Open Journal of Rheumatology and Autoimmune Diseases
Vol.4 No.1(2014), Article ID:42362,6 pages DOI:10.4236/ojra.2014.41008
Predicting Lung Function Decline with Serum Pneumoproteins: A Case Control Study
1Department of Medicine, Mount Sinai Hospital, Toronto, Ontario, Canada; 2Jewish General Hospital and McGill University, Montreal, Canada; 3Department of Medicine, Rheumatic Disease Unit, Section of Rheumatology, University of Manitoba, Winnipeg, Canada; 4Department of Medicine, Section of Respirology, University of Manitoba, Winnipeg, Canada.
Email: *smittoo@mtsinai.on.ca, marie.hudson@mcgill.ca, ernest.lo@mail.mcgill.ca, russell.steele.mcgill@gmail.com, skwong@cc.umanitoba.ca, DRobinson@exchange.hsc.mb.ca, zbshouty@exchange.hsc.mb.ca, mbaron@jgh.mcgill.ca
Copyright (c) 2014 Shikha Mittoo et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accor- dance of the Creative Commons Attribution License all Copyrights (c) 2014 are reserved for SCIRP and the owner of the intellectual property Shikha Mittoo et al. All Copyright (c) 2014 are guarded by law and by SCIRP as a guardian.
Received November 23rd, 2013; revised December 23rd, 2013; accepted December 30th, 2013
Keywords:Systemic Sclerosis; Interstitial Lung
ABSTRACT
Introduction: Predictors of lung function decline in systemic sclerosis (SSc) are unknown. Serum pneumoprotein levels, surfactant protein-D (SP-D) and Krebs von den Lungen-6 (KL-6), correlate with pulmonary damage. We aimed to test whether levels can predict rapid lung function decline in SSc. Methods: SSc patients who had serial pulmonary function tests (PFT) were analyzed for SP-D and KL-6 levels by enzyme linked immunosorbent assay. Levels were correlated with an annual rate of decline in % predicted forced vital capacity (FVC) of >-2% (out- come); controls did not experience this FVC decline. Uni- and multi-variate analysis, adjusting for age, disease duration, gender, baseline % predicted FVC, SP-D, and KL-6, was performed. Results are reported as mean ± SD. Results: Thirty three cases and 25 controls had a disease duration of 8.8 ± 7.3 and 8.3 ± 6.1 years, respectively. In adjusted analyses, lung function decline correlated with greater baseline FVC OR = 1.03 [95% CI of 1.00 - 1.07]; a trend towards significance was observed for greater levels of SP-D with FVC decline, OR = 1.37 [95% CI of 0.96 - 2.12]. Conclusion: Our data provide evidence that SSc patients with long-standing disease are still at risk for lung function decline and SP-D levels may predict lung function decline.
1. Introduction
Interstitial lung disease (ILD) is a significant complication and the leading cause of mortality in systemic sclerosis (SSc), but progression to end-stage restrictive lung disease is variable [1]. Predictors of a rapidly progressive lung function decline are unknown, but if found could potentially capture patients destined for a rapidly deteriorating course at an earlier point in their trajectory.
Randomized clinical trials using cyclophosphamide to treat SSc patients with ILD (SSc-ILD) have not been able to demonstrate a significant stabilization or improvement in lung function beyond one year [2-4]. The outcome of these trials may have been influenced, in part, by the fact that only a minority of patients may have been at risk of rapid ILD progression. As current treatments for SScILD carry significant toxicity, there is a pressing need for biomarkers that can identify patients at high risk for a rapid decline in lung function and in whom pharmacologic therapies would be of greatest benefit. Identifying such predictors would be of value to clinicians for clinical decision-making and to researchers when designing clinical trials for SSc-ILD.
Surfactant protein-D (SP-D) and Krebs von den Lungen-6 (KL-6) are glycoproteins found in a lipoprotein milieu that lines the lungs’ terminal airways (alveoli). Type II pneumocytes are responsible for their expression and, as a result, ensure alveolar stability and compliance [5,6]. Serum levels of these pneumoproteins are sensitive markers of SSc-ILD. Serum concentrations of these two pneumoproteins are significantly, inversely correlated with lung function parameters and positively correlated with radiographic changes of SSc-ILD [7-12]. It remains unknown whether SP-D and KL-6 levels correlate with the rate of lung function decline and are able to predict a rapid deterioration in lung function among SSc patients.
In a multi-center, prospective, study of a large cohort of SSc patients, we set out to determine whether serum levels of these pneumoproteins can predict a rapid decline in % predicted FVC.
2. Materials and Methods
2.1. Study Population
The Canadian Scleroderma Research Group (CSRG) Registry is a prospective, multi-center cohort comprised of 15 Canadian centers and greater than 1000 incident and prevalent SSc cases. Its purpose is to characterize clinical manifestations of and quality of life measures among SSc patients across Canada. Informed consent of registry patients was obtained and the research ethics board at each participating center approved the study protocol.
The CSRG registry was searched for evaluable subjects from September 2004 to April 2009. Patients were eligible if they satisfied the following criteria: 1) met the American College of Rheumatology criteria for the diagnosis of SSc, 2) had available baseline sera, 3) had at least two serial pulmonary function tests (PFTs) separated by a year, and 4) had available chest imaging (either chest X-rays (CXR) or chest computed tomography (CT) scans) [13].
2.2. Study Variables
Sociodemographic data, including self-reported ethnicity, clinical manifestations of SSc, and serum are collected on patients at baseline and annually; scleroderma subtype is defined according to criteria by LeRoy et al. [13,14]. Age of SSc onset was calculated from date of birth until the date of first non-Raynaud’s symptom; disease duration was defined as the duration from first non-Raynaud’s symptom until baseline registry visit. Results from pulmonary function tests (PFTs) and chest imaging (CXRs or CT scans) were recorded.
Smoking status was categorized by past use, never smoked, or current smoker at baseline visit. Current and past pharmacologic treatment with methotrexate, azathioprine, cyclophosphamide, and mycophenolate mofetil were noted at study entry and at the last available followup study visit. Antinuclear antibody (ANA), anti-centromere antibody, and anti-topoisomerase antibody were tested at study entry.
The absolute and % predicted forced vital capacity (FVC), total lung capacity (TLC) and diffusion capacity for carbon monoxide (DLCO) were recorded at baseline and at yearly registry visits. Restrictive lung disease was defined as a % predicted FVC or total lung capacity (TLC) of <80% predicted. Radiographic ILD was considered by the presence of interstitial infiltrates on chest imaging. Clinically significant ILD was defined as a % predicted FVC <80% and radiographic ILD.
A rapid decline in % predicted FVC was defined as an annual rate of decline in % predicted FVC of greater than 2% per year between the first and second PFT (cases). A normal rate of decline in % predicted FVC was defined as an annual rate of decline in % predicted FVC of equal to or less than 2%/year (controls).
2.3. Pneumoprotein Assays
After selection of cases and controls, a random number of serum samples was chosen for analysis; the number of samples analyzed was limited by financial contraints. Baseline serum from SSc patients and controls were analyzed, in duplicate, for levels of KL-6 (Sanko Junyako Co., Ltd, Tokyo, Japan) and SP-D (Biovendor, North Carolina, USA) by enzyme linked immunosorbent assay (ELISA) following the manufacturer’s guidelines by an experienced laboratory technician blinded to patient’s clinical data. KL-6 required a slight variation in technique, a sandwich ELISA method, but is a validated assay with an intra-assay coefficient of variation (cv) of 0.7% - 7.8% and inter-assay cv of 6.3% - 6.9% [15]. An additional 30 serum samples were obtained from nonsmoking, healthy controls collected under another protocol approved by the research ethics board at one of the participating CSRG sites (University of Manitoba, Winnipeg).
2.4. Statistical Analysis
Univariate and multivariate logistic regression was used to determine the factors associated with a rapid decline in lung function. Covariates used in the final multivariate model included age, disease duration, baseline % predicted FVC, SP-D, and KL-6. Multi-collinearity of serum pneumoproteins within each model was checked by examining the model variance inflation factors. In order to assess the impact of missing second visits for 9 of the patients, we also analyzed the data using inverse-probability weighting methods. We saw no important differences in the estimates or the conclusions, so we have omitted this data from this paper. Results are reported as mean ± SD except where indicated. Data analysis was performed using the R statistical package. (R Foundation for Statistical Computing, Vienna, Austria.)
3. Results
3.1. Baseline Characteristics: Cases and Controls
Of 954 SSc patients in the registry, 275 met eligibility criteria. Sera of 67 patients were analyzed. Of the 67 patients with analyzed sera, only 58 patients had measured FVC values within 12 months of the baseline appointment. There were 33 cases and 25 controls that attended their second visit, on average, 394 ± 44 days and 368 ± 37 days after the original baseline visit, respectively. Baseline demographic and pertinent clinical features of cases and controls are presented in Table 1. Cases had an age and disease duration of 53.2 ± 12.9 years and 8.8 ± 7.3 years compared with 50.1 ± 12.3 years and 8.3 ± 6.1 years, respectively. No differences were seen between cases and controls by gender, ethnicity, smoking status, autoantibody status, or disease duration (Table 1). There was a significantly higher proportion of diffuse cutaneous SSc subtype among controls than cases, 17 out of 25 patients (68%) compared with 12 out of 33 patients (36%), respectively.
Baseline mean ± SD % predicted FVC, TLC, and DLCO among cases was 97.8% ± 16.1%, 95.8 ± 14.2, and 68.3% ± 23.1%, respectively. Baseline mean ± SD % predicted FVC, TLC, and DLCO among controls was 90.4% ± 19.9%, 87.8% ± 18.9%, and 77.2% ± 24.4%,
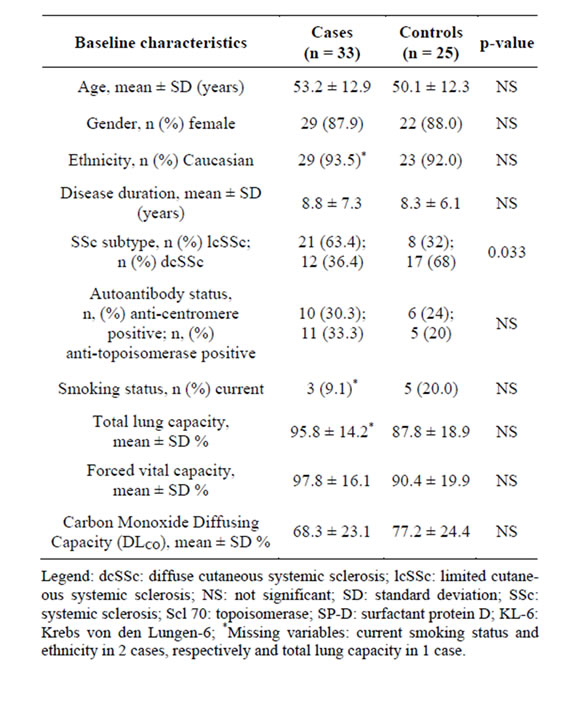
Table 1. Baseline clinical and serologic characteristics of scleroderma cases and controls*.
respectively (Table 1). Six cases (18%) and 10 (40%) controls had restrictive lung disease; 14 (42.4%) cases and 8 (32.0%) controls had radiographic ILD. Clinically significant ILD was seen in three cases (9.09%) and in six controls (24%). At study entry, one case was taking methotrexate and one was taking azathioprine. Among controls, four were taking methotrexate and two were taking azathioprine. None of the patients were on cyclophosphamide or mycophenolate mofetil at study entry. In the past, immunosuppressive medications other than steroids were prescribed to 5 cases (n = 2 methotrexate; n = 2 azathioprine; n = 1 cyclophosphamide) and 5 controls (n = 3 methotrexate; n = 1 azathioprine, n = 1 cyclophosphamide).
3.2. Baseline SP-D and KL-6 Levels
The median serum level of SP-D among SSc patients was 203.10 ng/mL (range, 22.1 - 935.40 ng/mL), which was significantly higher when compared with healthy controls (median level of 41.7 ng/mL, range, 20.9 - 99.4 ng/mL) (p < 0.0001). The median KL-6 levels of 658.22 U/mL (range, 0.03 - 3968.33 U/mL) were also significantly higher in SSc patients when compared with the level in healthy controls (median 36.2 U/mL (range, 33.5 - 40.6 U/mL) (p < 0.0001). There were no statistically significant differences among median serum levels of KL-6 between SSc cases (median 677 U/mL, range (0-3968.3 U/mL)) and SSc controls (median 551.8 U/mL, range (7.5 - 2958 U/mL)), nor were there statistically significantly higher levels of serum SP-D among SSc cases (203.10 ng/mL, range (22.1 - 935.4 ng/mL)) compared with SSc controls (159.3 ng/mL, range (37.4 - 618.7 ng/mL)).
3.3. Predictors of a Rapid Decline in % Predicted FVC
Next, we aimed to determine whether SP-D levels could predict rapid decline in % predicted FVC (Table 2). In univariate analysis, serum levels of KL-6 did not significantly predict a rapid decline in lung function. However, a trend was seen for levels of SP-D with outcome, OR = 1.22 [95% CI of 0.9 - 1.72]. Gender, ethnicity, autoantibody status, and smoking status did not predict a rapid decline in lung function among cases; lcSSc seems to be protective of lung function decline (Table 2).
Whereas patients defined as having clinically active ILD [OR = 2.00, 95% CI of 0.49 - 8.22, p = 0.34] or restrictive lung disease [OR = 0.95, 95% CI of 0.33 - 2.76, p = 0.93] were not at high risk for a rapid decline in % predicted FVC, there was a trend towards significance for patients with radiographic evidence of ILD [OR = 2.88, 95% CI of 0.96 - 8.65, p = 0.06]. After adjusting for age, disease duration, % predicted FVC at baseline, and KL-6 levels, a strong trend towards statistical signifi-

Table 2. Covariates associated with a rapid decline in lung function in systemic sclerosis on univariate analysis*.
cance was seen for higher SP-D levels and rapid lung function decline (OR = 1.44 with a 95% CI of 0.95 - 2.36, Table 3). This trend remained present even when SP-D was analyzed in a multivariate model with the same covariates except KL-6 (OR = 1.37 with a 95% CI of 0.96 - 2.10) (Table 3). In these adjusted analyses, there was no association between KL-6 and rapid decline in lung function.
Of note, in the multivariate analyses, baseline % predicted FVC also did significantly predicted rapid lung function decline [OR = 1.03, 95% CI of 1.00 - 1.07, Table 3]; this result also held in multivariate models where SP-D and KL-6 were analyzed separately.
Finally, if we were to use the outcome measure as >10% decrease of FVC and then examine whether SP-D predicts a decline of >10% predicted, we would have 14 cases and 44 controls. There would still be an increased risk whether we used >10% annual decrease of FVC or >2% annual decrease of FVC as the endpoint; the effect size, however, would change from an increased risk of 37% using the current endpoint of 2% FVC decline annually to a 4% increase in odds (95% CI: 0.70 - 1.51). We note that only 14 patients have a decline of >10% over one year, which decreases the number of events and makes it more difficult to show a difference between the two groups.
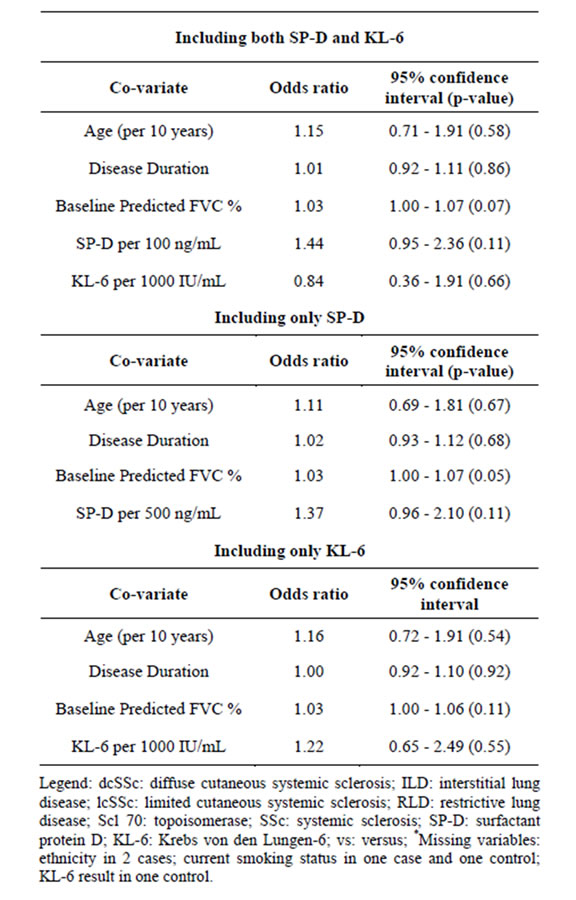
Table 3. Multivariate logistic regression analyses of rapid progression in systemic sclerosis.
4. Discussion
This is one of the largest series to date that examines the clinical and serologic predictors of lung function decline in SSc with a focus on serum glycoproteins. We found that there was a strong trend indicating an association between SP-D levels, but not KL-6, with rapid lung function decline. Baseline % predicted FVC also significantly predicted rapid FVC deterioriation.
Little is known about the predictors of a rapid deterioration in lung function in SSc. The literature is conflicting on whether baseline severity of lung involvement, as inferred by pulmonary physiologic parameters such as FVC, is a predictor of SSc-ILD progression. Randomized controlled trial data of SSc patients with a mean (±SD) % FVC at baseline of 68.1 ± 1.0 and disease duration of 3.2 ± 0.2 years, respectively, found that the severity of % predicted FVC at baseline was associated with radiographic progression of ILD [2]. On the contrary, in our population, with an average disease duration of slightly over 8 years and baseline % predicted FVC of greater than 90%, a better baseline in % predicted FVC predicted a rapid rate in lung function decline. In a study from the UK, Goh et al. found that among 215 SSc patients, lower baseline FVC and greater extent of radiographic damage on chest HRCT scans were predictive of a decline in FVC and/or Dlco in univariate analyses; in multivariate analyses, however, a threshold amount of radiographic damage at baseline was the most predictive of mortality [16]. The average disease duration in their study population is not well defined. Assassi et al. examined the predictors of a rapid rate of lung function decline in a cohort with an average disease duration of 2.5 years and found that the presence of anti-topoisomerase, but not baseline PFT parameters, significantly predicted a decline in FVC at 3 years, but not at long-term, follow-up [17].
It is widely appreciated that the greatest loss of lung volume occurs in the first few years after SSc disease onset, but lines of evidence also reveal that the rapid deterioration in lung function can occur among patients with long-standing disease, as seen in our cohort [1,18]. The importance of following the rate of FVC decline cannot be underscored. A faster deterioration in lung function is associated with mortality in SSc [17,19]. Our findings may indicate that patients with long-standing disease duration, who did not suffer a decline in lung function early in their disease course, are still at risk for a rapid lung function decline.
Previous studies evaluating the role of SP-D and KL-6 in SSc have demonstrated that these glycoproteins are positively, significantly correlated with the severity of radiographic fibrosis on chest imaging and inversely correlated with the % predicted FVC and DLCO on PFTs [7,11,12,20,21]. In a longitudinal analysis of 39 SSc patients with a mean follow-up of 2.9 years, Yanabe et al. also found that increasing serial KL-6 measurements corresponded with lung function deterioration over time [8,10]. However, the majority of studies evaluating the role of these glycoproteins have involved SSc patients of Japanese ancestry and in particular in patients positive for Scl 70 [8,10,20,21]. Differences in ethnicity and prevalence of anti-Scl 70 in our cohort may explain the fact that we did not find an association between KL-6 and rapid decline in lung function.
Due to budgetary constraints, we were unable to analyze a larger sample of serum or perform serial evaluation of the pneumoproteins. Such data may have provided more robust conclusions along with insights into how fluctuations of these markers correlate with changes in lung function over time. In addition, our results are not generalizable to all SSc patients since we excluded patients with less than a year of follow-up or those who did not have serial PFT measures. It is possible that some patients followed for less than a year had a rapid deterioration in lung function leading to death or lung transplant and would have not been captured in our study. On the other hand, the significance of our findings need to be emphasized. Identifying serum biomarkers to predict lung disease in SSc is of great importance. These markers may be more sensitive to change, are non-invasive, and can be repeatedly measured. They may also be useful for clinical trial purposes to identify SSc patients at high risk of rapid lung function decline. Our data provide a strong signal that serum SP-D may be a useful biomarker to predict rapid decline in lung function. Additional strengths of our study include the use of a multi-center, observational cohort of SSc patients and the fact that most study patients were not on any immunosuppressive therapy during the follow-up period. A rapid lung function decline was defined conservatively based on the fact that % predicted FVC should not decline in the general population, but that the unadjusted % FVC decline per year in the general population is approximately 1% [22,23]. Any rate of decline in % predicted FVC would be considered abnormal, but we chose to be even more conservative by using the definition of 2% predicted FVC/year to define a high risk SSc subgroup.
In conclusion, SSc patients with long-standing disease duration are still at risk for a rapid lung function decline and serum levels of SP-D hold promise for predicting rapid lung function decline in SSc. While larger studies are required to confirm our findings, baseline levels may be useful in identifying a high risk group of SSc patients for closer monitoring and therapeutic intervention
Acknowledgements and Funding
We would like to thank Dr. Charles Bernstein for providing control sera, Suzanne Taillefer, the CSRG national coordinator, and Mellissa Moyen, research coordinator. Grant support for SM was, in part, provided by the Manitoba Health Research Council for the study design, data collection, and for serum biomarker analysis. Grant support for the CSRG cohort is provided by the Canadian Institutes of Health Research.
REFERENCES
- P. D. Schneider, R. A. Wise, M. C. Hochberg and F. M. Wigley, “Serial Pulmonary Function in Systemic Sclerosis,” American Journal of Medicine, Vol. 73, No. 3, 1982, pp. 385-394. http://dx.doi.org/10.1016/0002-9343(82)90732-X
- D. P. Tashkin, R. Elashoff, P. J. Clements, J. Goldin, M. D. Roth, D. E. Furst, et al., “Cyclophosphamide versus Placebo in Scleroderma Lung Disease,” New England Journal of Medicine, Vol. 354, No. 25, 2006, pp. 2655- 2666. http://dx.doi.org/10.1056/NEJMoa055120
- D. P. Tashkin, R. Elashoff, P. J. Clements, M. D. Roth, D. E. Furst, R. M. Silver, et al., “Effects of 1-Year Treatment with Cyclophosphamide on Outcomes at 2 Years in Scleroderma Lung Disease,” American Journal of Respiratory and Critical Care Medicine, Vol. 176, No. 10, 2007, pp. 1026-1034. http://dx.doi.org/10.1164/rccm.200702-326OC
- R. K. Hoyles, R. W. Ellis, J. Wellsbury, B. Lees, P. Newlands, N. S. Goh, et al., “A Multicenter, Prospective, Randomized, Double-Blind, Placebo-Controlled Trial of Corticosteroids and Intravenous Cyclophosphamide Followed by Oral Azathioprine for the Treatment of Pulmonary Fibrosis in Scleroderma,” Arthritis & Rheumatology, Vol. 54, No. 12, 2006, pp. 3962-3970. http://dx.doi.org/10.1002/art.22204
- R. Mason, V. Broaddus, J. Murray and J. Nadel, “Murray & Nadel’s Textbook of Respiratory Medicine,” Philadelphia, 2005.
- J. Madsen, A. Kliem, I. Tornoe, K. Skjodt, C. Koch and U. Holmskov, “Localization of Lung Surfactant Protein D on Mucosal Surfaces in Human Tissues,” Journal of Immunology, Vol. 164, No. 11, 2000, pp. 5866-5870.
- Y. Asano, H. Ihn, K. Yamane, N. Yazawa, M. Kubo, M. Fujimoto, et al., “Clinical Significance of Surfactant Protein D as a Serum Marker for Evaluating Pulmonary Fibrosis in Patients with Systemic Sclerosis,” Arthritis & Rheumatology, Vol. 44, No. 6, 2001, pp. 1363-1369. http://dx.doi.org/10.1002/1529-0131(200106)44:6<1363::AID-ART229>3.0.CO;2-5
- K. Yanaba, M. Hasegawa, K. Takehara and S. Sato, “Comparative Study of Serum Surfactant Protein-D and KL-6 Concentrations in Patients with Systemic Sclerosis as Markers for Monitoring the Activity of Pulmonary Fibrosis,” Journal of Rheumatology, Vol. 31, No. 6, 2004, pp. 1112-1120.
- K. Yamane, H. Ihn, M. Kubo, N. Yazawa, K. Kikuchi, Y. Soma and K. Tamaki, “Serum Levels of KL-6 as a Useful Marker for Evaluating Pulmonary Fibrosis in Patients with Systemic Sclerosis,” Journal of Rheumatology, Vol. 27, No. 4, 2000, pp. 930-934.
- K. Yanaba, M. Hasegawa, Y. Hamaguchi, M. Fujimoto, K. Takehara and S. Sato, “Longitudinal Analysis of Serum KL-6 Levels in Patients with Systemic Sclerosis: Association with the Activity of Pulmonary Fibrosis,” Clinical and Experimental Rheumatology, Vol. 21, No. 4, 2003, pp. 429-436.
- F. N. Hant, A. Ludwicka-Bradley, H. J. Wang, N. Li, R. Elashoff, D. P. Tashkin, et al., “Surfactant Protein D and KL-6 as Serum Biomarkers of Interstitial Lung Disease in Patients with Scleroderma,” Journal of Rheumatology, Vol. 36, No. 4, 2009, pp. 773-780. http://dx.doi.org/10.3899/jrheum.080633
- S. Sato, T. Nagaoka, M. Hasegawa, C. Nishijima and K. Takehara, “Elevated Serum KL-6 Levels in Patients with Systemic Sclerosis: Association with the Severity of Pulmonary Fibrosis,” Dermatology, Vol. 200, No. 3, 2000, pp. 196-201. http://dx.doi.org/10.1159/000018382
- Preliminary Criteria for the Classification of Systemic Sclerosis (Scleroderma), “Subcommittee for Scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee,” Arthritis & Rheumatology, Vol. 23, No. 5, 1980, pp. 581-590.
- E. C. LeRoy, C. Black, R. Fleischmajer, S. Jablonska, T. Krieg, T. A. Medsger Jr., et al., “Scleroderma (Systemic Sclerosis): Classification, Subsets and Pathogenesis,” Journal of Rheumatology, Vol. 15, No. 2, 1988, pp. 202-205.
- A. Yokoyama, K. Kondo, M. Nakajima, T. Matsushima, T. Takahashi, M. Nishimura, et al., “Prognostic Value of Circulating KL-6 in Idiopathic Pulmonary Fibrosis,” Respirology, Vol. 11, No. 2, 2006, pp. 164-168. http://dx.doi.org/10.1111/j.1440-1843.2006.00834.x
- N. S. Goh, S. R. Desai, S. Veeraraghavan, D. M. Hansell, S. J. Copley, T. M. Maher, et al., “Interstitial Lung Disease in Systemic Sclerosis: A Simple Staging System,” American Journal of Respiratory and Critical Care Medicine, Vol. 177, No. 11, 2008, pp. 1248-1254. http://dx.doi.org/10.1164/rccm.200706-877OC
- S. Assassi, R. Sharif, R. E. Lasky, T. A. McNearney, Y. M. R. M. Estrada, H. Draeger, et al., “Predictors of Interstitial Lung Disease in Early Systemic Sclerosis: A Prospective Longitudinal Study of the GENISOS Cohort,” Arthritis Research & Therapy, Vol. 12, No. 5, 2010, p. R166. http://dx.doi.org/10.1186/ar3125
- V. D. Steen, C. Conte, G. R. Owens and T. A. Medsger Jr., “Severe Restrictive Lung Disease in Systemic Sclerosis,” Arthritis & Rheumatology, Vol. 37, No. 9, 1994, pp. 1283-1289. http://dx.doi.org/10.1002/art.1780370903
- D. P. Tashkin, P. J. Clements, R. S. Wright, H. Gong Jr., M. S. Simmons, P. A. Lachenbruch, et al., “Interrelationships between Pulmonary and Extrapulmonary Involvement in Systemic Sclerosis. A Longitudinal Analysis,” Chest, Vol. 105, No. 2, 1994, pp. 489-495. http://dx.doi.org/10.1378/chest.105.2.489
- M. Maeda, Y. Ichiki, Y. Aoyama and Y. Kitajima, “Surfactant Protein D (SP-D) and Systemic Scleroderma (SSc),” Journal of Dermatology, Vol. 28, No. 9, 2001, pp. 467-474.
- G. Kumanovics, T. Minier, J. Radics, L. Palinkas, T. Berki and L. Czirjak, “Comprehensive Investigation of Novel Serum Markers of Pulmonary Fibrosis Associated with Systemic Sclerosis and Dermato/Polymyositis,” Clinical and Experimental Rheumatology, Vol. 26, No. 3, 2008, pp. 414-420.
- J. E. Cotes, D. J. Chinn and M. R. Miller, “Lung Function: Physiology, Measurement, and Application in Medicine,” 6th Edition, Blackwell Publishing, 2006. http://dx.doi.org/10.1002/9781444312829
- H. I. Goldman and M. R. Becklake, “Respiratory Function Tests; Normal Values at Median Altitudes and the Prediction of Normal Results,” American Review of Tuberculosis, Vol. 79, No. 4, 1959, pp. 457-467.
NOTES
*Corresponding author.

