Open Journal of Anesthesiology
Vol.3 No.2(2013), Article ID:29612,4 pages DOI:10.4236/ojanes.2013.32023
Another Undiagnosed Pheochromocytoma Causes Hypertensive Crisis in the Operating Room—What about a Rapid Blood Metanephrine Test?
![]()
1Department of Anesthesiology, New Mexico Veterans Affairs Health Care System, Albuquerque, USA; 2Department of Anesthesiology and Critical Care, University of New Mexico Health Sciences Center, Albuquerque, USA.
Email: Carrie.Rosenberg@va.gov
Received January 20th, 2013; revised February 26th, 2013; accepted March 10th, 2013
Keywords: Pheochromocytoma; Metanephrines; Catecholamines; Perioperative Hypertension
ABSTRACT
Undiagnosed pheochromocytoma is a cause of hypertension intraoperatively. Hypertension from pheochromocytoma catecholamine secretion is potentially fatal and requires immediate treatment with cardiac afterload reduction and preload augmentation for end-organ protection. Diagnosis and management in the acute period can be complex in the context of co-existing disease, other possible causes of hypertension, and the anesthetized patient who is unable to provide historical clues. This case report highlights the plausibility that pheochromocytomas may be more likely to present subclinically in elderly patients with severe vascular disease, so we suggest the potential utility of studying whether reduced vascular responsiveness is protective in any way to catecholamine load. Above all, as an increasing number of case reports of pheochromocytoma discovery intra-operatively emerge, many with catastrophic outcomes, the development value and feasibility of a rapid, on-site blood metanephrine test should be considered. Existing diagnostic methods of pheochromocytoma are relatively time-consuming and not useful in the acute situation.
1. Introduction
Pheochromocytomas are somewhat rare catecholamineproducing neuroendocrine tumors arising from the chromaffin cells of the adrenal medulla or extra-adrenal paraganglia [1]. Hypertension is the most common presenting sign in patients with pheochromocytoma. However, only one in 400 - 800 hypertensive patients has a pheochromocytoma [2]. Age at diagnosis is variable, but usually is made in the fourth and fifth decades of life. Age at diagnosis is associated with tumor heredity, catecholamine phenotype, location, and/or whether the tumor is solitary or mutifocal [3]. However, as in our case, coincidence occasionally plays a part, as some pheochromocytomas are discovered while patients of various ages are undergoing surgery for unrelated issues or having unrelated imaging studies. In fact, a significant number of pheochromocytomas are found on autopsy, so the true incidence is not precisely known [4].
Hypertensive crisis under anesthesia related to pheochromocytoma can be abrupt, profound, and catastrophic. One case reported intraoperative patient blood pressure of 330/180 due to catecholamine crisis [4]. A 45-year-old female patient died while undergoing abdominal hysterectomy due to severe hypertension, tachycardia, and cardiovascular collapse from undiagnosed pheochromocytoma [5]. The mortality rate of pheochromocytomas discovered perioperatively has been reported to be about 80% [6]. For an elderly patient such as ours with coronary artery disease and peripheral vascular disease in the context of catecholamine excess, it seems reasonable to think his chance of mortality would be even higher. However, our patient survived, although he did require increased hospital care and interventions. His blood pressures, although elevated, did not reach levels as high as reported in some other cases. It is possible that his relatively less profound hypertensive crisis was due to other factors since catecholamine secretion profiles can vary [3]. However, perhaps his relatively reduced hypertensive response was at least somewhat associated with blood vessel aging, severe peripheral vascular disease, and resulting reduced overall vascular responsiveness to circulating catecholamines. After all, adrenoceptor desensitization can result in a subclinical presentation, even when catecholamine levels are high [7].
There are many case reports of pheochromocytomas being discovered intraoperatively [4,5,8-13]. With true incidence of pheochromocytoma unknown and as increasing case reports of pheochromocytoma discovery due to hypertension intraoperatively emerge, it seems that a rapid blood metanephrine test would be of value to the anesthesiologist. This is especially true because pheochromocytoma discovery by intraoperative hemodynamic crisis is often catastrophic. Choice of antihypertensive in this setting must be made promptly and can greatly affect morbidity and mortality.
The standard diagnostic process for pheochromocytoma usually involves several steps. First, there is usually some patient history of symptoms related to catecholamine excess such as diaphoresis, heart palpitations, and/ or headache. The patient may have family history of the tumor making genetic testing an option. Next, labarotory tests are done. The most common available laboratory tests include: 24-hour urine catecholamines, 24-hour urine metanephrines, and plasma metanephrines. Abnormal lab tests usually prompt imaging studies such as CT, MRI, and/or 131-I-MIBG scans.
Checking plasma free metanephrines by immunoassay has a high specificity as compared with urinary catecholamines [14]. In fact, in patients with pheochromocytoma, plasma free metanephrines displayed superior diagnostic sensitivity and specificity compared with urine and plasma catecholamines and urine catecholamine metabolites [15]. Unfortunately, rapid plasma metanephrine measurement is not currently possible, as plasma metanephrines measured by high-pressure liquid chromatography (HPLC) are available in specialized centers only [16]. Results are often not available for several days, rendering them useless in the acute situation.
2. Case Report
A 75-year-old man with history of coronary artery disease, hypertension, and peripheral vascular disease necessitating multiple lower extremity bypass procedures presented for elective left inguinal hernia repair. His vital signs prior to general anesthesia induction were: BP 134/ 90 and pulse 56. He had taken metoprolol 25 mg by mouth with a small sip of water that morning as instructed. Intravenous (IV) anesthesia induction was accomplished with fentanyl 50 mcg and propofol 160 mg. Size 4 laryngeal mask airway was easily placed and ventilation was adequate. The patient was placed on 2% sevoflurane for maintenance of anesthesia.
About 7 - 10 minutes after induction, approximately the time surgical incision was made, the patient developed hypertension, with systolic blood pressures of 210 to 250 mmHg. In response, the anesthetic was deepened with intravenous propofol, fentanyl and increased inspired concentration of sevoflurane. The hypertension continued, although heart rate was in the 70’s and respiratory rate remained in the normal range. The patient was given 5 mg metoprolol IV in the context of his cardiovascular disease. As the severe hypertension persisted over the next few minutes, he was given 20 mg hydralazine IV and was started on a sodium nitroprusside infusion of 100 mcg/min while a radial arterial line was placed. The surgical procedure was well under way by this point, and the patient’s systolic blood pressure began to decrease to the 180’s with sodium nitroprusside and hydralazine treatment. Labs were sent.
The surgical procedure was rather brief. The patient was allowed to emerge from anesthesia, still somewhat hypertensive with systolic blood pressures in the 160’s. He remained on sodium nitroprusside infusion. He received 1300 ml of isotonic crystalloid in the operating room. In the post-anesthesia recovery unit (PACU), he was awake and oriented. He reported minimal surgical incisional pain, but no chest pain, shortness of breath or other complaints. Shortly after arrival to PACU, his heart rate increased over several minutes to about 120 beats per minute. He was started on an esmolol infusion in addition to the sodium nitroprusside. Central venous catheter was placed for infusion of his medications. Chest X-ray was obtained. A bed in the intensive care unit was reserved. A summary of his condition can be viewed in Table 1.
While studies were being obtained and interventions carried out in the PACU, the patient complained of middle back pain. In the context of hypertension and vascular disease, a computed-tomography (CT) scan was obtained to look for aortic dissection. Although no dissection was appreciated, a left-sided 3.1 cm suprarenal mass with a density of about 52 Hounsfield units was discovered.
Table 1. Patient’s condition one hour after onset of hypertensive crisis.
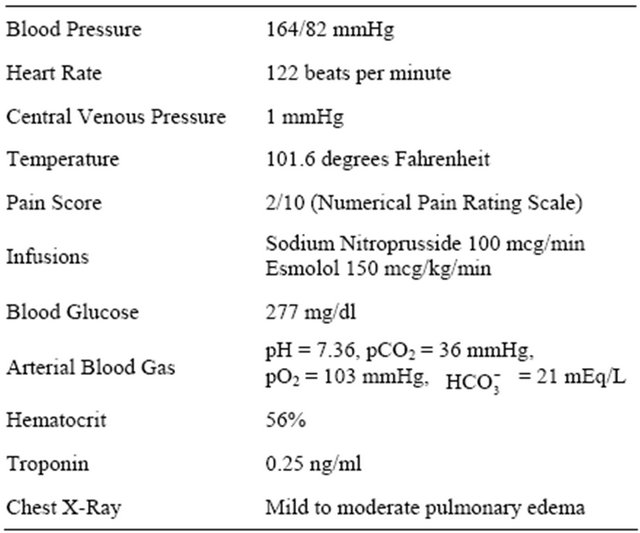 Patient’s clinical status mostly supported pheochromocytoma, but more confirmatory diagnosis required laboratory and imaging studies not immediately available.
Patient’s clinical status mostly supported pheochromocytoma, but more confirmatory diagnosis required laboratory and imaging studies not immediately available.
Endocrinology consultants elicited a patient history of recent weight loss, episodic diaphoresis, and orthostatic hypotension previously not reported by the patient. Plasma free metanephrines were sent, although it took almost two weeks to get the results. 24-hour urine catecholamine collection began. MRI adrenal imaging was scheduled. The patient was started on phenoxybenzamine. He recovered overnight in the intensive care unit with resolution of his hypertension, tachycardia and pulmonary edema. Consultation with a surgeon for pheochromocytoma resection planning was arranged.
In summary, what was intended to be straightforward, elective left inguinal hernia repair became a clinical picture of resistant hypertension, tachycardia, hyperglycemia, hyperthermia, pulmonary edema, troponin increase, and intravascular volume contraction, all in the context of a left pheochromoctyoma appreciated on CT scan which was obtained for a different reason. Lab findings can be viewed in Table 2.
3. Discussion
Although discovery of a pheochromocytoma intraoperatively is reported extensively in the literature and is not novel, suggesting specific methods to rapidly confirm or eliminate pheochromocytoma as a cause of hemodynamic emergency and therefore improve outcomes via pharmacological choice is novel, as is thinking about protectivity of severe vascular disease against catecholamine crisis.
Pheochromocytoma should be in the differential diagnosis for intraoperative hypertension, even if the patient is elderly or outside the normal tumor diagnosis age range. Choice of antihypertensive should be made carefully and beta-blockade should be performed with caution, as reduced inotropy can leave intense vasoconstriction unopposed in catecholamine crisis, causing cardioTable 2. Laboratory results indicative of pheochromocytoma.
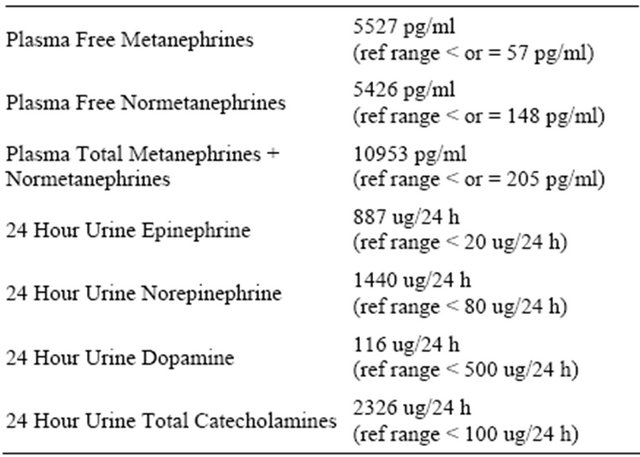 Laboratory results were consistent with pheochromocytoma, but were not available for days and/or weeks, rendering them useless in the acute situation.
Laboratory results were consistent with pheochromocytoma, but were not available for days and/or weeks, rendering them useless in the acute situation.
vascular collapse. Fortunately, our patient’s heart rate demonstrated a delayed rise, so we chose to primarily perform cardiac afterload reduction first with hydralazine and sodium nitroprusside.
Pheochromocytoma may present subclinically in elderly patients with peripheral vascular disease. Older hypertensive patients are less likely to be diagnosed due to absence of classical symptoms of sympathetic overactivity and confounding effects of aging, comorbidities, and medications [17]. Most case reports and studies thus far have focused on tumor properties such as size, location, malignancy, and secretory patterns rather than intrinsic adrenoceptor-related properties of the vasculature in relation to severity of hemodynamic crises. Perhaps severe peripheral vascular disease in our patient was protective to his quantitatively high catecholamine load. One patient with severe cardiovascular disease survived off pump coronary artery bypass grafting with a previously undiagnosed pheochromocytoma [10], so it does not seem to necessarily be the healthy patients who are in the 20% survivability group of perioperative pheochromocytoma discovery.
It is well-known that plasma free metanephrines provide the best test for excluding or confirming pheochromocytoma and should be the test of first choice for diagnosis of the tumor [18]. However, it took several days to get our patient’s plasma metanephrine results from the lab, as these plasma metanephrine samples currently are only analyzed at special facilities. All other existing methods of pheochromocytoma diagnosis are relatively timeconsuming as well, rendering them useless in the acute hemodynamic situation such as we often encounter as anesthesia providers. Without a rapid, reliable, on-site test which would point to pheochromocytoma, all we had was an index of suspicion among an extensive list of possible causes of the hypertension. A rapid on-site blood metanephrine test would be useful for hypertensive crisis management and is currently unavailable.
REFERENCES
- J. W. M. Lenders, G. Eisenhofer, M. Mannelli and K. Pacak, “Phaeochromocytoma,” Lancet, Vol. 366, No. 9486, 2005, pp. 665-675. doi:10.1016/S0140-6736(05)67139-5
- C. Prys-Roberts, “Phaeochromocytoma—Recent Progress in Its Management,” British Journal of Anaesthesia, Vol. 85, No. 1, 2000, pp. 44-57. doi:10.1093/bja/85.1.44
- G. Eisenhofer, H. J. Timmers, J. W. M. Lenders, S. R. Bornstein, et al., “Age at Diagnosis of Pheochromocytoma Differs According to Catecholamine Phenotype and Tumor Location,” Journal of Clinical Endocrinology and Metabolism, Vol. 96, No. 2, 2011, pp. 375-384.
- H. J. Holldack, “Induction of Anesthesia Triggers Hypertensive Crisis in a Patient with Undiagnosed Pheochromocytoma: Could Rocuronium Be to Blame?” Journal of Cardiothoracic and Vascular Anesthesia, Vol. 21, No. 6, 2007, pp. 858-862. doi:10.1053/j.jvca.2006.11.007
- A. Dabbous, S. Siddik-Sayyid and A. Baraka, “Catastrophic Hemodynamic Changes in a Patient with Undiagnosed Pheochromocytoma Undergoing Abdominal Hysterectomy,” Anesthesia and Analgesia, Vol. 104, No. 1, 2007, pp. 223-224. doi:10.1213/01.ane.0000249812.87527.91
- O. F. Sellevold, J. Raeder and R. Stenseth, “Undiagnosed Phaeochromocytoma in the Perioperative Period. Case Reports,” Acta Anaesthesiologica Scandinavica, Vol. 29, No. 5, 1985, pp. 474-479. doi:10.1111/j.1399-6576.1985.tb02236.x
- M. Mannelli, J. W. Lenders, K. Pacak, G. Parenti and G. Eisenhofer, “Subclinical Phaeochromocytoma,” Best Practice & Research Clinical Endocrinology & Metabolism, Vol. 26, No. 4, 2012, pp. 507-515. doi:10.1016/j.beem.2011.10.008
- D. J. Myklejord, “Undiagnosed Pheochromocytoma: The Anesthesiologist Nightmare,” Journal of Clinical Medicine Research, Vol. 2, No. 1, 2004, pp. 59-62. doi:10.3121/cmr.2.1.59
- K. J. Crowley, A. J. Cunningham, B. Conroy, P. R. O’Connell and P. G. Collins, “Phaeochromocytoma—A Presentation Mimicking Malignant Hyperthermia,” Anaesthesia, Vol. 43, No. 12, 1988, pp. 1031-1032. doi:10.1111/j.1365-2044.1988.tb05703.x
- U. Sartipy, J. Lindvall, J. Van Der Linden and G. Dellgren, “Successful of Pump Coronary Bypass Grafting in a Patient with an Undiagnosed Pheochromocytoma,” Acta Anaesthesiol Scand, Vol. 47, No. 8, 2003, pp. 1044-1046. doi:10.1034/j.1399-6576.2003.00191.x
- M. Bensghir, A. Elwali, S. J. Lalaoui, N. D. Kamili, H. Alaoui, J. Laoutid, H. Azendour, H. Balkhi, C. Haimeur and M. Atmani, “Management of Undiagnosed Pheochromocytoma with Acute Appendicitis,” World Journal of Emergency Surgery, Vol. 15, No. 4, 2009, p. 35. doi:10.1186/1749-7922-4-35
- S. Lewis, M. Dirnhuber and J. Soar, “An Unusual Presentation of a Pheochromocytoma,” J Cardiothorac Vasc Anesth, Vol. 20, No. 3, 2006, pp. 390-393. doi:10.1053/j.jvca.2005.03.029
- G. C. Allen and H. Rosenberg, “Phaeochromocytoma Presenting as Acute Malignant Hyperthermia—A Diagnostic Challenge,” Canadian Journal of Anesthesia, Vol. 37, No. 5, 1990, pp. 593-595. doi:10.1007/BF03006334
- T. T. Christensen, J. Frystyk and P. L. Poulsen, “Comparison of Plasma Metanephrines Measured by a Commercial Immunoassay and Urinary Catecholamines in the Diagnosis of Pheochromocytoma,” Scandinavian Journal of Clinical & Laboratory Investigation, Vol. 71, No. 8, 2011, pp. 695-700. doi:10.3109/00365513.2011.622410
- P. E Hickman, M. Leong, J. Chang, S. R. Wilson and B. McWhinney, “Plasma Free Metanephrines Are Superior to Urine and Plasma Catecholamines and Urine Catecholamine Metabolites for the Investigation of Phaeochromocytoma,” Pathology, Vol. 41, No. 2, 2009, pp. 173- 177.
- N. Unger, T. Deutschbein, M. K. Walz, K. Mann and S. Petersenn, “The Value of Immunoassays for Metanephrines in the Biochemical Diagnosis of Pheochromocytomas,” Hormone and Metabolic Research, Vol. 41, No. 9, 2009, pp. 676-679. doi:10.1055/s-0029-1224133
- K. J. Joo-Ching, Au V. Shu-Chuan and C. R. Yuan-Tud, “Recurrent Urosepsis and Cardiogenic Shock in an Elderly Patient with Pheochromocytoma,” Case Reports in Endocrinology, Vol. 2011, 2011. Article ID 759523. doi:10.1155/2011/759523
- J. W. Lenders, K. Pacak, M. M. Walther, W. M. Linehan, M. Mannelli, P. Friberg, H. R. Keiser, D. S. Goldstein and G. Eisenhofer, “Biochemical Diagnosis of Pheochromocytoma: Which Test Is Best?” Journal of American Medical Association, Vol. 287, No. 11, 2002, pp. 1427- 1434. doi:10.1001/jama.287.11.1427

