Surgical Science
Vol. 4 No. 2 (2013) , Article ID: 27873 , 6 pages DOI:10.4236/ss.2013.42030
Patterns of Recurrence and Their Significance in Patients with Endometrial Carcinoma*
—For Improved Follow-Up after Initial Treatment
Department of Obstetrics and Gynecology, Nagoya University Graduate School of Medicine, Nagoya, Japan
Email: #kajiyama@med.nagoya-u.ac.jp
Received November 9, 2012; revised December 8, 2012; accepted December 17, 2012
Keywords: Endometrial Carcinoma; Recurrence Site; Postrecurrence Survival; Symptomatic; Vaginal Vault Cytology
ABSTRACT
Objectives: The aim of this study was to identify the patterns of recurrence and their significance in patients with endometrial carcinoma (EMCA). Patients and Methods: After a search of the medical records from single institutions, a total of 49 patients with relapsed endometrial carcinoma were retrospectively evaluated. Various clinical information was examined, including the site of recurrence, detection procedure, and presence or absence of any symptom at the time of recurrence. Furthermore, the postrecurrence survival analysis was based on the Kaplan-Meier method. Results: The median follow-up period of all patients was 39.4 months (5.8 - 293.1). In all, twenty-five (51.0%) patients experienced recurrence within 12 months after the final treatment. At the time of recurrence, 15 (30.6%) and 34 (69.4%) patients were symptomatic and asymptomatic, respectively. Among the 34 asymptomatic patients, recurrence was detected by CT scan in 14 (28.6%), tumor markers alone in 14 (28.6%), and pelvic examination/ultrasound scan in 5 (10.2%). There was no relapsed case detected by vaginal vault cytology alone. The 5-year postrecurrence survival rates in symptomatic and asymptomatic patients were 57.5 and 36.6 months, respectively (P = 0.2973). After recurrence, 12 patients underwent debulking surgery, and 37 received salvage chemotherapy or radiotherapy. The postrecurrence survival of patients receiving surgery did not differ from that of those with chemotherapy/radiotherapy (P = 0.9198). Conclusion: Although imaging studies and tumor marker measurement contributed to the early detection of recurrence, they did not necessarily improve the prognosis postrecurrence.
1. Introduction
According to Cancer Statistics in 2008, it was estimated that 287,100 women were newly diagnosed with endometrial carcinoma (EMCA) worldwide [1], and it is the leading cause of death in developed countries. This tumor is generally known to be associated with an acceptable prognosis due to the fact that the majority of EMCA are diagnosed at an early stage. However, patients with advanced stage, deep myometrial invasion, a poorly differentiated tumor, and the presence of lymph node metastasis are considered to be at a higher risk of recurrence [2,3]. Indeed, based on previous large-scale studies, the risk of recurrence was reported to range from 13% to 17% [4-6]. Consequently, 10% - 20% of EMCA patients without extrauterine spread and 40% - 60% of those with metastatic lesions experienced recurrence [6-9].
In general, most recurrences occur within 3 years and tend to be accompanied by physical symptoms [8,10]. While intensive surveillance after initial treatment is commonly practiced in many institutions, there are no established guidelines for the optimal follow-up of EMCA patients. Moreover, it is now controversial whether the early detection of an asymptomatic recurrence during routine post-treatment surveillance leads to a survival benefit [11]. For example, several studies that have examined the usefulness of routine vaginal vault cytology demonstrated that the diagnostic modality rarely detects asymptomatic recurrence [8].
The aim of this study was to identify the patterns of recurrence to assess standard follow-up including routine vaginal vault cytology for detecting asymptomatic recurrence and the possibility of improving the prognosis.
2. Materials and Methods
2.1. Patients
Between 2001 and 2010, 290 patients were treated for EMCA in Nagoya University Hospital. Based on data from the medical records, we identified 49 patients who experienced recurrence.
Patients were excluded from this study if they had insufficient clinical data or a history of other malignancies, were lost to follow-up immediately after surgery, or had a sarcoma/carcinosarcoma histology. Stages were defined according to the 1988 International Federation of Gynecology and Obstetrics (FIGO) staging system.
2.2. Treatment
In principle, standard primary surgical treatment consisted of hysterectomy, bilateral salpingo-oophorectomy, and pelvic and/or paraaortic lymphadenectomy/sampling. Peritoneal washing was routinely carried out in all patients. If patients were of an advanced age, showed severe complications, and had a tumor showing well-differentiated/shallow myometrial invasion, retroperitoneal lymphadenectomy was omitted.
Among them, five patients received preoperative chemotherapy. Selected patients with a preoperative diagnosis of suspected cervical invasion sometimes underwent radical hysterectomy. Thirty-three patients underwent adjuvant chemotherapy, and three patients received adjuvant radiotherapy. Adjuvant therapy was not indicated for the thirteen patients without high-risk factors. Postoperative adjuvant chemotherapy regimens included carboplatin and paclitaxel/docetaxel in 24 patients. Other patients received CAP (cyclophosphamide, adriamycin, and cisplatin), CP (cyclophosphamide and cisplatin), AP (adriamycin and cisplatin), single agent of paclitaxel, etc.
2.3. Follow-Up and Analysis
The standard surveillance for EMCA patients following primary treatment consisted of physical examination, cytology from the vaginal vault, measurements of tumor markers, and transvaginal/transabdominal ultrasound. In principle, chest X-ray and CT scans of the abdomen and pelvis were conducted every 6 months until the fifth year. With or without a CT scan, positron emission tomogramphy (PET) and magnetic resonance imaging (MRI) were occasionally added according to individual circumstances.
The overall survival (OS) was defined as the time between the date of surgery and last date of follow-up or death from any cause. The postrecurrence survival (PRS) was defined as the time interval between the date of recurrence and last date of follow-up or death due to the disease. The survival curves were based on the Kaplan-Meier method and compared employing the Log-rank test. Multivariable analysis was carried out with the Cox proportional hazards model to evaluate independent factors affecting survival. A P-value of <0.05 was considered significant.
3. Results
3.1. Patients’ Characteristics
The clinical characteristics of the 49 patients who experienced recurrence are described in Table 1. The median follow-up was 39.4 months, ranging from 5.8 and 293.1 months.
The median age at primary treatment was 61 years old (33 - 78 years). The distribution of the FIGO stage was as follows: 26.5% of patients in stage I (n = 13), 12.2% in stage II (n = 6), 53.1% in stage III (n = 26), and 8.2% in stage IV (n = 4). Regarding the histological type, the endometrioid type was the most frequently observed (39/ 49: 79.6%). A serous histology was the second most frequently identified (7/49: 14.3%). Of all, fifteen (30.6%) patients were symptomatic. The most frequent symptom was vaginal bleeding, which was noted in six patients. Thirty-four (69.4%) recurrences were asymptomatic and detected on routine follow-up.
The distribution of the timing of recurrence after the final treatment is shown in Figure 1. Twenty-five (51.0%) recurrences were detected within 12 months after the initial treatment. There were 4 (8.2%) patients who experienced recurrence 5 years after the initial treatment.
Detection methods in asymptomatic patients with regards to the site of recurrence are shown in Figure 2. Among the 34 asymptomatic patients, fourteen recurrences were detected by CT scan (vagina: 1, pelvis: 2, paraaortic lymph node: 8, distant organ: 3). In fourteen patients, recurrence was detected based on elevated tumor markers (vagina: 1, pelvis: 5, paraaortic lymph node: 4, distant organ: 4). There was no recurrence detected by vaginal vault cytology alone.
3.2. The Survival of Recurrent EMCA Patients
In all patients, the 5-year overall and postrecurrence survival rates were 50.9% and 43.1%, respectively. In addition, the median time to recurrence from the initial
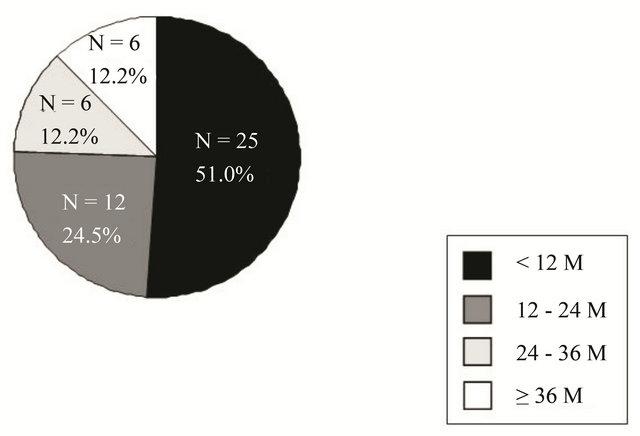
Figure 1. Distribution of the timing of recurrence after final treatment.
Table 1. Patients’ characteristics.
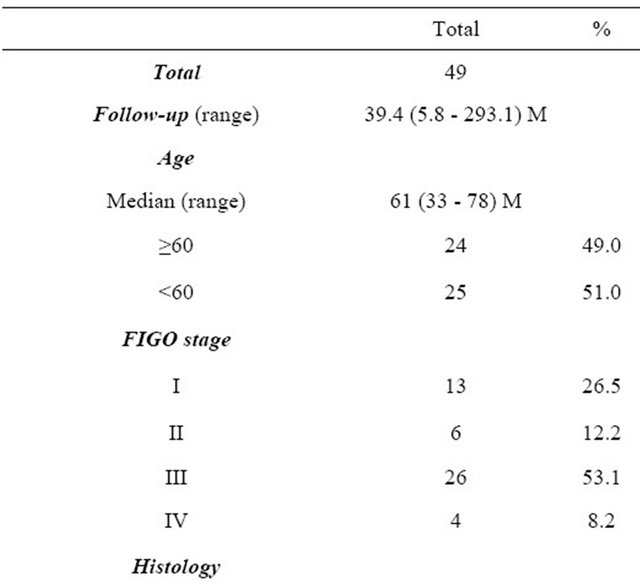
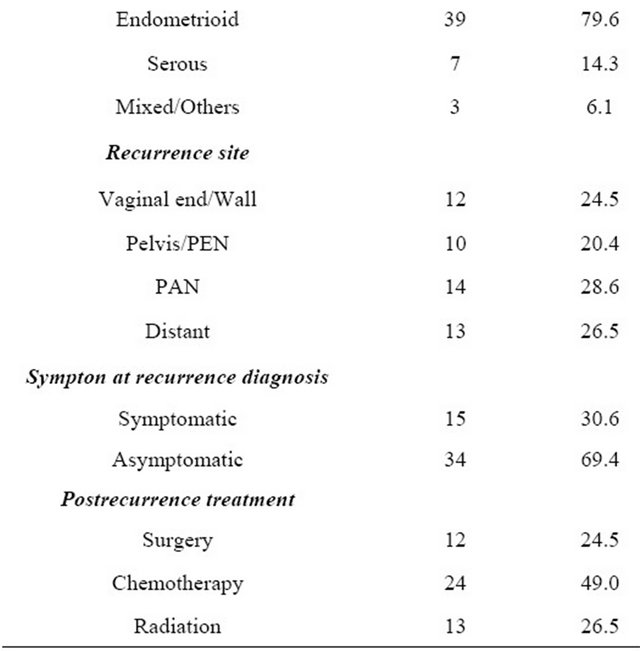
PEN: Pelvic lymph node; PAN: Paraaortic lymph node.
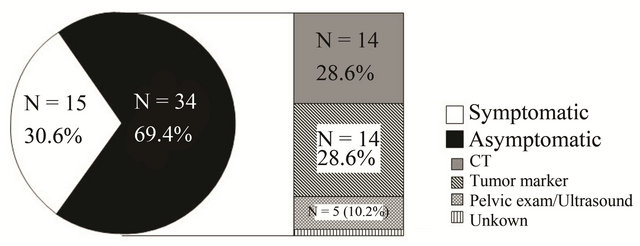
Figure 2. The presence or absence of any physical symptom at the time of recurrence. Fifteen (30.6%) and 34 (69.4%) patients were symptomatic and asymptomatic, respectively. Among the 34 asymptomatic patients, recurrence was detected by CT scan in 14 (28.6%), tumor markers alone in 14 (28.6%), and pelvic examination/ultrasound scan in 5 (10.2%).
treatment of symptomatic and asymptomatic patients was 10.5 and 12.3 months, respectively. On stratifying by physical symptom, the 5-year postrecurrence survival rates in symptomatic and asymptomatic patients were 57.5 and 36.6 months, respectively. There was no significant difference between the two groups (P = 0.2973) (Figure 3).
After recurrence, 12 patients underwent debulking surgery, and 37 received salvage chemotherapy or radiotherapy. The postrecurrence survival of patients receiving surgery did not differ from that of those with chemotherapy/radiotherapy (P = 0.9198) (Figure 4).
3.3. Multivariable Analyses
To eliminate selection bias from a number of clinicopathologic factors as thoroughly as possible, the age (≤60 vs. >60), FIGO stage (I/II vs. III/IV), recurrence site (pelvis/vaginal cavity vs. distant/paraaortic lymph node),
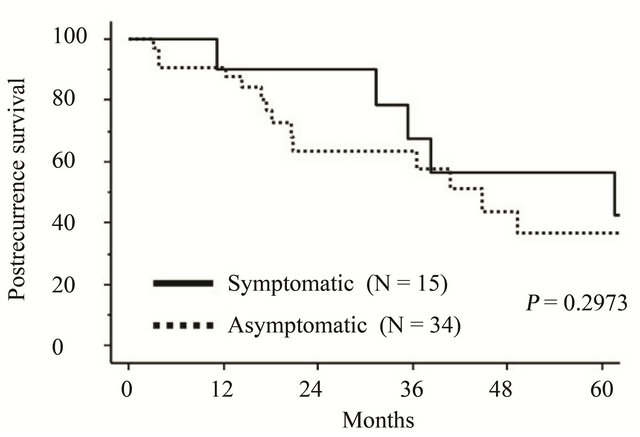
Figure 3. Kaplan-Meier-estimated postrecurrence survival of recurrent EMCA patients with or without any physical symptom at the time of recurrence. Solid line: symptomatic (N = 15), dotted line: asymptomatic (N = 34).
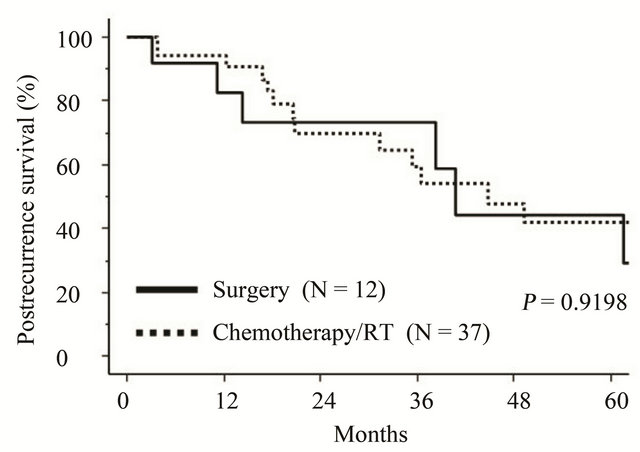
Figure 4. Kaplan-Meier-estimated postrecurrence survival of recurrent EMCA patients on stratifying by the postrecurrence treatment. Solid line: debulking surgery (N = 12), dotted line: Chemotherapy/radiotherapy (N = 37).
symptoms at the diagnosis of recurrence (symptomatic vs. asymptomatic), and time to recurrence (<12 M vs. ≥12 M) were entered into the multivariable analyses (Table 2). Among those factors, there was no independent prognostic indicator for postrecurrence survival.
3.4. Long-Term Survivors 5 Years after Recurrence
We finally show 9 patients surviving for more than 5 years after recurrence in Table 3. Of these cases, 6 patients were asymptomatic at the time of recurrence diagnosis. Furthermore, 5 patients showed no evidence of disease at the final follow-up.
4. Discussion
There has been no established recommendation regarding the optimal follow-up protocols in patients with EMCA owing to a lack of sufficient evidence to suggest that intensive surveillance after initial treatment results in improvement of the postrecurrence oncologic outcome. In our study, we detected recurrence in 49 (16.9%) of 290 patients, and, regarding the timing of relapse, 25 (51.0%) and 43 (87.7%) patients experienced recurrence within 1 and 3 years after primary treatment, respectively. These results are consistent with those in previous studies [2,5,11,12]. In addition, approximately 70% of all patients were asymptomatic at the recurrence diagnosis, and the majority of recurrences were identified by the periodical assessment of radiologic images and/or tumor markers. However, there was no patient whose asymptomatic recurrence was detected by vaginal vault cytology alone. Most patients with vaginal recurrence will have various symptoms of disease, and the additional effect of the periodical vaginal vault cytology remains to be elucidated. According to a report from Cooper et al., among 717 women who were diagnosed with uterine cancer, total of 36 women had recurrence in the vagina, 31 (86%) were apparent clinically, and only 5 (14%) were asymptomatic and identified by vaginal vault cytology. Based on several previous studies, vaginal vault cytology uncommonly identified vaginal recurrences in
Table 2. Multivariable analyses of clinicopathologic parameters in relation to postrecurrence survival.
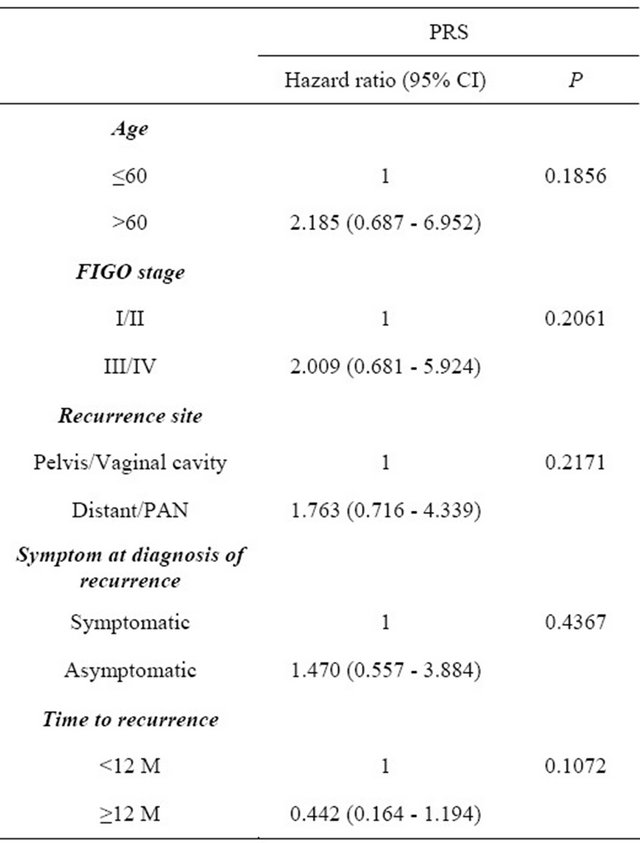
PRS: Postrecurrence survival; HR: Hazard ratio; CI: Confidence interval; FIGO: International federation of gynecology and obstetrics; PAN: Paraaortic lymph node.
Table 3. Long-term survivors 5 years after recurrence.
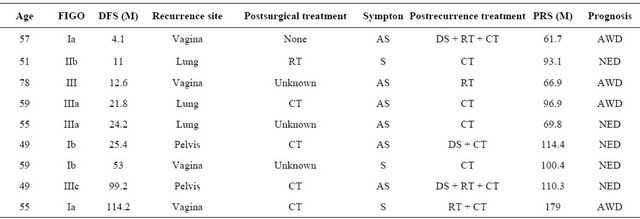
DFS: Disease-free survival; RT: Radiation; CT: Chemotherapy; DS: Debulking surgery; AS: Asymptomatic; S: Symptomatic; PRS: Postrecurrence survival; AWD: Alive with disease; NED: No evidence of disease.
patients without any disease symptom ranging from 0% to 25% [8,13-16]. We think that vaginal vault cytology in asymptomatic EMCA patients may be omissible from the point of cost-effectiveness.
In particular, it is still uncertain whether detecting recurrence before patients present any physical symptoms would lead to amelioration of the survival rate. In the present study, 34 (69.4%) asymptomatic cases were identified during a routine follow-up at the outpatient clinic. There was no significant difference in postrecurrence survival rates between symptomatic and asymptomatic patients. Namely, the early detection of recurrence through imaging studies and tumor marker measurement did not necessarily improve the postrecurrence prognosis. Ueda et al. reported that the median progression-free survival after recurrence of 16 asymptomatic patients was 9 months, which was significantly longer than the 3 months for the 13 symptomatic patients; however, the significance disappeared for the overall postrecurrence survival {median: 37 months (asymptomatic), 20 months (symptomatic)} [12]. On the other hand, according to a study from Sartori et al. involving 84 EMCA patients, there was a significant prognostic advantage in the postrecurrence survival when recurrence was detected in asymptomatic patients than in those who presented symptoms [5]. Presumably, the difference in such studies may be based on the limitations associated with any retrospective study. In our analysis, although the early detection of asymptomatic recurrence through the periodical follow-up surveillance cannot improve the prognosis as a whole, we think that it is meaningful for a certain proportion of patients with solitary recurrent disease which is curable by local surgical resection or radiotherapy. In fact, we identified long-term surviving patients after the retreatment of asymptomatic recurrence lesions in a distant organ. Smith et al. stated that patients at low/intermediate risk of recurrence may benefit from intensive follow-up [17]. In contrast, Dijkstra et al. pointed out a paradox considering the curability: patients with a low risk of recurrence have a high possibility of curable disease, while those with a high risk of recurrence have a low possibility of curable disease [18]. Simultaneously, as Tjalma et al. stated, there is little value in routine follow-up in terms of improving the survival rate for EMCA patients, and follow-up should be tailored to lowand high-risk patients [4]. At any rate, it is desirable for further investigations to accumulate EMCA patients who experienced recurrence with a large-scale prospective study in the future.
In summary, this study demonstrated that, although imaging studies and tumor marker measurement contributed to the early detection of recurrence, they did not necessarily improve the postrecurrence prognosis. Consequently, our current results highlighted similar clinical issues to previous studies. Indeed, whether the early detection of recurrence leads to improvement of the prognosis of patients with EMCA is still controversial. Nevertheless, in times of increasing concern over rising health care costs, it is an urgent task to establish an effective follow-up surveillance system for this tumor accompanying the omission of less meaningful modalities for the diagnosis. The present study had several limitations owing to its retrospective nature, such as the possibility of type II error, and variable follow-up length during the study period. However, we could overview the actual state regarding recurrent EMCA, so that a variety of issues surrounding this tumor were comprehended.
REFERENCES
- A. Jemal, R. Siegel, E. Ward, et al., “Cancer Statistics, 2006,” CA: A Cancer Journal for Clinicians, Vol. 56, No. 2, 2006, pp. 106-130. doi:10.3322/canjclin.56.2.106
- M. Fung-Kee-Fung, J. Dodge, L. Elit, H. Lukka, A. Chambers and T. Oliver, “Follow-Up after Primary Therapy for Endometrial Cancer: A Systematic Review,” Gynecologic Oncology, Vol. 101, No. 3, 2006, pp. 520-529. doi:10.1016/j.ygyno.2006.02.011
- W. T. Creasman, C. P. Morrow, B. N. Bundy, H. D. Homesley, J. E. Graham and P. B. Heller, “Surgical Pathologic Spread Patterns of Endometrial Cancer. A Gynecologic Oncology Group Study,” Cancer, Vol. 60, No. S8, 1987, pp. 2035-2041. doi:10.1002/1097-0142(19901015)60:8+<2035::AID-CNCR2820601515>3.0.CO;2-8
- W. A. Tjalma, P. A. van Dam, A. P. Makar and D. J. Cruickshank, “The Clinical Value and the Cost-Effectiveness of Follow-Up in Endometrial Cancer Patients,” International Journal of Gynecological Cancer, Vol. 14, No. 5, 2004, pp. 931-937. doi:10.1111/j.1048-891X.2004.014532.x
- E. Sartori, B. Pasinetti, L. Carrara, A. Gambino, F. Odicino and S. Pecorelli, “Pattern of Failure and Value of Follow-Up Procedures in Endometrial and Cervical Cancer Patients,” Gynecologic Oncology, Vol. 107, No. 1, 2007, pp. S241-S247. doi:10.1016/j.ygyno.2007.07.025
- C. P. Morrow, B. N. Bundy, R. J. Kurman, et al., “Relationship between Surgical-Pathological Risk Factors and Outcome in Clinical Stage I and II Carcinoma of the Endometrium: A Gynecologic Oncology Group Study,” Gynecologic Oncology, Vol. 40, No. 1, 1991, pp. 55-65. doi:10.1016/0090-8258(91)90086-K
- I. Otsuka, M. Uno, A. Wakabayashi, S. Kameda, H. Udagawa and T. Kubota, “Predictive Factors for Prolonged Survival in Recurrent Endometrial Carcinoma: Implications for Follow-Up Protocol,” Gynecologic Oncology, Vol. 119, No. 3, pp. 506-510. doi:10.1016/j.ygyno.2010.08.013
- A. Berchuck, C. Anspach, A. C. Evans, et al., “Postsurgical Surveillance of Patients with FIGO Stage I/II Endometrial Adenocarcinoma,” Gynecologic Oncology, Vol. 59, No. 1, 1995, pp. 20-24. doi:10.1006/gyno.1995.1262
- E. Podczaski, P. Kaminski, K. Gurski, et al., “Detection and Patterns of Treatment Failure in 300 Consecutive Cases of ‘Early’ Endometrial Cancer after Primary Surgery,” Gynecologic Oncology, Vol. 47, No. 3, 1992, pp. 323-327. doi:10.1016/0090-8258(92)90134-5
- H. B. Salvesen, L. A. Akslen, T. Iversen and O. E. Iversen, “Recurrence of Endometrial Carcinoma and the Value of Routine Follow up,” BJOG: An International Journal of Obstetrics & Gynaecology, Vol. 104, No. 11, 1997, pp. 1302-1307. doi:10.1111/j.1471-0528.1997.tb10979.x
- I. Otsuka, M. Uno, A. Wakabayashi, S. Kameda, H. Udagawa and T. Kubota, “Predictive Factors for Prolonged Survival in Recurrent Endometrial Carcinoma: Implications for Follow-Up Protocol,” Gynecologic Oncology, Vol. 119, No. 3, 2010, pp. 506-510. doi:10.1016/j.ygyno.2010.08.013
- Y. Ueda, T. Enomoto, T. Egawa-Takata, et al., “Endometrial Carcinoma: Better Prognosis for Asymptomatic Recurrences than for Symptomatic Cases Found by Routine Follow-Up,” International Journal of Clinical Oncology, Vol. 15, No. 4, pp. 406-412. doi:10.1007/s10147-010-0080-7
- A. G. Shumsky, G. C. Stuart, P. M. Brasher, J. G. Nation, D. I. Robertson and S. Sangkarat, “An Evaluation of Routine Follow-Up of Patients Treated for Endometrial Carcinoma,” Gynecologic Oncology, Vol. 55, No. 2, 1994, pp. 229-233. doi:10.1006/gyno.1994.1282
- J. M. Reddoch, T. W. Burke, M. Morris, C. Tornos, C. Levenback and D. M. Gershenson, “Surveillance for Recurrent Endometrial Carcinoma: Development of a Follow-Up Scheme,” Gynecologic Oncology, Vol. 59, No. 2, 1995, pp. 221-225. doi:10.1006/gyno.1995.0012
- P. Morice, C. Levy-Piedbois, S. Ajaj, et al., “Value and Cost Evaluation of Routine Follow-Up for Patients with Clinical Stage I/II Endometrial Cancer,” European Journal of Cancer, Vol. 37, No. 8, 2001, pp. 985-990. doi:10.1016/S0959-8049(01)00066-1
- O. O. Agboola, E. Grunfeld, D. Coyle and G. A. Perry, “Costs and Benefits of Routine Follow-Up after Curative Treatment for Endometrial Cancer,” CMAJ, Vol. 157, No. 7, 1997, pp. 879-886.
- C. J. Smith, M. Heeren, J. L. Nicklin, et al., “Efficacy of Routine Follow-Up in Patients with Recurrent Uterine Cancer,” Gynecologic Oncology, Vol. 107, No. 1, 2007, pp. 124-129. doi:10.1016/j.ygyno.2007.06.002
- J. R. Dijkstra, G. H. De Bock and M. J. Mourits, “The Clinical Value and the Cost-Effectiveness of Follow-Up in Endometrial Cancer Patients,” International Journal of Gynecological Cancer, Vol. 15, No. 5, 2005, pp. 991-992. doi:10.1111/j.1525-1438.2005.00257.x
NOTES
*All authors declare that there are no conflicts of interest.
#Corresponding author.

