Open Journal of Endocrine and Metabolic Diseases
Vol.2 No.4(2012), Article ID:24555,4 pages DOI:10.4236/ojemd.2012.24015
Inhibited 131I Uptake but Normal Release of Thyroid Hormone by Thyroid Gland in Response to TSH Administration in Subclinical Hypothyroidism
Endocrine Section, Medical Service, Veterans Affairs Medical Center, Des Moines, University of Iowa, Iowa City, USA
Email: udaya.kabadi@va.gov, ukabadi@gmail.com
Received September 12, 2012; revised October 13, 2012; accepted November 15, 2012
Keywords: Subclinical Hypothyroidism; 131I Thyroid Uptake; Thyroid Hormone Release
ABSTRACT
Background: Subclinical hypothyroidism is characterized by normal circulating thyroid hormone levels with super-normal TSH concentrations in absence of clinical manifestations. In majority of subjects, an etiologic factor is often identified. Moreover, therapy with levothyroxine normalizes serum TSH concentration while maintaining normal thyroid hormone concentrations. However, the exact pathophysiology of these thyroid hormone alterations is not well defined. Objective: Major steps in synthesis i.e. iodine uptake and the release of thyroid hormones in response to SC TSH administration were assessed in subjects with subclinical hypothyroidism. Methods: 10 men and 5 women with subclinical hypothyroidism, ages 42 - 76 years and 10 euthyroid men (39 - 70 years) participated. 24 hr 131Iodine thyroid uptake and serum T3, T4 and TSH concentrations were determined prior to and after SC administration of recombinant human TSH, 0.9 mg for two consecutive days. Comparisons were conducted for 24 hour uptake values as well as serum T3, T4 and TSH levels obtained prior to and after TSH administration. Results: In subjects with subclinical hypothyroidism 24 hour 131I thyroidal uptakes were normal (10% - 30%). However, the mean value was significantly lower, (p < 0.05) as compared to euthyroid volunteers. 24 hr uptakes rose following TSH administration in both groups. However, the rise was significant (p < 0.01) only in normal subjects. Furthermore, both the mean absolute uptake and the mean % rise following TSH administration were markedly significantly lower in subjects with subclinical hypothyroidism in comparison to normal subjects. Serum T3 and T4 concentrations in subjects with subclinical hypothyroidism were not significantly different in comparison to normal subjects. Serum TSH concentrations were supernormal and therefore were significantly higher in subjects with subclinical hypothyroidism in comparison to normal subjects and rose markedly in both groups following TSH administration with no significant difference among groups. Serum T4 and T3 rose significantly from PreTSH levels in both groups (p < 0.05). However, the rises were not significantly different between groups. Conclusion: In subjects with subclinical hypothyroidism secondary to Hashimoto’s thyroiditis, 24 hour 131I Thyroid uptake is inhibited prior to as well as following SC TSH administration in comparison to normal subjects with maintenance of normal hormone release.
1. Introduction
Subclinical hypothyroidism is a syndrome characterized by normal circulating thyroid hormone levels with simultaneous supra-normal TSH concentrations frequently in absence of clinical manifestations of hypothyroidism [1-18]. In majority of these subjects, a known etiologic factor i.e. presence of antithyroid antibodies, previous treatment for hyperthyroidism, external neck radiation or surgery and use of drugs known to induce hypothyroiddism, etc. is frequently identified [6-10,13-15]. Moreover, therapy with levothyroxine normalizes serum TSH concentration while continuing to maintain normal thyroid hormone concentration [6-10,12-19]. However, the exact pathophysiology of these unusual thyroid hormone alterations is not clearly defined.
It is also well established that several physiologic steps have to be intact to induce synthesis and release of thyroid hormones and TSH promotes each of these steps beginning with iodine uptake and ending with release of thyroid hormones by the thyroid gland [20,21]. Moreover it is also well documented that the major aberration of a single step in thyroid hormone synthesis or release results in clinical hypothyroidism, whereas, a minor inhibition of any of these individual steps induces a goiter without a clinical hypothyroidism [20-24]. Therefore we studied the major steps in synthesis i.e. iodine uptake and the release of thyroid hormone in response to administration of TSH in subjects with subclinical hypothyroidism.
2. Subjects and Methods
10 men and 5 women with ages 42 - 76 years with established diagnosis of subclinical hypothyroidism, manifested by normal T4 and T3 and supra normal TSH levels, participated in the study after obtaining informed consent. 10 euthyroid men with ages 39 - 70 years participated as controls, also after providing informed consent. Normal serum T4, T3 and TSH levels were documented prior to participation. The study protocol was approved by the research and development committee as well as the human studies subcommittee. The study was conducted at Veteran’s Affairs Medical Center in Des Moines, Iowa. The subjects were diagnosed to manifest Hashiomoto’s thyroiditis as documented by elevated titers of antithyroperoxidase antibody.
24 hr 131I thyroid uptake and serum TSH, T4 and T3 concentrations were determined on two occasions at interval of 2 - 3 weeks prior to and after SC administration of recombinant human TSH (Thyrogen, Genzyme Laboratories), 0.9 mg for two consecutive days. 131I uptakes were conducted in the local nuclear medicine department. Serum T4, T3, and TSH concentrations were determined by local clinical laboratory using well established commercial assays. During the procedure of TSH stimulation, 131I was administered on the day prior to 2nd TSH injection; 24 hr thyroidal uptake and serum TSH, T3 and T4 levels were determined on the next day. Comparisons were conducted between subjects with subclinical hypothyroidism and euthyroid volunteers for 24 hour uptake values as well as serum T3, T4 and TSH levels obtained prior to and after TSH administration. The comparisons were performed by statistical analyses using paired student’s t-test and analysis of variance. All values are provided as mean ± standard error of mean (SEM).
3. Results
In all subjects with subclinical hypothyroidism 24 hour 131I thyroidal uptakes were within the normal range [10% - 30%] as defined previously by the local nuclear medicine department. However, the mean value was significantly lower, (p < 0.05) as compared to euthyroid volunteers (Table 1). These 24 hr uptake values rose following TSH administration in both groups of participants. However, the rise was significant (p < 0.01) only in normal subjects. Furthermore, both the mean absolute uptake value and the mean % rise following TSH administration from preTSH uptake values were markedly significantly lower in subjects with subclinical hypothyroidism in comparison to normal subjects (Table 1).
Serum T3 and T4 concentrations in subjects with subclinical hypothyroidism were within normal range as established by the clinical laboratory. Moreover, the mean levels were also not significantly different in comparison to normal subjects. (Table 2) Serum TSH concentrations were supernormal and therefore were significantly higher (p < 0.01) in subjects with subclinical hypothyroidism (8.6 ± 1.5 uU/ml) in comparison to normal subjects (2.3 ± 0.8 uU/ml) and rose markedly in both groups following TSH administration with no significant difference among the elevations between both groups (78 ± 7 uU/ml Vs 70 ± 9 uU/ml). Serum T4 and T3 rose significantly from PreTSH levels in both groups (p < 0.05). However, the rises were not significantly different between both groups of subjects (Table 2).
4. Discussion
The presence of iodine organification defect in subjects with Hashiomoto’s thyroiditis has been well documented [22,25]. Moreover, this defect is attributed to destruction of the enzyme thyroperoxidase induced by the antibody.
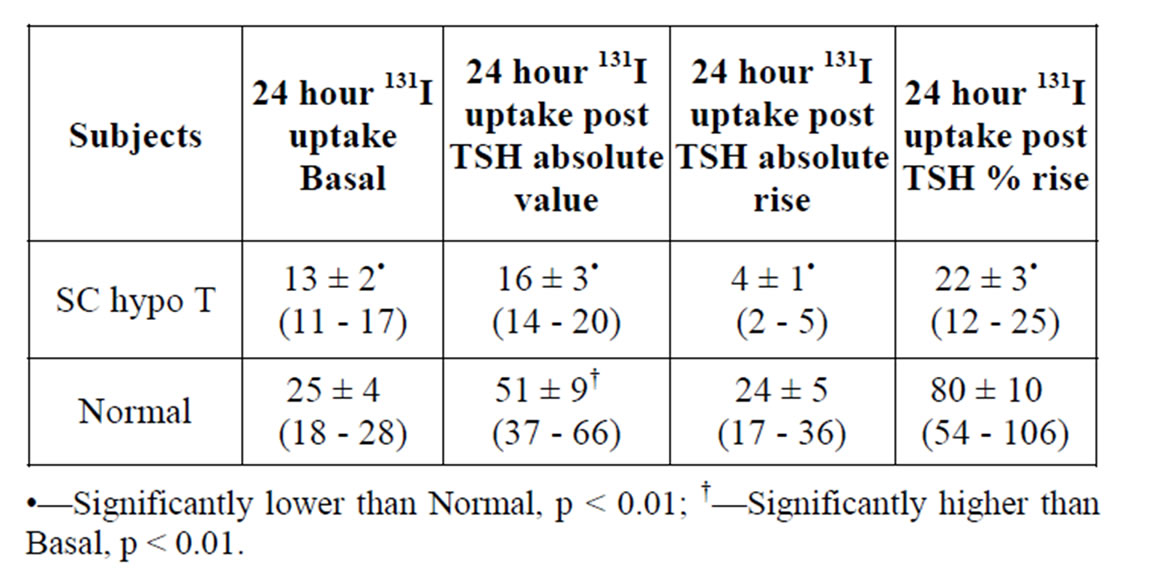
Table 1. Mean 24 hour 131I Thyroid uptake values prior to (PreTSH) and following SC administration of recombinant human TSH 0.9 mg daily for 2 days (postTSH) in 15 subjects with Subclinical Hypothyroidism (SC HypoT) and 10 Euthyroid Volunteers (Normal).
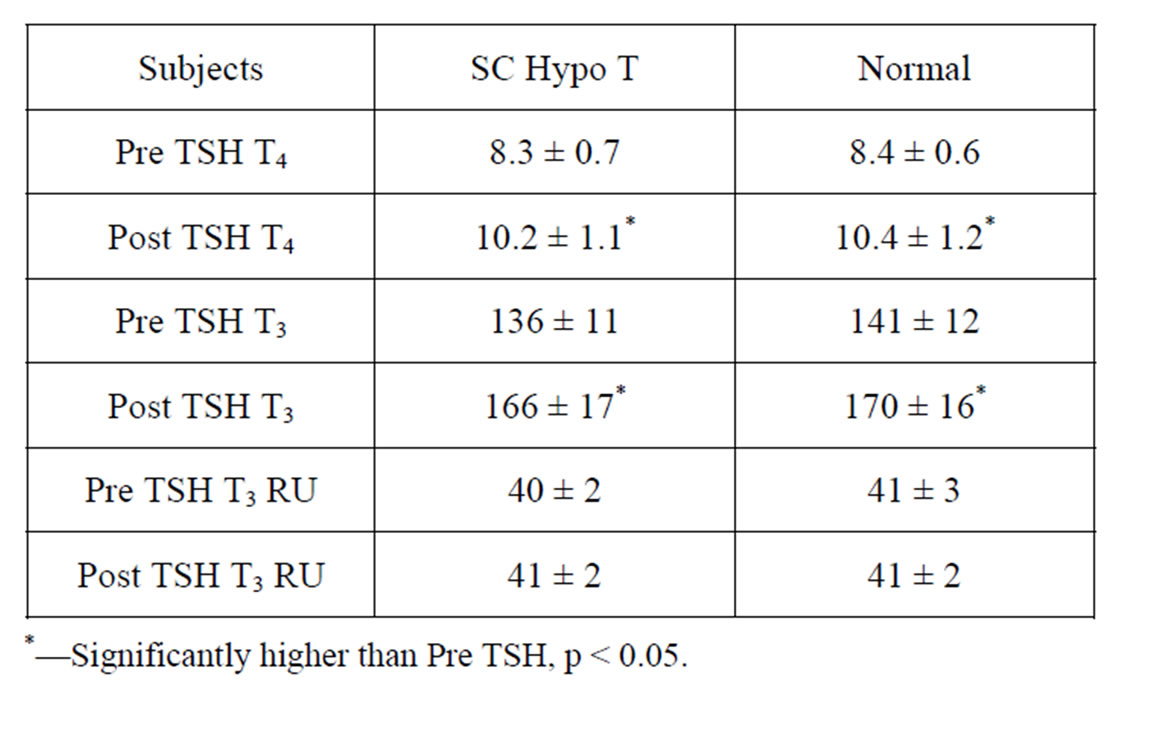
Table 2. Mean Serum T4 (ug/dl), T3 (ng/dl) and T3 Resin Uptake (%) Levels prior to (Pre TSH) and following SC administration of recombinant human TSH 0.9 mg daily for 2 days (postTSH) in 15 subjects with Subclinical Hypothyroidism (SC HypoT) and 10 Euthyroid Volunteers (Normal).
However, all subjects with Hashimoto’s thyroiditis do not manifest clinical hypothyroidism with low T4 and high TSH levels. Some present with goiters without thyroid hormonal changes while others manifest subclinical hypothyroidism whereas still others manifest no abnormalities in terms of thyroid hormone concentrations or goiters. The variability in presentations may be attributed to either the magnitude of a sole defect of organification, or may be due to the presence of multiple defects of minor degrees [20-22,25]. A similar pathophysiologic milieu may explain the variability of manifestation of hypothyroidism in subjects treated with certain drugs i.e. Lithium, Amiodarone etc. [26,27]. It is likely that these drugs inhibit release of thyroid hormones by the thyroid, thus adding injury to the insult in subjects susceptible by the presence of a minor inhibition of on step in thyroid hormone synthesis and release i.e. subjects with Hashiomoto’s thyroiditis with organification defect or subjects with minor iodine deficiency, and therefore manifest hypothyroidism [25]. Alternatively, subjects who do not manifest hypothyroidism may lack any other abnormality in synthesis of thyroid hormone i.e. absence of Hashimoto’s thyroiditis or iodine deficiency [25-29].
This study demonstrates that in subjects with Hashimoto’s thyroiditis manifesting subclinical hypothyroidism, iodine uptake by the thyroid gland is inhibited but release of the thyroid hormones is still intact. Thus, it is plausible that blocking another step i.e. release of the thyroid hormones by the thyroid gland would induce multiple step abnormality in these subjects and promote progression to clinical hypothyroidism as noted with administration of drugs [25-27]. Therefore we believe that manifestation of clinical hypothyroidism may be attributed to a major abnormality in a single step in synthesis and release of thyroid hormone by the thyroid gland i.e. long term Hashimoto’s thyroiditis, major iodine deficiency or other major individual step, e.g. congenital hormonogenetic defect. Alternatively, clinical hypothyroidism may manifest with presence of minor defects in multiple steps in thyroid hormone synthesis and release. Finally, a single step minor abnormality may be responsible for goiter without clinical hypothyroidism or with subclinical hypothyroidism.
REFERENCES
- W. M. Tunbridge, D. C. Evered, R. Hall, D. Appleton, M. Brewis, F. Clark, J. G. Evans, E. Young, T. Bird and P. A. Smith, “The Spectrum of Thyroid Disease in a Community: The Whickham Survey,” Journal of Clinical Endocrinology, Vol. 7, No. 6, 1977, pp. 481-493. doi:10.1111/j.1365-2265.1977.tb01340.x
- A. Gordin and B. A. Lamberg, “Spontaneous Hypothyroidism in Symptomless Autoimmune Thyroiditis. A Long-Term Follow-Up Study,” Journal of Clinical Endocrinology, Vol. 15, No. 6, 1981, pp. 537-543.
- U. M. Kabadi, “Subclinical Hypothyroidism: Natural Course of the Syndrome during a Prolonged Follow-Up Study,” Archives of Internal Medicine, Vol. 153, No. 8, 1993, pp. 958-961.
- M. P. J. Vanderpump, W. M. G. Tunbridge, J. M. French, et al., “The Incidence of Thyroid Disease in the Community. A Twenty Year Follow-Up of the Whickham Survey,” Clinical Endocrinology, Vol. 43, No. 1, 1995, pp. 55-68.
- U. M. Kabadi and R. Cech, “Normal Thyroxine and Elevated Thyrotropin Concentrations: Evolving Hypothyroidism or Persistent Euthyroidism with Reset Thyrostat,” Journal of Endocrinological Investigation, Vol. 20, No. 6, 1997, pp. 319-326.
- N. Clarke and U. M. Kabadi, “Optimizing Treatment of Hypothyroidism,” Treatments in Endocrinology, Vol. 3, No. 4, 2004, pp. 217-221. doi:10.2165/00024677-200403040-00003
- U. M. Kabadi, “Optimal Daily L-Thyroxine Dosage in Primary Hypothyroidism: The Role of Pathogenetic Causes,” Iowa Medicine, Vol. 84, No. 12, 1994, pp. 535- 537.
- M. Helfand, “Preventive Services Task Force. Screening for Subclinical Thyroid Dysfunction in Nonpregnant Adults: A Summary of the Evidence for the US Preventive Services Task Force,” Annals of Internal Medicine, Vol. 140, No. 2, 2004, pp. 128-141.
- M. I. Surks, E. Ortiz, G. H. Daniels, C. T. Sawin, N. F. Col, R. H. Cobin, J. A. Franklyn, J. M. Hershman, K. D Burman, M. A. Denke, C. Gorman, R. S. Cooper and N. J. Weissman, “Subclinical Thyroid Disease: Scientific Review and Guidelines for Diagnosis and Management,” The Journal of American Medical Association, Vol. 291, No. 2, 2004, pp. 228-238. doi:10.1001/jama.291.2.228
- N. F. Col, M. I. Surks and G. H. Daniels, “Subclinical Thyroid Disease: Clinical Applications,” The Journal of American Medical Association, Vol. 291, No. 2, 2004, pp. 239-243. doi:10.1001/jama.291.2.239
- J. G. Hollowell, N. W. Staehling, W. D. Flanders, W. H. Hannon, E. W. Gunter, C. A. Spencer and L. E. Braverman, “Serum TSH T4, and Thyroid Antibodies in the United States Population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III),” Journal of Clinical Endocrinology & Metabolism, Vol. 87, No. 2, 2002, pp. 489-499. doi:10.1210/jc.87.2.489
- J. Karmisholt, S. Andersen and P. Laurberg, “Variation in Thyroid Function in Subclinical Hypothyroidism: Importance of Clinical Follow-Up and Therapy,” European Journal of Endocrinology, Vol. 164, No. 3, 2011, pp. 317-323. doi:10.1530/EJE-10-1021
- M. J. Boomsma, H. P. Bijl and J. A. Langendijk, “Radiation-Induced Hypothyroidism in Head and Neck Cancer Patients: A Systematic Review,” Radiotherapy & Oncology, Vol. 99, No. 1, 2011, pp. 1-5. doi:10.1016/j.radonc.2011.03.002
- B. Gopinath, J. J. Wang, A. Kifley, J. R. Wall, C. J. Eastman, S. R. Leeder and P. Mitchell, “Five-Year Incidence and Progression of Thyroid Dysfunction in an Older Population,” European Journal of Internal Medicine, Vol. 40, No. 9, 2010, pp. 642-649. doi:10.1111/j.1445-5994.2009.02156.x
- D. D. Jones, K. E. May and S. A. Geraci, “Subclinical Thyroid Disease,” American Journal of Medicine, Vol. 123, No. 6, 2010, pp. 502-504. doi:10.1016/j.amjmed.2009.12.023
- G. Ceresini, S. Morganti, M. Maggio, E. Usberti, I. Fiorino, A. Artoni, G. Teresi, S. Belli, V. Ridolfi, G. Valenti and G. P. Ceda, “Subclinical Thyroid Disease in Elderly Subjects,” Acta BioMedica, Vol. 81, No. S1, 2010, pp. 31-36.
- D. S. Cooper, R. Halpern, L. C. Wood, A. A. Levin and E. C. Ridgway, “L-Thyroxine Therapy in Subclinical Hypothyroidism. A Double-Blind, Placebo-Controlled Trial,” Annals of Internal Medicine, Vol. 101, No. 1, 1984, pp. 18-24.
- E. Nyström, K. Caidahl, G. Fager, C. Wikkelsö, P. A. Lundberg and G. Lindstedt, “A Double-Blind Cross-Over 12-Month Study of L-Thyroxine Treatment of Women with ‘Subclinical’ Hypothyroidism,” Journal of Clinical Endocrinology, Vol. 29, No. 1, 1988, pp. 63-75. doi:10.1111/j.1365-2265.1988.tb00250.x
- E. C. Ridgway, D. S. Cooper, H. Walker, D. Rodbard and F. Maloof, “Peripheral Responses to Thyroid Hormone before and after L-Thyroxine Therapy in Patients with Subclinical Hypothyroidism,” Journal of Clinical Endocrinology & Metabolism, Vol. 53, No. 6, 1981, pp. 1238- 1242. doi:10.1210/jcem-53-6-1238
- J. T. Dunn and A. D. Dunn, “Update on Intrathyroidal Iodine Metabolism,” Thyroid, Vol. 11, No. 5, 2001, pp. 407-414. doi:10.1089/105072501300176363
- W. E. Winter and M. R. Signorino, “Review: Molecular Thyroidology,” Annals of Clinical and Laboratory Science, Vol. 31, No. 3, 2001, pp. 221-244.
- B. Rapoport, “Pathophysiology of Hashimoto’s Thyroiditis and Hypothyroidism,” Annual Review of Medicine, Vol. 42, 1991, pp. 91-96. doi:10.1146/annurev.me.42.020191.000515
- M. Nakhjavani and H. Gharib, “Diffuse Nontoxic and Multinodular Goiter,” Current Therapy in Endocrinology and Metabolism, Vol. 6, 1997, pp. 109-112.
- M. Derwahl and H. Studer, “Nodular Goiter and Goiter Nodules: Where Iodine Deficiency Falls Short of Explaining the Facts,” Experimental and Clinical Endocrinology & Diabetes, Vol. 109, No. 5, 2001, pp. 250-260. doi:10.1055/s-2001-16344
- W. Reinhardt, M. Luster, K. H. Rudorff, C. Heckmann, S. Petrasch, S. Lederbogen, R. Haase, B. Saller, C. Reiners, D. Reinwein and K. Mann, “Effect of Small Doses of Iodine on Thyroid Function in Patients with Hashimoto’s Thyroiditis Residing in an Area of Mild Iodine Deficiency,” European Journal of Endocrinology, Vol. 139, No. 1, 1998, pp. 23-28. doi:10.1530/eje.0.1390023
- J. H. Lazarus, “The Effects of Lithium Therapy on Thyroid and Thyrotropin-Releasing Hormone,” Thyroid, Vol. 8, No. 10, 1998, pp. 909-913. doi:10.1089/thy.1998.8.909
- G. Barbesino, “Drugs Affecting Thyroid Function,” Thyroid, Vol. 20, No. 7, 2010, pp. 763-770. doi:10.1111/j.1365-2265.1991.tb00314.x
- C. C. Chow, D. I. Phillips, J. H. Lazarus and A. B. Parkes, “Effect of Low Dose Iodide Supplementation on Thyroid Function in Potentially Susceptible Subjects: Are Dietary Iodide Levels in Britain Acceptable?” Journal of Clinical Endocrinology, Vol. 34, No. 5, 1991, pp. 413-416. doi:10.1111/j.1365-2265.1991.tb00314.x
- Z. Sang, P. P. Wang, Z. Yao, J. Shen, B. Halfyard, et al., “Exploration of the Safer Upper Level of Iodine Intake in Euthyroid Chinese Adults: A Randomized Double Blind Trial,” The American Journal of Clinic Nutrition, Vol. 95, No. 2, 2012, pp. 367-373.
NOTES
*Corresponding author.

