humans. On account of this, the present article aims to consider past and recent data related to vitamin E in dairy cow nutrition.
1. Introduction
Vitamin E is one of the most important components of cellular antioxidant systems. In nature it is present under eight different forms, four tocopherols (α-, β-, γ-, δ) and four tocotrienols (α-, β-, γ-, δ), of which α-tocopherol is the most bioactive. As an antioxidant, α-tocopherol is able to prevent free-radical mediated tissue damage, and thus to prevent or delay the development of degenerative and inflammatory diseases; in such a role it has been extensively investigated in many species, humans included. About dairy cows, several reviews have discussed extensively on this topic over the past decades [1-4]. According to different surveys vitamin E supplementation helps to enhance animal health and production and, when dietary selenium is adequate, it significantly reduces the incidence of intramammary infections (IMI) and clinical mastitis [5]. Vitamin E also seems to be crucially involved in immune system function so that supplementation with supra-nutritional levels of the vitamin, at least in some instances, results in improved immune responses [4]. These benefits, particularly in a context of much-reduced use of fresh forage (vitamin E-rich) in dairy cow nutrition, have led to a substantial increase in recommended intake levels for this animal [6]. Vitamin E supplementation is a common practice in animal nutrition, and increasing the feed content of synthetic or natural vitamins is the best way to maintain adequate plasma levels for animal health.
Objective of this paper is to consider bioavailability, bioactivity and transfer into milk of different vitamin forms. First, we discuss the new insights on the utilisation of vitamin E isomeric forms and formulations, and then we consider the dietary role of vitamin E to improve animal health and milk quality.
2. Vitamin E Bioavailability and Utilisation
2.1. Natural versus Synthetic Vitamin E
When α-tocopherol is synthesised chemically, a racemic mixture of all 8 possible isomers of α-tocopherol in equimolar concentrations is produced (synthetic form, also referred as all-rac α-tocopherol and historically labeled dl-α-tocopherol), with four stereoisomers showing a 2R configuration (RRR, RRS, RSS, RSR, having R configuration at position 2’ of the phytyl tail) and four stereoisomers possessing a 2S configuration (SRR, SSR, SRS, and SSS, having S configuration at position 2’ of the phytyl tail, Figure 1). Among possible different forms of α-tocopherol, RRR α-tocopherol is the only isomeric form of vitamin E produced by plants (natural form, historically labeled d-α-tocopherol) and is therefore the only one naturally present in feedstuffs.
Since the free forms are easily oxidized, more stable forms—such as acetate and succinate esters of α-tocopherol (RRR α-tocopheryl acetate, RRR α-tocopheryl succinate, all-rac α-tocopheryl acetate, all-rac α-tocopheryl succinate)—have been synthesized to be used as feed additives. The ester forms are very stable to in vitro oxidation; however, they need to be hydrolysed in the animal gut to free tocopherol which exerts its activity in vivo [1]. The acetate ester of all-rac α-tocopherol (all-rac α-tocopheryl acetate) is the most common form of vitamin E supplementations, due to its cost and stability in animal feeds.
The different stereoisomers of vitamin E have different biopotencies, with the RRR form having a greater activeity than the all-rac form. The terms biopotency or bioactivity usually refer to the amount of a nutrient associated with some measured physiological endpoints, e.g. prevention of specific deficiency symptoms [7]. The relative activity of RRR and all-rac vitamin E was estimated by the United States Pharmacopeia (USP) [8] based on the rat foetal resorption bioassay and the USP conversion factor of 1.36 equating RRR to all-rac α-tocopheryl acetate was extended to other species. Recently, the FEEDAP Panel (EFSA Panel on Additives and Products or Substances Used in Animal Feed) confirmed the conversion factors stated by USP in 1979 for livestock, reporting that all-rac α-tocopheryl acetate, RRR α-tocopherol and RRR α-tocopheryl acetate are efficacious in all animal species and have different biopotencies: one International Unit (IU) of vitamin E is defined as 1 mg all-rac α-tocopheryl acetate, as 0.74 mg of RRR α-tocopheryl acetate and as 0.67 mg of RRR α-tocopherol [9].
It is noteworthy that in humans the Institute of Medicine (IOM) redefined the USP conversion factors on the basis of studies indicating that the 2S stereoisomers of all-rac α-tocopherol were not maintained in human plasma [10-12] or in tissues [13] and published new conversion factors (Table 1), limiting the active form of vitamin E to the 2R stereoisomeric forms of α-tocopherol, which represent 50% of all-rac α-tocopheryl acetate and 100% of RRR α-tocopheryl acetate formulations [14].
Some recent comprehensive reviews of literature data suggest that bioavailability of RRR α-tocopheryl acetate should be reconsidered for livestock animals as well, and that new conversion factors, in line with human studies, are needed [15,16]. A suggestion deriving from a deeper understanding of bioavailability concerning stereoisomeric forms of vitamin E, achieved by studying their distribution in plasma and tissues of various animal species by analytical methods based on chiral HPLC procedures [15].
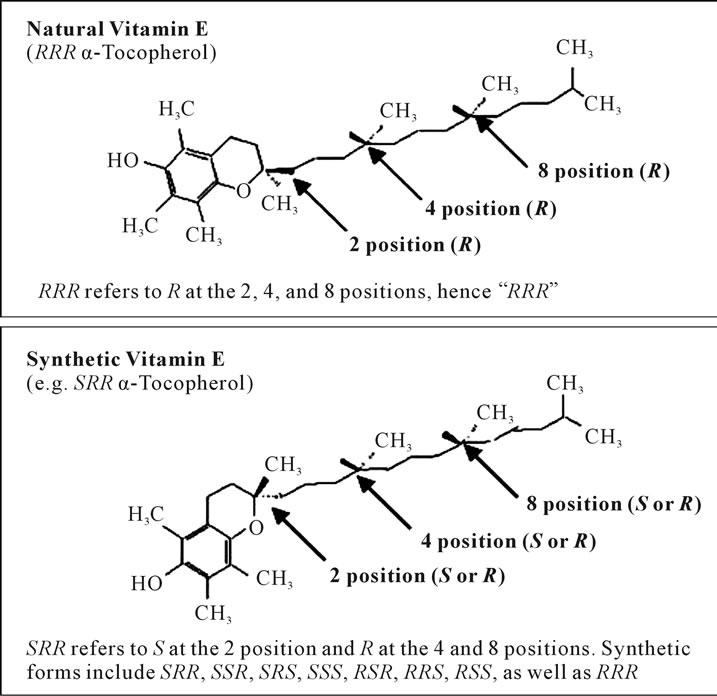
Figure 1. Natural and synthetic vitamin E.
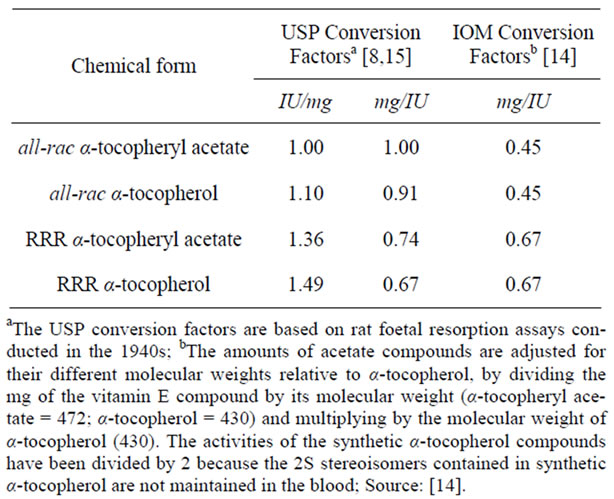
Table 1. Factors for converting international units of vitamin E to α-tocopherol to meet recommended intake in humans.
Distribution of α-tocopherol isomers in plasma and milk has been recently studied when feeding all-rac or RRR forms to periparturient dairy cows. Meglia et al. [17] showed that RRR α-tocopherol is the predominant form (more than 86% of the total) found in plasma of cows fed 1000 IU/day of either supplemental all-rac α-tocopheryl acetate, RRR α-tocopheryl acetate, or RRR α-tocopherol. Weiss et al. [18], after supplementation of 2500 IU/day of vitamin E from all-rac α-tocopheryl acetate or RRR α-tocopheryl acetate, found concentrations of α-tocopherol in cow plasma ranging from 1.2 to 1.4 times greater for RRR treatment than for all-rac treatment.
In both studies, very low concentrations of 2S isomers were detected in plasma of cows fed all-rac α-tocopheryl acetate. As the 2S isomers represent 50% of the isomers present in the all-rac α-tocopherol, such data suggest that 1 g of all-rac α-tocopheryl acetate is equivalent to 0.5 g of RRR α-tocopheryl acetate in dairy cows.
A study evaluating plasma concentration and distribution of α-tocopherol stereoisomers after intramuscular injection of 2.5 g all-rac α-tocopheryl acetate [19] evidenced difference in turnover rate of different stereoisomers in cow plasma: the 2S stereoisomers had showed the faster disappearance from blood compared to the other forms; however, considering the plasma concentration of stereoisomers one day after injection, the 2S forms showed also a faster increase related to their initial level, when compared to the other forms. Based on plasma concentrations at 10 days after injection, Dersjant-Li and Peisker [20] calculated a higher relative bioavailability of RRR α-tocopheryl acetate over all-rac α-tocopheryl acetate. However, considering that plasma distribution of stereoisomers is different over time, sampling time (e.g. day 1 or day 5) chosen to determine the relative bioavailability is crucial. As a general rule, the ratio of bioavailability, being not constant but variable [21], should be estimated considering the plasma profile over time (and relative area under the concentration curve, AUC) rather than on a single timepoint.
Methodologies used in the evaluation of α-tocopherol forms bioavailability frequently diverge according to different studies, making it difficult to compare sometimes dissimilar results [20]. To estimate true relative bioavailability of RRR over all-rac α-tocopheryl acetate, it is also important to consider the contribution given by α-tocopherol originating from basal ingredients, consisting only of RRR form and responsible for the basal plasma level.
Bioactivity underpinning different bioavailability of vitamin E isomers is still under debate. In fact, the RRR form, while being more bioavailable, does not appear to affect neutrophil phagocytosis and killing. In the previously-mentioned study, Weiss et al. [18] found that, although cows fed on supplemental RRR α-tocopherol showed higher concentration of α-tocopherol in neutronphils, phagocytosis was greater in neutrophils from cows supplied with supplemental all-rac vitamin E (Table 2).
Not only its chemical form, but also different formulations and delivery systems can greatly affect vitamin E bioavailability [22-24]. A formulation can have a major influence on intestinal availability of vitamin E, which in dairy cows may be limited by ruminal degradation. Encapsulation technologies made it possible to formulate products that deliver specific nutrients to small intestine absorption sites by protecting them from degradation within the rumen. Baldi et al. [23] compared the pharmacokinetic parameters of vitamin E after intraruminal administration of oil-based, silica-adsorbed and microencapsulated preparations and found that all-rac α-tocopheryl acetate adsorbed on silica or microencapsulated
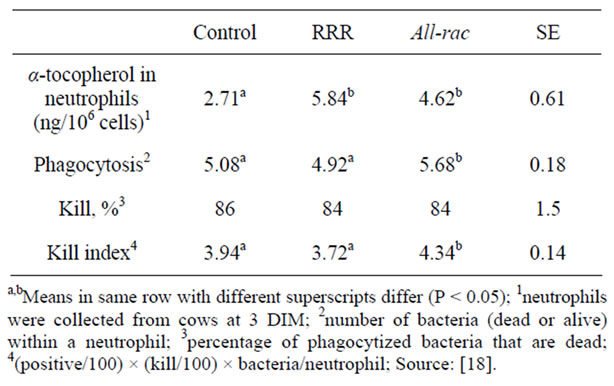
Table 2. Effect of supplemental vitamin E (2500 IU) on concentration of total α-tocopherol in blood neutrophils and neutrophil function.
resulted in different pharmacokinetic profiles and in a greater relative bioavailability than the oil-based preparation. Bontempo et al. [24], after oral administration of α-tocopherol in oil, encapsulated in liposomes or cyclodextrin, found that encapsulated preparations had a longer persistence in blood and a slightly greater availability than the oil-based preparation.
In general, it has been observed (Table 3) that plasma total response (AUC) increases linearly with increasing dose, while the maximal plasma α-tocopherol reached does not increase to the same extent.
2.2. Vitamin E Interactions with Other Nutrients
Vitamin E interacts with other nutrients that can influence its bioavailability. Several papers have examined this topic deeply, as reported below. Vitamin E absorption is closely tied to the digestion and absorption of fats, thus the type and amount of fat in the gut has an important influence on the absorption efficiency of the vitamin. Mid-lactation cows receiving 25, 125 or 250 IU of vitamin E and diets containing no supplemental fat or 2.25% added fat from roasted soybeans or tallow, showed a rate of increase of plasma α-tocopherol 1.9 greater when fat was supplemented [25]. Nevertheless, Baldi et al. [26] showed that when 0.2 kg/day of calcium-soaps (relative to isoenergetic starch) were fed to early lactation dairy cows in addition to vitamin E, this had no effect on the vitamin E status. These different results can be explained in two ways: first, the amount of fat in the basal diet may affect vitamin E absorption efficiency and, hence, plasma levels; second, absorption might also be affected by differences in the rate of digesta flow through the gut at different stages of lactation.
Dairy cows receiving 20 g/day of rumen-protected choline showed higher plasma concentrations of α-tocopherol than control cows during the first 30 days of lactation (2.46 vs 1.85 μg/ml), suggesting that choline supply can reduce the physiological drop of vitamin E in the transition period [27]. The mechanism by which choline maintains vitamin E levels in this period has not been completely understood yet. Anyway, as α-tocopherol is preferentially incorporated in to nascent VLDLs, a reasonable hypothesis is that choline, a vital component of lipoproteins, influences vitamin E status by promoting VLDL formation [28].
Bioactivity of vitamin E is closely related to the supplementation of adequate dietary levels of other nutrients involved in the cell antioxidant system. In particular, as an integral component of the enzyme glutathione peroxidase (GSH), selenium is important to maintain proper levels of the components of the tissue defence mechanisms against free-radical damage and its metabolic function is closely linked to vitamin E. When vitamin E exerts its antioxidant activity it is converted into tocopheroxyl radical that needs to be converted back to tocopherol in order to prevent α-tocopherol free-radical related damage. Regeneration of tocopherol involves multiple reactions and interacting molecules [29], including vitamin C and the selenium-containing enzyme GSH.
Several studies have shown that contemporary administration of vitamin E and selenium may result into a synergistic enhancement of both immune response and disease resistance in domestic animals, particularly in ruminants [30,31]. In dairy cows, selenium nutritional requirement is 0.3 mg/kg dry matter [6] and selenium supplements are available in form of inorganic mineral salts, such as sodium selenite or selenate and organic forms, such as selenium-enriched yeasts. Effects of selenium supplementation on immune function and bovine mammary gland health have been recently reviewed by Salman et al. [32] and scientific evidence related to the effect of oral supplementation of organic and inorganic forms on milk selenium concentration in cattle have been summarised by a systematic review and meta-analysis [33].
3. Vitamin E Function and Activity
3.1. Vitamin E and Mammary Gland Health
Based on health and immune function in cows, vitamin E intake has been generally considered adequate when α-tocopherol plasma levels are above 3 - 3.5 µg/ml. In
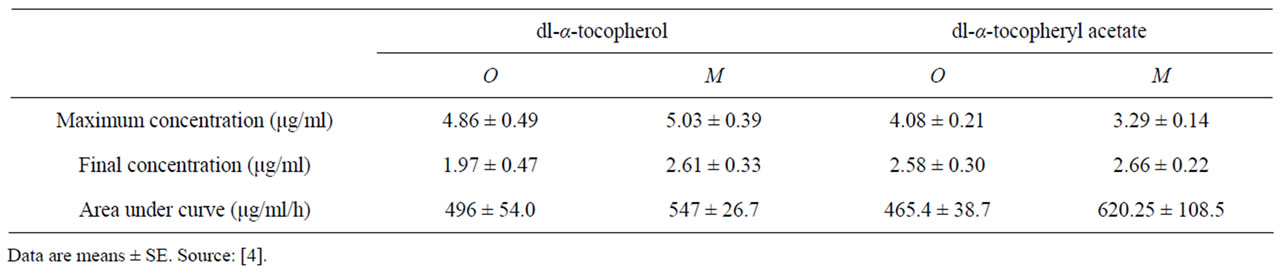
Table 3. Pharmacokinetic parameters of α-tocopherol after intraruminal administration of dl-α-tocopherol or dl-α-tocopheryl acetate in oil-based (O) and microencapsulated (M) preparations.
order to maintain these blood values, non-grazing dairy cows are suggested to be fed on 1000 international units (IU) of supplemental vitamin E daily during the dry period and 500 IU during lactation [6].
It is established that plasma vitamin E levels fall significantly around parturition in dairy cows so that it is difficult to maintain levels considered adequate for their health in this period.
During the periparturient period, dairy cows experience alterations in immune responsiveness and a conesquent greater susceptibility to various metabolic and infectious diseases including mastitis, metritis and retained foetal membranes. Several components of the host defence system are impaired during this period, in particular a decreased activity of neutrophils has been associated with increased susceptibility to mastitis [34,35].
Several studies reported positive effects of supplementation with supranutritional levels of vitamin E on the immune system, specifically a higher killing ability, superoxide production and u-PA activities of neutrophils and improved macrophage function [36-42].
Benefits of vitamin E and selenium supplementation in this critical period on mammary gland health have been registered since 1984 [43] and most of the studies published over the past decades clearly indicate that adequate dietary vitamin E and selenium intakes, above normal nutritive requirements and supplemented during the dry period, reduce milk SCC and influence the prevalence and severity of IMI in dairy herds [26,42,44,45]. A recent meta-analysis [5] of 34 papers published between 1984 and 2003 that addressed the relationship between vitamin E and udder health, confirmed that different levels of supplementation, up to a maximum of 4000 IU during the dry and early lactation periods, were associated with lower IMI, somatic cell counts (SCCs) and clinical mastitis. On average, vitamin E supplementation was associated with a 14% reduction in the risk of IMI, with a reduction of milk SCC by a factor of 0.70 and a 30% decrease in the risk of occurrence of clinical mastitis.
Other studies showed no positive effects of vitamin E supplementation on udder health. LeBlanc et al. [46] found no association between serum α-tocopherol concentration after injection of 3000 IU of vitamin E one week before expected calving and the risk of mastitis at or soon after calving. Similarly, a study [47] performed in European commercial dairy herds with a high incidence of veterinary-treated clinical mastitis did not find any significant effect of extra daily supplementation of 1610 mg of RRR α-tocopheryl acetate around calving on udder health or on other cow health problems.
A recent experiment conducted on five farms with historically high rates of mastitis in the Netherlands reported for the first time higher incidences of clinical and subclinical mastitis in cows receiving a daily supplement of 3000 IU of vitamin E during the dry period, compared to cows receiving a daily supplement of 135 IU [48]. The mean vitamin E level at dry off for the cows involved in this study was 2 to 3 times higher than starting levels for cows in other studies [46,49] and in the opinion of the authors this is the most reasonable explanation for the results observed. In this study, an initial blood vitamin E level at dry off above 6.42 μg/ml was considered a risk factor for developing clinical mastitis. However in another study with average blood vitamin E levels equal to or higher than 6 μg/ml during the periparturient period, a positive rather than negative effect of supplementation on the immune function and SCC/ml levels in milk was found [41].
The plasma concentration of α-tocopherol at dry off is obviously a critical factor in determining the positive effect of vitamin E supplementation on the incidence of mastitis. Additionally, different levels of oxidative stress may explain the variation in results from different studies. As previously discussed, the importance of establishing the levels of other nutrients interacting with vitamin E has to be considered to determine whether supplementation will be beneficial or not [29,50]. Finally, differences in the conclusions from different studies may be explained by differences in farm management, differences in the way vitamin E is supplemented (oral v. i.m. injecttion), and even in the type of microorganism causing the disease.
3.2. Vitamin E and Milk Quality
It is well known that vitamin E supplementation has a positive effect not only on animal health, but also on milk quality. Results from most studies [26,39,42] strongly suggest that high levels of vitamin E supplementation (2000-3000 UI/cow per day) during the periparturient period are effective in reducing milk SCC by about 29% in treated cows.
Furthermore, as an antioxidant vitamin E helps to slow lipid peroxidation and to maintain oxidative stability and flavour of milk. Several studies reported a positive effect of vitamin E on oxidative stability of milk [51-53]. Bovine milk is susceptible to auto-oxidation when the level of vitamin E falls below 20 μg/g of fat [54] and this leads to the development of an “oxidised flavour” (OF), described as cardboard-like, metallic or tallow-like. The fatty acid profile of milk fat is a major factor in the development of OF, and milk with a high concentration of polyunsaturated fatty acids, as linoleic or linolenic acid, is more likely to develop OF [55]. Recently, there has been a considerable interest in developing specific animal feeding regimes in order to increase the polyunsaturated lipid content of milk, and in particular the content of fatty acids thought to benefit human health, such as conjugated linoleic acids. Milk with a high content of these lipids may benefit human health, but at the same time is more vulnerable to oxidation. In this context, increasing the vitamin E content of milk may represent a useful way to protect lipids from peroxidation and to maintain nutritional and organoleptic qualities of milk. It has also been shown that the uptake of plasma vitamin E by the mammary gland of dairy cows increases when diets enriched in polyunsaturated fatty acids are fed [56].
Available data on the amount of supplemental vitamin E required to ensure oxidative stability of milk with a high unsaturated fat content are not conclusive. Weiss [55] suggested supplementing at least 3000 IU of vitamin E per day, when OF is a problem. By contrast, Slots et al. [57] found that supplementation of the feed with 2600 and 3400 IU of all-rac α-tocopheryl acetate for 16 days did not improve oxidative stability of milk.
Although several studies [25,26,55] indicated that transfer of vitamin E from feed to milk is low, around 1.6% - 2.2%, supra-nutritional supplementation to transition cows can increase the vitamin E content of milk (Figure 2). Vitamin E supplementation at 2000 UI/day from 14 days before expected calving to 7 days after was found to produce an increase of vitamin E content of milk of about 40%, compared to supplementation at 1000 UI/day [26].
The rather low transfer efficiency of α-tocopherol in milk could also be dependent on the form of vitamin E supplemented. In the previously-mentioned studies [17, 18], the distribution of α-tocopherol stereoisomers in milk in relation to the supplementation of natural and synthetic vitamin E to periparturient dairy cows has also been investigated. Data from these studies showed that concentrations of α-tocopherol in milk was 1.24 to 1.43 times greater for cows fed the RRR supplement compared to cows fed the all-rac supplement. Moreover, irrespective of dietary treatment, α-tocopherol with natural stereochemistry (RRR) was by far the most predominating form in milk of cows (at least 86% of the total, [17]). Similarly, Slots et al. [57] reported that after supplementation with 2600 IU and 3400 IU of all-rac α- tocopheryl acetate, only the four 2R stereoisomers were excreted into the milk, with the RRR isomer dominating over others (84.3% and 88% of the isomers, respecttively).
A preferential uptake or transfer of the RRR form from plasma into milk is also suggested by recent studies investigating the effect of farming system type on vitamin E content of milk. Even in the absence of feed supplementation with synthetic vitamins, milk and milk products from organic farms were found to contain more or as much vitamin E than conventional milk [60-62] and
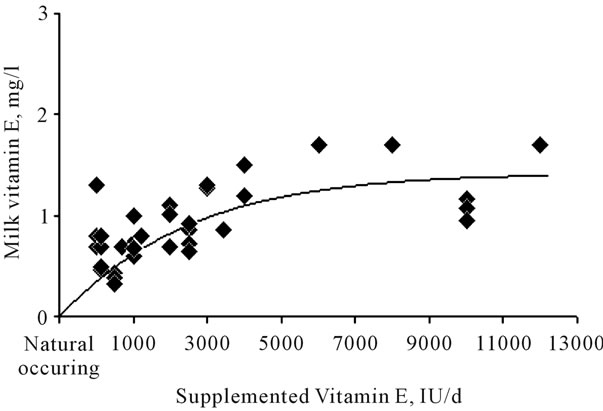
Figure 2. Milk content of vitamin E in relation to levels of vitamin E supplementation in dairy cows. Data from [17,18, 25,26,57-59].
concentrations of the 2R stereoisomers in milk were not significantly different between highand low-input conventional systems and organic low-input system [63].
High levels of vitamin E are effective in maintaining milk quality and safety in general, and a further advantage in increasing vitamin content of milk by animal nutrition rather than fortification is that it contributes to animal health safeguard, a primary factor in determining safety, quality and health benefits of food of animal origin [64].
4. Conclusion and Implications
Defining the relative bioequivalence between RRR and all-rac forms is fundamental to determine proper recommended intakes of different supplemental forms of vitamin E on the basis of α-tocopherol conversion factors, in dairy cows as in humans.
In literature, recent data clearly show that bioavailability of RRR α-tocopheryl acetate relative to all-rac α- tocopheryl acetate in dairy cows is 2:1. Nonetheless, the use of bioavailability studies in order to determine the ratio of potency of the different vitamin E stereoisomers is currently under debate. In the absence of clinical endpoints, no studies have been performed in cattle and pigs, as well as in humans, to assay the potency of RRR relative to all-rac α-tocopherol so far. On one hand, being bioavailability a precondition for any biological activity, some authors based the determination of utilisation efficiency and efficacy of vitamin E stereoisomers on bioavailability studies. Other authors disagree with such inference arguing that, as RRR and all-rac are not chemically identical, any ratio of bioavailability calculated in this way cannot be a valid substitute for the ratio of potency. Indeed, as discussed above, a greater bioavailability of the RRR form does not necessarily correspond to a higher bioactivity, as evidenced by no effect of this form shown on neutrophil function. Furthermore, an essential requisite frequently not considered in vitamin E feeding trials for the estimation of the true relative bioavailability of RRR over all-rac α-tocopheryl acetate, is the correction of α-tocopherol intake originating from basal ingredients.
REFERENCES
- L. R. McDowell, S. N. Williams, N. Hidiroglou, C. A. Njeru, G. M. Hill, L. Ochoa and N. S. Wilkinson, “Vitamin E Supplementation for the Ruminant,” Animal Feed Science and Technology, Vol. 60, No. 3-4, 1996, pp. 273- 296. doi:10.1016/0377-8401(96)00982-0
- W. P. Weiss, “Requirements of Fat-Soluble Vitamins for Dairy Cows: A Review,” Journal of Dairy Science, Vol. 81, No. 9, 1998, pp. 2493-2501. doi:10.3168/jds.S0022-0302(98)70141-9
- R. D. Allison and R. A. Laven, “Effect of Vitamin E Supplementation on the Health and Fertility of Dairy Cows: A Review,” Veterinary Record, Vol. 147, No. 25, 2000, pp. 703-708.
- A. Baldi, “Vitamin E in Dairy Cows,” Livestock Production Science, Vol. 98, No. 1-2, 2005, pp. 117-122. doi:10.1016/j.livprodsci.2005.10.004
- N. Moyo, M. Nielen, C. Kruitwagen and A. C. Beynen, “Vitamin E Supplementation and Udder Health: A Meta-Analysis,” In: H. Hogeveen, Ed., Mastitis in Dairy Production. Current Knowledge and Future Solutions, Wageningen Academic Publishers, Wageningen, 2005, pp. 159-165.
- National Research Council, “Nutrient Requirements of Dairy Cattle,” 7th Edition, National Academy Press, Washington DC, 2001, pp. 167-177.
- H. Scherf, L. J. Machlin, T. M. Frye, B. A. Krautmann and S. N. Williams, “Vitamin E Biopotency: Comparison of Various ‘Natural-Derived’ and Chemically Synthesized Alpha-Tocopherols,” Animal Feed Science and Technology, Vol. 59, No. 1-3, 1996, pp. 115-126. doi:10.1016/0377-8401(95)00892-6
- United States Pharmacopeial Convention, “The United States Pharmacopeia: The National Formulary,” Rockville, 1979.
- EFSA Panel on Additives and Products or Substances Used in Animal Feed (FEEDAP), “Scientific Opinion on the Safety and Efficacy of Vitamin E as a Feed Additive for All Animal Species,” EFSA Journal, Vol. 8, No. 6, 2010, p. 1635.
- R. V. Acuff, S. S. Thedford, N. N. Hidiroglou, A. M. Papas and T. A. Odom Jr., “Relative Bioavailability of RRRand All-Rac-Alpha-Tocopheryl Acetate in Humans: Studies Using Deuterated Compounds,” American Journal of Clinical Nutrition, Vol. 60, No. 3, 1994, pp. 397- 402.
- G. Burton, “Vitamin E: Molecular and Biological Function,” Proceedings of the Nutrition Society, Nottingham, 12-15 July 1993, pp. 251-262.
- C. Kiyose, R. Muramatsu, Y. Kameyama, T. Ueda and O. Igarashi, “Biodiscrimination of Alpha-Tocopherol Stereoisomers in Humans after Oral Administration,” American Journal of Clinical Nutrition, Vol. 65, No. 3, 1997, pp. 785-789.
- G. W. Burton, M. G. Traber, R. V. Acuff, D. N. Walters, H. Kayden, L. Hughes and K. U. Ingold, “Human Plasma and Tissue Alpha-Tocopherol Concentrations in Response to Supplementation with Deuterated Natural and Synthetic Vitamin E,” American Journal of Clinical Nutrition, Vol. 67, No. 4, 1998, pp. 669-684.
- National Academy of Sciences, Institute of Medicine, Food and Nutrition Board, “DRI Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids,” The National Academies Press, Washington DC, 2000, pp. 186-283.
- S. K. Jensen and C. Lauridsen, “Alpha-Tocopherol Stereoisomers,” Vitamins and Hormones, Elsevier Academic Press Inc., San Diego, 2007, pp. 281-308.
- Y. Dersjant-Li and M. Peisker, “Utilization of Stereoisomers from Alpha-Tocopherol in Livestock Animals,” Journal of Animal Physiology and Animal Nutrition, Vol. 94, No. 4, 2009, pp. 413-421.
- G. E. Meglia, S. K. Jensen, C. Lauridsen and K. P. Waller, “Alpha-Tocopherol Concentration and Stereoisomer Composition in Plasma and Milk from Dairy Cows Fed Natural or Synthetic Vitamin E around Calving,” Journal of Dairy Research, Vol. 73, No. 2, 2006, pp. 227-234. doi:10.1017/S0022029906001701
- W. P. Weiss, J. S. Hogan and D. J. Wyatt, “Relative Bioavailability of All-Rac and RRR Vitamin E Based on Neutrophil Function and Total Alpha-Tocopherol and Isomer Concentrations in Periparturient Dairy Cows and Their Calves,” Journal of Dairy Science, Vol. 92, No. 2, 2009, pp. 720-731.
- S. K. Jensen, N. B. Kristensen, C. Lauridsen and K. Sejersen, “Enrichment of Cows’ Milk with Natural or Synthetic Vitamin E,” Proceedings of Vitamin and Additives in the Nutrition of Man and Animal, Jena, 28-29 September 2005, pp. 78-83.
- Y. Dersjant-Li and M. Peisker, “A Critical Review of Methodologies Used in Determination of Relative BioAvailability Ratio of RRR-Alpha-Tocopheryl Acetate and All-Rac-Alpha-Tocopheryl Acetate,” Journal of the Science of Food and Agriculture, Vol. 90, No. 10, 2010, pp. 1571-1577. doi:10.1002/jsfa.3994
- P. P. Hoppe, “Letter to the Editor Regarding the Review Paper by Dersjant-Li and Peisker,” Journal of Animal Physiology and Animal Nutrition, Vol. 94, No. 4, 2010, pp. 547-548.
- M. Hidiroglou, “Pharmacokinetic Profile of Plasma Tocopherol Following Intramuscular Administration of Acetylated Alpha-Tocopherol to Sheep,” Journal of Dairy Science, Vol. 79, No. 6, 1996, pp. 1027-1030. doi:10.3168/jds.S0022-0302(96)76455-X
- A. Baldi, V. Bontempo, F. Cheli, S. Carli, C. S. Rossi and V. Dell’Orto, “Relative Bioavailability of Vitamin E in Dairy Cows Following Intraruminal Administration of Three Different Preparations of DL-Alpha-Tocopheryl Acetate,” Veterinary Research, Vol. 28, No. 6, 1997, pp. 517-524.
- V. Bontempo, A. Baldi, F. Cheli, F. Fantuz, I. Politis, S. Carli and V. Dell’Orto, “Kinetic Behavior of Three Preparations of Alpha-Tocopherol after Oral Administration to Postpubertal Heifers,” American Journal of Veterinary Research, Vol. 61, No. 5, 2000, pp. 589-593. doi:10.2460/ajvr.2000.61.589
- W. P. Weiss and D. J. Wyatt, “Effect of Dietary Fat and Vitamin E on Alpha-Tocopherol in Milk from Dairy Cows,” Journal of Dairy Science, Vol. 86, No. 11, 2003, pp. 3582-3591. doi:10.3168/jds.S0022-0302(03)73964-2
- A. Baldi, G. Savoini, L. Pinotti, E. Monfardini, F. Cheli and V. Dell’Orto, “Effects of Vitamin E and Different Energy Sources on Vitamin E Status, Milk Quality and Reproduction in Transition Cows,” Journal of Veterinary Medicine. Series A, Vol. 47, No. 10, 2000, pp. 599-608. doi:10.1046/j.1439-0442.2000.00323.x
- L. Pinotti, A. Baldi, I. Politis, R. Rebucci, L. Sangalli and V. Dell’Orto, “Rumen-Protected Choline Administration to Transition Cows: Effects on Milk Production and Vitamin E Status,” Journal of Veterinary Medicine. Series A, Vol. 50, No. 1, 2003, pp. 18-21. doi:10.1046/j.1439-0442.2003.00502.x
- A. Baldi and L. Pinotti, “Choline Metabolism in HighProducing Dairy Cows: Metabolic and Nutritional Basis,” Canadian Journal of Animal Science, Vol. 86, No. 2, 2006, pp. 207-212. doi:10.4141/A05-061
- E. U. Nwose, H. F. Jelinek, R. S. Richards and P. G. Kerr, “The Vitamin E Regeneration System’ (VERS) and an Algorithm to Justify Antioxidant Supplementation in Diabetes—A Hypothesis,” Medical Hypotheses, Vol. 70, No. 5, 2008, pp. 1002-1008. doi:10.1016/j.mehy.2007.07.048
- J. M. Finch and R. J. Turner, “Effects of Selenium and Vitamin E on the Immune Responses of Domestic Animals,” Research in Veterinary Science, Vol. 60, No. 2, 1996, pp. 97-106. doi:10.1016/S0034-5288(96)90001-6
- J. W. Spears and W. P. Weiss, “Role of Antioxidants and Trace Elements in Health and Immunity of Transition Dairy Cows,” Veterinary Journal, Vol. 176, No. 1, 2008, pp. 70-76. doi:10.1016/j.tvjl.2007.12.015
- S. Salman, A. Khol-Parisini, H. Schafft, M. LahrssenWiederholt, H. W. Hulan, D. Dinse and J. Zentek, “The Role of Dietary Selenium in Bovine Mammary Gland Health and Immune Function,” Animal Health Research Reviews, Vol. 10, No. 1, 2009, pp. 21-34. doi:10.1017/S1466252308001588
- A. Ceballos, J. Sanchez, H. Stryhn, J. B. Montgomery, H. W. Barkema and J. J. Wichtel, “Meta-Analysis of the Effect of Oral Selenium Supplementation on Milk Selenium Concentration in Cattle,” Journal of Dairy Science, Vol. 92, No. 1, 2009, pp. 324-342. doi:10.3168/jds.2008-1545
- T. Q. Cai, P. G. Weston, L. A. Lund, B. Brodie, D. J. McKenna and W. C. Wagner, “Association between Neutrophil Functions and Periparturient Disorders in Cows,” American Journal of Veterinary Research, Vol. 55, No. 7, 1994, pp. 934-43.
- B. A. Mallard, J. C. Dekkers, M. J. Ireland, K. E. Leslie, S. Sharif, C. L. Vankampen, L. Wagter and B. N. Wilkie, “Alteration in Immune Responsiveness during the Peripartum Period and Its Ramification on Dairy Cow and Calf Health,” Journal of Dairy Science, Vol. 81, No. 2, 1998, pp. 585-595. doi:10.3168/jds.S0022-0302(98)75612-7
- J. S. Hogan, K. L. Smith, W. P. Weiss, D. A. Todhunter and W. L. Schockey, “Relationships among Vitamin E, Selenium, and Bovine Blood Neutrophils,” Journal of Dairy Science, Vol. 73, No. 9, 1990, pp. 2372-2378. doi:10.3168/jds.S0022-0302(90)78920-5
- J. S. Hogan, W. P. Weiss, D. A. Todhunter, K. L. Smith and P. S. Schoenberger, “Bovine Neutrophil Responses to Parenteral Vitamin E,” Journal of Dairy Science, Vol. 75, No. 2, 1992, pp. 340-399. doi:10.3168/jds.S0022-0302(92)77775-3
- J. S. Hogan, W. P. Weiss and K. L. Smith, “Role of Vitamin E and Selenium in Host Defence against Mastitis,” Journal of Dairy Science, Vol. 76, No. 9, 1993, pp. 2795- 2908. doi:10.3168/jds.S0022-0302(93)77618-3
- I. Politis, N. Hidiroglou, T. R. Batra, J. A. Gilmore, R. C. Gorewit and H. Scherf, “Effects of Vitamin E on Immune Function of Dairy Cows,” American Journal of Veterinary Research, Vol. 56, No. 2, 1995, pp. 179-184.
- I. Politis, N. Hidiroglou, J. H. White, J. A. Gilmore, S. N. Williams, H. Scherf and M. Frigg, “Effects of Vitamin E on Mammary and Blood Leukocyte Function, with Emphasis on Chemotaxis, in Periparturient Dairy Cows,” American Journal of Veterinary Research, Vol. 57, No. 4, 1996, pp. 468-471.
- I. Politis, N. Hidiroglou, F. Cheli and A. Baldi, “Effects of Vitamin E on Urokinase-Plasminogen Activator Receptor Expression by Bovine Neutrophils,” American Journal of Veterinary Research, Vol. 62, No. 12, 2001, pp. 1934-1938. doi:10.2460/ajvr.2001.62.1934
- I. Politis, I. Bizelis, A. Tsiaras and A. Baldi, “Effect of Vitamin E Supplementation on Neutrophil Function, Milk Composition and Plasmin Activity in Dairy Cows in a Commercial Herd,” Journal of Dairy Research, Vol. 71, No. 3, 2004, pp. 273-278. doi:10.1017/S002202990400010X
- K. L. Smith, J. H. Harrison, D. D. Hancock, D. A. Todhunter and H. R. Conrad, “Effect of Vitamin E and Selenium Supplementation on Incidence of Clinical Mastitis and Duration of Clinical Symptoms,” Journal of Dairy Science, Vol. 67, No. 6, 1984, pp. 1293-1300. doi:10.3168/jds.S0022-0302(84)81436-8
- W. P. Weiss, J. S. Hogan, D. A. Todhunter and K. L. Smith, “Effect of Vitamin E Supplementation in Diets with a Low Concentration of Selenium on Mammary Gland Health of Dairy Cows,” Journal of Dairy Science, Vol. 80, No. 8, 1997, pp. 1728-1737. doi:10.3168/jds.S0022-0302(97)76105-8
- N. Bourne, D. C. Wathes, K. E. Lawrence, M. McGowan and R. A. Laven, “The Effect of Parenteral Supplementation of Vitamin E with Selenium on the Health and Productivity of Dairy Cattle in the UK,” The Veterinary Journal, Vol. 177, No. 3, 2008, pp. 381-387. doi:10.1016/j.tvjl.2007.06.006
- S. J. LeBlanc, T. F. Duffield, K. E. Leslie, K. G. Bateman, J. TenHag, J. S. Walton and W. H. Johnson, “The Effect of Prepartum Injection of Vitamin E on Health in Transition Dairy Cows,” Journal of Dairy Science, Vol. 85, No. 6, 2002, pp. 1416-1426. doi:10.3168/jds.S0022-0302(02)74209-4
- K. Persson Waller, C. Hallen Sandgren, U. Emanuelson and S. K. Jensen, “Supplementation of RRR-Alpha-Tocopheryl Acetate to Periparturient Dairy Cows in Commercial Herds with High Mastitis Incidence,” Journal of Dairy Science, Vol. 90, No. 8, 2007, pp. 3640-3646. doi:10.3168/jds.2006-421
- R. J. Bouwstra, M. Nielen, J. A. Stegeman, P. Dobbelaar, J. R. Newbold, E. H. J. M. Jansen and T. van Werven, “Vitamin E Supplementation during the Dry Period in Dairy Cattle. Part I: Adverse Effect on Incidence of Mastitis Postpartum in a Double-Blind Randomized Field Trial,” Journal of Dairy Science, Vol. 93, No. 12, 2010, pp. 5684-5695. doi:10.3168/jds.2010-3159
- W. P. Weiss, D. A. Todhunter, J. S. Hogan and K. L. Smith, “Effect of Duration of Supplementation of Selenium and Vitamin E on Periparturient Dairy Cows,” Journal of Dairy Science, Vol. 73, No. 11, 1990, pp. 3187-3194. doi:10.3168/jds.S0022-0302(90)79009-1
- R. J. Bouwstra, M. Nielen, J. R. Newbold, E. H. J. M. Jansen, H. F. Jelinek and T. van Werven, “Vitamin E Supplementation during the Dry Period in Dairy Cattle. Part II: Oxidative Stress Following Vitamin E Supplementation May Increase Clinical Mastitis Incidence Postpartum,” Journal of Dairy Science, Vol. 93, No. 12, 2010, pp. 5696-5706. doi:10.3168/jds.2010-3161
- J. W. G. Nicholson, A. M. St-Laurent, R. E. McQueen and E. Charmley, “The Effect of Feeding Organically Bound Selenium and α-Tocopherol to Dairy Cows on Susceptibility of Milk to Oxidation,” Canadian Journal of Animal Science, Vol. 71, No. 1, 1991, pp. 135-143.
- E. Charmley, J. W. G. Nicholson and J. A. Zee, “Effect of Supplemental Vitamin E and Selenium in the Diet on Vitamin E and Selenium Levels and Control of Oxidized Flavor in Milk from Holstein Cows,” Canadian Journal of Animal Science, Vol. 73, No. 2, 1993, pp. 453-457. doi:10.4141/cjas93-049
- R. M. Al-Mabruk, N. F. G. Beck and R. J. Dewhurst, “Effects of Silage Species and Supplemental Vitamin E on the Oxidative Stability of Milk,” Journal of Dairy Science, Vol. 87, No. 2, 2004, pp. 406-412. doi:10.3168/jds.S0022-0302(04)73180-X
- A. S. Atwal, M. Hidiroglou, J. K. G. Kramer and M. R. Binns, “Effects of Feeding Alpha-Tocopherol and Calcium Salts of Fatty-Acids on Vitamin E and Fatty-Acid Composition of Cows Milk,” Journal of Dairy Science, Vol. 73, No. 10, 1990, pp. 2832-2841. doi:10.3168/jds.S0022-0302(90)78971-0
- W. P. Weiss, “Antioxidant Nutrients, Cow Health, and Milk Quality,” Proceedings of the Penn State Dairy Cattle Nutrition Workshop, Grantville, 9-10 November 2005, pp. 11-18.
- D. Durand, V. Scislowski, D. Gruffat, Y. Chilliard and D. Bauchart, “High-Fat Rations and Lipid Peroxidation in Ruminants: Consequences on the Health of Animals and Quality of Their Products,” In: J. F. Hocquette and S. Gigli, Eds., Indicators of Milk and Beef Quality, Wageningen Academic Publishers, Wageningen, 2005, pp. 137- 150.
- T. Slots, L. H. Skibsted and J. H. Nielsen, “The Difference in Transfer of All-Rac-Alpha-Tocopherol StereoIsomers to Milk from Cows and the Effect on Its Oxidative Stability,” International Dairy Journal, Vol. 17, No. 7, 2007, pp. 737-745. doi:10.1016/j.idairyj.2006.09.010
- R. D. Allison and R. A. Laven, “Vitamin E for Milk Production in Dairy Cows: A Review,” Nutrition Abstracts and Reviews. Series B, Livestock Feeds and Feeding, Vol. 71, No. 12, 2001, pp. 43- 51.
- M. S. Havemose, M. R. Weisbjerg, W. L. P. Bredie, H. D. Poulsen and J. H. Nielsen, “Oxidative Stability of Milk Influenced by Fatty Acids, Antioxidants, and Copper Derived from Feed,” Journal of Dairy Science, Vol. 89, No. 6, 2006, pp. 1970-1980. doi:10.3168/jds.S0022-0302(06)72264-0
- P. Bergamo, E. Fedele, L. Iannibelli and G. Marzillo, “Fat-Soluble Vitamin Contents and Fatty Acid Composition in Organic and Conventional Italian Dairy Products,” Food Chemistry, Vol. 82, No. 4, 2003, pp. 625-631. doi:10.1016/S0308-8146(03)00036-0
- J. H. Nielsen, T. Lund-Nielsen and L. Skibsted, “Higher Antioxidant Content in Organic Milk than in Conventional Milk Due to Feeding Strategy,” Newsletter from Danish Research Centre for Organic Farming, No. 3, 2004.
- K. A. Ellis, A. Monteiro, G. T. Innocent, D. Grove-White, P. Cripps, W. G. McLean, C. V. Howard and M. Mihm, “Investigation of the Vitamins A and E and Beta-Carotene Content in Milk from UK Organic and Conventional Dairy Farms,” Journal of Dairy Research, Vol. 74, No. 4, 2007, pp. 484-491. doi:10.1017/S0022029907002816
- G. Butler, J. H. Nielsen, T. Slots, C. Seal, M. D. Eyre, R. Sanderson and C. Leifert, “Fatty Acid and Fat-Soluble Antioxidant Concentrations in Milk from Highand Low-Input Conventional and Organic Systems: Seasonal Variation,” Journal of the Science of Food and Agriculture, Vol. 88, No. 8, 2008, pp. 1431-1441. doi:10.1002/jsfa.3235
- A. Baldi and L. Pinotti, “Lipophilic Microconstituents of Milk,” In: Z. Bösze, Ed., Bioactive Components of Milk, Springer-Verlag, Berlin, Vol. 606, Part 1, 2008, pp. 109- 125. doi:10.1007/978-0-387-74087-4_3

