Journal of Materials Science and Chemical Engineering
Vol.02 No.11(2014), Article ID:51520,5 pages
10.4236/msce.2014.211008
Effect of Milling Time on Co0.5Zn0.5Fe2O4 Microstructure and Particles Size Evolution via the Mechanical Alloying Method
Abubakar Yakubu1*, Zulkifly Abbas1, Mansor Hashim2, Ahmad Fahad3
1Departament of Physics, Universiti Putra Malaysia, Serdang, Malaysia
2Institute of Advance Material Science, Universiti Putra Malaysia, Serdang, Malaysia
3Institute of Mathematical Research, Universiti Putra Malaysia, Serdang, Malaysia
Email: *abulect73@yahoo.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 25 August 2014; revised 30 September 2014; accepted 10 October 2014
ABSTRACT
Nanocrystalline CoZn-ferrite was fabricated by a high-energy milling method by mixing Fe3O4 + CoO + ZnO. The structural properties of the milled powder at different milling times were analysed so as to ascertain the diffusion of CoO and ZnO into the tetrahedral and octahedral sites using mechanical alloying method. The effect of mechanical alloying towards particle size was also investigated. The XRD spectra indicated the precursors reacted during milling with the diffusion of ZnO and followed by CoO into their respective crystallographic sites. SEM micrographs showed the agglomeration of powders due to high energy milling and TEM images confirmed that the particles of the materials were of nanosize dimension. In addition, the results show that the grain possesses a single-phase CoZn-ferrite structure in a typical size of ~16 - 30 nm. The experiment reveals that nanosize CoZn-ferrite can be obtained after the powder is milled for about 8 hours at room temperature. The mechanism and efficiency of the synthesis of the technique are also discussed in this paper.
Keywords:
Mechanical Alloying, CoZn-Ferrite, Nanocrystalline, XRD Profile

1. Introduction
Nanocrystalline materials have attracted considerable interest in recent years because of the possibility of improved macroscopic properties of materials by varying the crystallite sizes. Development in the electronic sectors has put a pressure on the manufacturers to develop smaller and lighter products. A reduction in size and weight can be achieved by focusing on the development of nanosized particles by various techniques.
The useful properties of CoZn-ferrites in low- and high-frequency equipment and their roles in microwave devices, power transformers, rod antennas and read/write heads for high speed digital tapeshas have attracted much interest of researches in recent years [1] . The interesting properties of CoZn ferrites are having small eddy current at higher frequencies, high resistivity, chemical stability and low dielectric losses [2] .
Common methods for preparation of nanocrystalline materials include the inert gas condensation (IGC), hydrothermal method, chemical vapor position (CVD), solid state technique, sol gel method and high energy ball milling [3] . In order to study grain size effects, the average grain size has to be varied over a large range; this can be readily achieved by varying the milling times used during alloying. In order to avoid impurities in the samples during milling, appropriate milling parameters have to be chosen (e.g., material of the milling vial, ball- to-powder weight ratio and optimum milling time) [4] . The advantage of ball milling is easy handling, the possibility to produce large quantities and the applicability to a wide range of different classes of materials. Mechanical alloying via high-energy ball milling has now become one of the conventional methods for producing nano/non-crystalline materials. In mechanical alloying, materials in powder form undergo multiple collisions with balls and vial wall of the grinding media through the process of high energy collision. Various attempts have been made to improve the structural, dielectric and magnetic properties of materials using this milling process. In this study, a compound comprising of three constituent elements was milled to study the effect of milling time on the compound, morphology and particle size of the nanopowder produced.
2. Materials and Methods
A nominal composition of powder for Co0.5Zn0.5Fe2O4 was prepared by mechanical alloying of a mixture of metallic oxides. The materials used were Fe2O3 (Sigma Aldrich) (99.95%), CoO (Sigma Aldrich) (99.99%) and ZnO (Sigma Aldrich) (99.99%) weighed according to the composition formula. The chemicals were mixed with chosen molar ratio of 1:0.5:0.5. High energy milling was carried out in a SPEX 8000D shaker mill in ambient atmosphere for 1, 4, 8, 12, and 17 hours. The ball-to-powder mass-charge ratio (BPR) was approximately 10:1. All the samples were examined with X-ray diffraction (Phillips Expert Pro PW3040) using CuKa. Three samples were selected which are of 1, 4 and 12 h of milling and sent for Scanning Electron Microscope(SEM) images (JEOL 6400) and were further examined under a Transmission Electron Microscope (TEM) (LEO 912AB).
3. Results and Discussion
FTIR spectrum of as-made ferrite nanoparticles is presented in Figure 1. The spectrum showed that the as prepared nanoparticle has a transmittance of about 97.0%. In spinels and ferrites, there are two main broad metal-oxygen bands which are seen in the FTIR spectra. The bands consist of the highest and lowest bands.
The highest wave number (v1) is generally observed in the range 600 - 550 cm−1 which corresponds to intrinsic stretching vibrations of the metal at the tetrahedral site, Mtetra ↔ O, whereas the lowest wavenumber (v2) is usually observed in the range 430 - 385 cm−1, and is assigned to octahedral metal stretching, Mocta ↔ O [5] [6] . The absorption bands observed at ~3490 and ~1610 cm−1 confirms the presence of adsorbed water on the surface of the ferrite nanoparticles.
In the Co0.5Zn0.5Fe2O4 nanocrystals, Zinc ion occupies the tetrahedral site while cobalt and iron have two valences; hence they partially occupy both the tetrahedral and octahedral sites [6] . It is thus concluded that the vibrational mode of tetrahedral clusters is higher than the octahedral clusters, which is attributed to the shorter bond length of tetrahedral clusters.
XRD serves to identify phase transitions or chemical reactions by indicating the presence of new crystalline phases. The average grain size D of the samples was determined by the line broadening of XRD profiles. The ball-milled samples show increasing peak widths with longer milling times (Figure 2). As standard procedure we used the Scherrer Equation [7] :
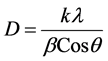 (1)
(1)
where k is a constant equal to 0.9, λ is the wavelength of the X-ray radiation (all diffraction patterns shown in
Figure 1. FTIR spectrum of prepared CoZn-ferrite nanoparticles.
Figure 2. Variation in XRD spectra of CoZn-ferrites powder prepared by mechanical alloying.
this paper were performed with CuKa radiation), θ is the diffraction angle, and β is the full width half maximum (FWHM).
The XRD profile in Figure 2 shows powder patterns recorded from unmilled and mechanically alloyed (BPR = 10:1) homogeneous powder mixtures at different milling times. The result in Figure 2 is further used to calculate the crystallite sizes of samples at different milling hours. Careful observations on the figure showed that there are individual reflections of CoO-, ZnO- and Fe2O3-phases for the powder milled at 1 hour. Further milling up to 8 hours resulted in the disappearance of starting materials phases completely and the CoZn-ferrite phase began to appear.
It is evident from the spectra shown in Figure 2 that in the course of milling of these three individual powders, the CoZn-ferrite phase was formed and its amount increased gradually with increasing milling time. There is a significant reduction of the ZnO phase to a large extent compared to the CoO and Fe2O3 phases after 1 hour of milling which subsequently vanished after 4 hours of milling. It shows that the ZnO phase is much more prone to deformation fault as all the reflections are sufficiently broadened in comparison to the other two phases [8] . Conclusion can be drawn that the rate of solid-state diffusion of ZnO into Fe2O3 lattice is higher than that of CoO. From the spectra, it suggests that ZnO diffused into Fe2O3 and the Zn-Fe2O3 phase is formed. This reaction was then followed by CoO diffusion which phase diffused slowly into the ZnFe2O3 as the milling process continues, finally forming the CoZn-ferrite phase. Careful observation in Figure 2 shows that ZnO diffusion into Fe2O3 is very prominent compared to CoO diffuison. This could be explained by looking at the preferences of Zn2+ ions into the tetrahedral site. It is also possible that longer hour of milling may not affect the crystal size of the materials under test. [9] stated that constant crystallite size from the longer milling time is a reflection of the lattice strain which no longer increases and remains almost constant till the end of milling. This shows that high-energy impact produces enormous amount of lattice imperfections to the sample.
Based on the result shown in Table 1 for the crystallite sizes, it shows two stages of crystallite size attainment from the milling of samples. The first stage of the mechanical alloyed samples from 1 - 4 hours showed variation of sizes from 15.9 - 36.5 nm. The second stage started from 12 hours and up to 17 hours which showed the crystallite size ranges from 16.12 - 24.4 nm.
For the analysis using SEM and TEM, three samples were selected which were from 1, 4, and 12 hours of milling. Figure 3 shows SEM images of CoZn-ferrite after being milled for 1, 4, and 12 hours. The size for the 12 hour milling ranges from 19.5 - 24 nm with an average grain size of 21.8 nm. The powders obtained after the milling showed high agglomeration of the materials as seen in the SEM micrograph.
Table 1. Crystallite size of CoZn-ferrite powder mechanically alloyed at various milling times.


Figure 3. SEM micrograph of milled powders after (a) 1 h, (b) 4 h and (c) 12 h.
The particle size obtained using TEM (Figure 4) for 1, 4 and 12 hours of milling are 30 nm, 20.5 nm, and 13.8 nm which are smaller than the crystallite size obtained from the XRD pattern which implies that the particles exist as nonporous aggregates, due to their high surface energy [10] . From the crystallite size shown in Table 1, it is evidently clear that the result are not in close agreement with the particle size result gathered from the micrograph shown in Figure 4. The Scherer equation used in deducing the crystallite size may also contribute some error to the calculations.
Additional effects such as crystal strain or defects can make significant contributions to line broadening of the FWHM values [11] . It is known that if the particle size of a powder material is as big as its crystallite size, it shows that the material is a single crystal. However in this study, the TEM results for the mechanically alloyed materials showed a smaller crystallite size than that calculated from the Scherer equation. It is thus speculated that this might be due to an instrumental error of the XRD machine. In [12] , they reported that instrumental factors governed the line broadening of the FWHM for particle size above 0.1 μm.
4. Conclusion
The mechanical alloying method was employed to prepare CoZn-ferrite nanoparticles at milling time as low as 1 hour at room temperature which was successfully used to study grain size effects and make reasonable comparison. FT-IR spectra indicate that successive esterification reaction and formation of metal-oxygen bands are attributed to the spinel phase. XRD patterns reveal that the mechanical alloying method can produce cobalt zinc ferrite at relatively low milling time while the spectra from XRD indicates the diffusion of ZnO into the tetrahedral sites followed by CoO into the octahedral sites. The diffusion occurs during the early stage of the milling process. The crystal size calculated exhibits the nanosized regime of powder due to high energy ball milling and it shows 2 crystal-size attainment stages of the materials. SEM images show that the samples at different milling hours have nearly homogeneous grain distribution. The 12-hour-milled sample has an average particle size of about 19 - 24 nm as analysed by the SEM. Despite the procedure used, agglomeration is observed as the milling time increased above 12 hours. The study confirms that the technique used is able to produce materials in nanosize dimension as evident in TEM analysis.


Figure 4. TEM micrograph of milled powders after (a) 1 h, (b) 4 h and (c) 12 h.
Acknowledgements
The researchers wish to thank the Universiti Putra Malaysia, Malaysia for the enabling environment to carry out this study.
References
- Globus, A. and Duplex, P. (1966) Separation of Susceptibility Mechanisms for Ferrites of Low Anisotropy. IEEE Transaction on Magnetics, 2, 441-445. http://dx.doi.org/10.1109/TMAG.1966.1065867
- Zaag, V.D., Ruigrok, J.J.M., Noordermeer, A. and Van Delden, M.H.W.M. (1993) The Initial Permeability of Polycrystalline MnZn Ferrites: The Influence of Domain and Microstructure. Journal of Applied Physics, 74, 4085-4095. http://dx.doi.org/10.1063/1.354454
- Indris, S., Bork, D. and Heitjans, P. (2000) Nanocrystalline Oxide Ceramics Prepared by High-Energy Ball Milling. Journal of Materials Synthesis and Processing, 8, 245-250.
- Yu, L.M., Zhang, J.C., Liu, Y.S., Jing, C. and Cao, S.X. (2005) Fabrication, Structure and Magnetic Properties of nanocrystalline NiZn-Ferrite by High-Energy Milling. Journal of Magnetism and Magnetic Materials, 288, 54-59. http://dx.doi.org/10.1016/j.jmmm.2004.08.024
- Nguyen, K.D. and Nguyen, H.T. (2009) The Effect of Cobalt Substitution on Structure and Magnetic Properties of Nickel Ferrite. VNU Journal of Science, Mathematics—Physics, 25, 153-159.
- Köseoğlu, Y., Baykal, A., Gözüak, F. and Kavas, H. (2009) Structural and Magnetic Properties of CoxZn1−xFe2O4 Nanocrystals Synthesized by Microwave Method. Polyhedron, 28, 2887-2892. http://dx.doi.org/10.1016/j.poly.2009.06.061
- Shanahan, A.E., Sullivan, J.A., McNamara, M. and Byrne, H.J. (2011) Preparation and Characterization of a Composite of Gold Nanoparticles and Single-Walled Carbon Nanotubes and Its Potential for Heterogeneous Catalysis. New Carbon Materials, 26, 347-355. http://dx.doi.org/10.1016/S1872-5805(11)60087-5
- Banerjee, A., Bid, S., Dutta, H., Chaudhuri, S., Das, D. and Pradhan, S.K. (2012) Microstructural Changes and Effect of Variation of Lattice Strain on Positron Annihilation Lifetime Parameters of Zinc Ferrite Nanocomposites Prepared by High Energy Ball-Milling. Materials Research, 15, 1022-1028. http://dx.doi.org/10.1590/S1516-14392012005000135
- Ismayadi, I., Hashim, M., Khamirul, A.M. and Alias, R. (2009) The Effect of Milling Time on Ni0.5Zn0.5Fe2O4 Compositional Evolution and Particle Size Distribution. American Journal of Applied Sciences, 6, 1548-1552.
- Mukhtar, N.Z.F., Borhan, M.Z., Rusop, M. and Abdullah, S. (2014) Nanozeolite Produced by Wet Milling at Different Milling Time. In: Recent Trends in Nanotechnology and Materials Science, Springer International Publishing, Berlin, 41-47.
- Moshtaghioun, B.M., Monshi, A., Abbasi, M.H. and Karimzadeh, F. (2013) A Study on the Effects of Silica Particle Size and Milling Time on Synthesis of Silicon Carbide Nanoparticles by Carbothermic Reduction. International Journal of Refractory Metals and Hard Materials, 29, 645-650.
- O’Connor, B.H. and Jaklevic, J.M. (1981) Line Broadening Effect in X-Ray Powder Diffraction Analysis of Particulate Ammonium Sulfate. Atmospheric Environment, 5, 19-22. http://dx.doi.org/10.1016/0004-6981(81)90120-7
NOTES

*Corresponding author.




