Open Journal of Marine Science
Vol.06 No.01(2016), Article ID:62982,14 pages
10.4236/ojms.2016.61008
Distribution of Heavy Metals and Rare Earth Elements in the Surface Sediments of Penang River Estuary, Malaysia
M. C. Ong1,2, F. M. Fok1, K. Sultan1, B. Joseph3
1School of Marine and Environmental Sciences, Universiti Malaysia Terengganu, Kuala Terengganu, Malaysia
2Sunda Shelf Research Group, Universiti Malaysia Terengganu, Kuala Terengganu, Malaysia
3Institute of Oceanography and Environment, Universiti Malaysia Terengganu, Kuala Terengganu, Malaysia

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 13 October 2015; accepted 22 January 2016; published 25 January 2016
ABSTRACT
Geochemical sediment of the tropical Pinang River, Malaysia was carried out with the aim at documenting elemental concentrations and pollution level assessment. Concentration of selected heavy metals (Cu, Cd, Cr, Pb, Zn and Mn), rare earth elements, TOC and grain size distribution of sediments were determined at 100 m sampling interval along the river. Sediment size showed a positive correlation with ƩREE and Mn and medium correlations with TOC, Zn, Cu, Cr and Pb contents showing enrichment in the clay size fraction. Results of enrichment factor and geoaccumulation index showed that most of the elemental sources were natural (especially REE) and mostly likely represented background values. However, pollution load index revealed the higher levels of Cr, Cd, Zn and Pb, and, therefore, indicating to the anthropogenic sources (i.e. fishing activities) especially in the downstream locations. Thus, the Pinang River is classified as moderately to highly polluted.
Keywords:
Heavy Metals, REE, Tropical River, Sediment, Pollution, Penang, Malaysia

1. Introduction
Heavy metals and rare earths elements (REEs) are potentially toxic inorganic substances in the environment. Persistence and bioaccumulation of such elements may reach a certain threshold of toxicity to aquatic life and therefore, to the food chain systems [1] - [4] . River sediments carry potential of being repository of metals and may serve as a sink first by sorption of metals from the water column [3] and then as a source at elevated levels under the varying conditions (e.g. pH, Eh, temperature). Generally, heavy metals and REE are added to the riverine system by natural processes such as rock weathering, volcanic eruption and long distance atmospheric dust [5] . However, in recent times, the major source of metals is related to the human activities including industry, agriculture, urban development and waste discharge [1] [6] [7] .
Rare earth elements are being widely exploited due to the ever rising demand in modern gadgetry manufacturing such as magnet, catalyst, alloy, glasses, and related electronics [8] . Particularly at risk are the benthic communities that accumulate the toxic contents in sediments and transfer them to the higher trophic level [3] [9] . Thus, the health of living organisms on higher trophic level of food chain in aquatic and terrestrial ecosystems (e.g. human) will be affected as most protein sources of human are derived from aquatic ecosystems [2] [9] [10] . River and estuarine sediments can be used to assess the pollution level of heavy metals and REEs as the surface sediments interact with water column and record the depositional pollution history [11] .
Pinang River is one of the seven most contaminated river basins in Malaysia and is classified as Class-IV by the Interim National Water Quality Standards for Malaysia [12] [13] . Mostly the wastewater discharges do not undergo appropriated water treatment. Land cover and land use change have also enhanced the removal, transport and accumulation of metals in sediments. The shore has been extended outwards through successive land reclamation which has moved the estuary seaward and the resultant changes of saline water intrusion during high tides which may affect the distribution of heavy metals and REE [14] . In tropical settings (marked by high rainfall and temperature), the contaminated river sediments pose even greater risk of becoming the secondary source of pollutants to the ocean as it may re-dissolve back from sediments into water column via remobilization through disturbance of physical, chemical and biological process. This work is important because Penang River provides many services such as drinking water, source of proteins (e.g. fish), transportation, agriculture, electricity generation and tourism. The primary aim of this study is, therefore, to determine the concentration of heavy metals and rare earth elements in surficial sediments of Penang River, and to assess the pollution level by using enrichment factor (EF) and Index of Geoaccumulation (Igeo).
2. Material and Methods
2.1. Study Area
Pinang River is located in the northeast of Penang Island, Malaysia, as shown in Figure 1. Pinang River meanders and flows eastward into the sea and is the sub-basin of the larger Pinang River basin (total area ~50.97 km2; length ~3.1 km) [15] . Headwaters of Penang River drain hilly granitic rock which is island’s bedrock. Middle and lower catchment areas consist of Quaternary deposits (clay, silt, sand, peat and minor gravel). The highest peak is 833 m ASL located in the north of island. Study area experiences tropical rainforest climate with average annual rainfall of 2670 mm and average annual air temperature of 27˚C. Transport and deposition of dust particles by wind from perennial but transient forest fires create haze and is a source of atmospheric input of elements.
2.2. Sampling
Sampling cruises were made aboard the research vessel that also served as sample processing platform. Surface sediments (0 - 10 cm depth) were collected with an interval of 100 m in July 2013 from 24 locations (Figure 1) using Ponar grab that minimized the fine sediments from being washed out by water. The systematic sampling campaign allowed not missing any hotspots that might contain extreme level of potentially toxic metals. Distance between the upstream sampling station and the downstream station was less than 3 km. Sampling locations were recorded using Global Positioning System (GPS) and in-situ data included pH, EC and other parameters (e.g. temperature) using Quanta Hydrolab. The apparatus used were soaked in 10% of nitric acid overnight and rinsed with distilled water for sampling and laboratory analysis as per QA/QC program (EPA, 1997). Buffer solutions (pH 3, 7 and 10) were used in calibration for Quanta hydrolab.
2.3. Analytical
Sediment samples were dried at 60˚C in a clean oven for one week. Coarse debris and gravel in the sample were removed manually. The dried sediments samples were used to determine the concentration of heavy metals, REEs, TOC and grain size of sediments. For the total digestion about 50 mg of sample was transferred into the
Figure 1. Study area map showing surface sediment sampling stations along the Pinang River, Malaysia.
Teflon cup along with 2 mL of mixed acids of HNO3, HCl and HF in the ratio of 3:3:1, respectively. The Teflon cup was enclosed in a pressure bomb and heated at 100˚C for 7 hours until a clear solution without residue obtained. After cooling down, sample was brought to volume of 10 mL using Milli-Q water (18.2 MΩ・cm). Concentration of heavy metals and REE were then measured by the multi-elements technique using Inductively Coupled Plasma Mass Spectrometry (ICP-MS) (Method EPA 6020A; EPA, 2007).
Precautions were taken and appropriate procedures were followed as part of the QA/QC program throughout the analysis. Blank solution was prepared which only contained reagent to assess the impurity. Standard Reference Material (SRM-1646a) of sediments was used in the recovery test by comparing the measured values with the certified values to ensure accuracy. Recovery of analysis varied from 83% to 108% depending upon the metal as shown in Table 1.
The organic carbon content (TOC) was determined in a 0.5 g of sample, avoiding contact with iron or steel, by wet oxidation and digestion by the Walkley-Black method. Oxidisable matter in the sediment was oxidised by 10.0 mL of 1N potassium dichromate (K2Cr2O7) solution. The reaction was assisted by the heat generated when two volumes of concentrated sulphuric acid (H2SO4) was mixed with one volume of the dichromate. The remaining dichromate was titrated with ferrous sulphate (Fe2SO4・7H2O). The titre is inversely related to the
Table 1. Result of accuracy and precision analysis for Standard References Material (SRM), NBS 1646a estuarine sediments.
amount of carbon present in the sediment sample.
For the grain size analysis, sediment samples were oven dried (105˚C for 24 hours) and about 200 g of sample was passed through 14 different mesh size sieves arranged consecutively downward from 4000 µm to <63 µm. For the fine fraction (<63 µm), about 2 g of sample was diluted with distilled water and 10% of Calgon solution (Na6P6O18) was added to disperse bonded particles in sediments. The grain size sediments of sediments were analysed using laser Particle Size Analyzer (PSA). Statistical analysis was carried out using SPSS software. Geographical Information System (GIS) was used to develop spatial variation maps. All data is reported as dry weight of sediments.
3. Results
3.1. Physico-Chemical Parameters
In situ measurements of water column (bottom layer) included several parameters such as pH which ranged from 6.91 to 8.15, DO from 0.13 to 4.92 mg/L and salinity from 15.6‰ to 30.81‰. Upstream sampling locations (P1 to P11) registered sand size (63 - 2000 µm) fraction only (Figure 2). The highest proportion of silt size (4 - 63 µm) was found at P23 (81.39%) while for clay grain size (<4 µm) is found in P21 (4.72%). The average mean size of sediments of all sampling stations was 2.625 ɸ. Grain size generally decreased from the upstream towards the river mouth. Clay size fraction is less than 5%.
TOC ranged from 5.33% at P10 to 0.36% at P4 with mean value of 2.16%. TOC contents remained nearly unchanged at the upstream locations (P1 to P9; <1%) but increased sharply at P10 and then remained consistent before gradually decreasing closer to the estuary. TOC (%) in Pinang River is higher than the Langat River, Yangtze River and Pearl River [1] [16] indicating higher input under the tropical environment.
3.2. Heavy Metals
Concentrations of Cd, Cr, Cu, Mn, Pb and Zn in surficial sediments are given in Table 2 and spatial distribution maps are shown in Figure 3. Concentration of Cd varied between 0.07 µg・g−1 and 1.52 µg・g−1 with mean value of 0.53 µg・g−1. The highest concentration of Cd was recorded at location P12 which is closer to jetty with intense boat activities. Concentration of Cr ranged from 18.8 to 122 µg・g−1 with mean value of 58.4 µg・g−1. The highest Cr concentration was measured at location P18 near the housing area. Both upstream and river mouth sampling locations showed higher Cr contents than the middle part of river. Concentration of Cu varied between 3.57 µg・g−1 and 62.1 µg・g−1 with mean value of 21.3 µg・g−1 and showed a trend of increasing concentration from the upstream to downstream. The concentration of Pb varied between 8.42 µg・g−1 and 83.5 µg・g−1 with mean value of 25.9 µg・g−1. Bothe upstream and downstream locations recorded lower Pb contents. Middle catchment locations (e.g. P17) close to the housing area registered higher contents. The concentrations of Zn varied from 46.6 to 317 µg・g−1 with the mean value of 131 µg・g−1. Mn concentration ranged from 39.8 to 375 µg・g−1
Figure 2. Percentage of sand, silt and clay size fractions of surface sediments and the change of mean size (ɸ) from upstream to the downstream of Pinang River.
with the mean value of 142 µg・g−1. The highest Mn contents were recorded location, P24, which is close to the estuary. Mn contents showed high correlation with mean fine size sediments (r = 0.83) and Zn, Cu, Cr and Pb showed medium correlation with (r = 0.64), (r = 0.62), (r = 0.41) and (r = 0.52) respectively. Cd showed a week correlation with mean sediments size (r = 0.37). Strong correlation between Zn and Cu (0.87) points to the same source which is probably anti-fouling paints contain about 15% - 30% of metals [17] [18] .
TOC showed a medium to strong correlations with studied metals, Zn (r = 0.71), Cu (r = 0.70), Pb (r = 0.63), Mn (r = 0.51), Cd (r = 0.45). TOC such as humic substances and fulvic acid provides absorption, ion exchange and forms complex (chelate compound) with metal re-deposition process [19] [20] . Synergic effect between fine sediments and TOC provide larger cation exchange capacities and higher surface to volume ratio for absorption process of metals ion in water column [1] [21] .
3.3. REE
Concentration of REE and related statistical data in surficial sediments of Pinang River is shown in Table 3. Figure 4 shows spatial distribution maps of ƩREE and selected REE concentration in surface sediments. The highest concentration among REEs was 982 µg・kg−1 of Sm at the downstream location P22 and the lowest concentration of 0.1 µg・kg−1 of Lu at the upstream location P2. The mean content of REE was 102 µg・kg−1. In general, the downstream locations registered higher concentration of ƩREE compared to the upstream locations. The sum of light REE (ƩLREE; La-Eu) contents of sediments was measured to be 16069.3 µg・kg−1 with the mean value of 669.6 µg・kg−1. The sum of heavy REEs (ƩHREE; Gd-Lu) contents was measured to be 898.0 µg・kg−1 with the mean value of 37.4 µg・kg−1. The highest concentration of ƩHREE was located at P19 (138.3 µg・kg−1) while for the lowest concentration was located P5 with 6.8 µg・kg−1. REEs contents showed positive correlations (0.42 to 0.83) with mean grain size of sediments (except Sc, r = −0.66).
Both grain size and TOC contents of sediments showed a positive correlation with ƩREE, r = 0.82 and r = 0.70, respectively, and various metals (e.g. Zn) suggesting important controlling factors of metal distributions
Table 2. Concentration of heavy metals (µg・g−1 dry weight) in surficial sediments in Pinang River, Penang, Malaysia.
(Figure 5). This observation is in agreement with findings of river sediments of Brahmaputra River [22] . Coarser sediments tend to contain higher fraction of quartz, carbonate and feldspar and lower contents of mafic minerals [23] . Also, finer grain sizes has higher surface to volume ratio and clay minerals and, therefore, higher capacity of absorption of REE ions [24] . Downstream zone tends to have higher ƩREE which may also due to high proportion of heavy minerals (e.g. zircon, monazite). Dissolved REE ions are removed from water column by sorption on to suspended particles and sediments coated with Fe and Mnoxyhydroxides [24] .
Organic matter contains humic substances which provide high affinity for REE ion especially at near-neutral pH aquatic environments [24] . Compared to upper catchment, downstream zone has high percentage of TOC possibly due to continuous supply and accumulation of organic matter from terrestrial, urban runoff, untreated waste effluent.
4. Discussion
Texturally river sediments are classified as silt-sand and the sand size (>2000 µm) fraction decreases towards
Figure 3. Spatial distribution maps of Cd, Cr, Cu, Pb, Zn and Mn contents in Penang River sediments. Midstream and downstream sampling locations generally registered higher levels.
river mouth. Upstream locations tend to have coarser grain size sediments due the removal of fine particles and deposition towards downstream locations deepening upon hydraulic energy and gradient. Tropical rain events are intense and significantly remove fine sediments to the sea.
Mn concentration increased gradually towards the river mouth. Downstream zone contains higher fraction of fine sediments and potentially provides larger binding sites for dissolved Mn ions in water column [20] . Moreover, downstream river water registered higher DO (up to 4.39 mg/L) and pH (up to 8.15) that leads to precipitation of dissolved Mn2+ on surficial sediments under oxide environment.
Highest concentrations of Cu and Cd were determined at location P12 closer to the jetty. The input is most likely due to the fishing boat activities such as fish delivering, leakage of petrol and lubricant as well as antifouling paint residue [17] [25] . Besides, effluents from industrial activities such as metal plating and vehicle waste, and fuel leakage might cause addition of metals including Cu [26] . The highest content of Pb was found at location P17 which is closer to the industrial area (i.e. cable, paint and battery) and possibly the source of elevated levels of metals. An average Zn concentration of 130.6 µg・g−1 is 2.5 times above the 52 µg・g−1 value [27] of upper continental crust. Housing area at downstream zone was observed and suggested that wastewater such as shampoo and detergent [28] discharged from urban activities might be the factor of Zn inputs in sediments. About 2 - 7 fold higher concentration of Zn was observed in the study area compared to Terengganu River, Port Klang and Kelantan River in Malaysia.
Table 3. Concentration of REEs (µg・kg−1) and characteristic parameter in surficial distribution of Cu concentration in the surficial sediments of Pinang River.
Figure 4. Spatial distribution of selected REE in surface sediments of Pinang River. Most REE showed higher concentration close to the river mouth with the exception of a few (e.g. Sc).
Heavy metal concentration changes showed more or less three distinct trends along the river flow path (Figure 6). Zn, Pb, Cu and Cd all registered lower concentrations in the upstream than the downstream locations. Cr concentration was higher at upstream and downstream locations but much lower in the middle catchment of the river. Mn concentration systematically increased along the river with the higher values close to the estuary pointing to precipitation and/or adsorption onto sediments.
The order of abundance of REEs in sediments was observed to be Sc > Ce > La > Nd > Y > Pr > Sm > Gd > Dy > Yb > Er > Eu > Tb > Ho > Tm > Lu. The enrichment of LREE over HREE and nearly flat pattern of
Figure 5. Plots of grain size and TOC against REEs, Mn and Zn in sediments of Pinang River. Rectangles with broken lines enclose samples with high concentration of REEs and Mn and fine grain size fraction at the downstream locations.
Figure 6. Graphs showing various trends in concentration changes along the Penang river from upstream to downstream. Note gradual increase of few metals and an abrupt change at location P12.
HREE is shown in Figure 7 of Chondrite normalized concentrations. The various patterns of chondrite normalized REEs point to the origin and in situ processes of enrichment or depletion. Chondrite meteorite composition was used to normalize REEs as it represents the primordial earth and also considering the granitoids as the dominant at the study area. A similar pattern was reported in Terengganu River Basin [29] and marine sediments of the South China Sea [30] . Slightly positive Ce anomaly and negative Eu anomaly was also observed.
ƩLREE is more abundant as compared to ƩHREE with an average ƩLREE/ƩHREE ratio of 18.0. Estuarine environment have higher content of carbonate that leads to the enrichment ƩLREE in surficial sediments [32] . The mean value of ƩREE (707.0 ug・kg−1) in Pinang River sediments is observed to be lower than UCC [27] which suggested low contents of REE containing rare earth mineral inputs. Ratio of (La/Yb) N ranged from 4.06 to 51.3 with the mean value of 25.6. Higher (La/Yb) N ratio with Eu anomaly also suggests sediment source of granite. River sediments showed a slightly positive Ce anomaly, 1.63, mostly likely due to the change of soluble Ce (III) into insoluble Ce (IV) in water column under oxidizing conditions. In general, sediment Ce anomaly decreased towards estuary. Under the prevailing pH-redox conditions, the insoluble Eu (II) tends to form soluble Eu (III), hence change in Eu anomaly from upstream to the downstream.
A multivariate assessment of surface sediments contamination included determination of enrichment factor (EF), Index of Geoaccumulation (Igeo) and Pollution Load Index (PLI). Assessments were calculated using upper continental crust (UCC) values of elements as background.
For Enrichment Factor (EF) calculation, Li was used a reference element to normalize the metal concentrations. Concentration of Li showed a positive correlation with mean sediment grain size and most of the metals measured. EF was used to estimate whether metal input is natural or anthropogenic An EF value of less than 2.0 is considered natural. The EF is calculated as presented by Buat-Menard and Chesselet [33] :
where,(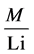 ) Sample is the ratio of concentration metal and Li of the sample.(
) Sample is the ratio of concentration metal and Li of the sample.(
Figure 7. Chondrite-normalized REE patterns of the surface sediments of Pinang River. Chondrite values are after Anders and Grevesse [31] .
EF value followed order Cd > Zn > Cr > Pb > Cu > Mn. The moderate anthropogenic of metal Cd at P2 (2.43), P4 (2.67), P9 (3.01), P12 (4.16), P13 (4.56), P20 (2.28), P22 (4.29) might relate to local point discharge. EF for REE was less than 2 and therefore, all sources of REE are natural. Anthropogenic enrichment of REE is mainly caused by ore mining activities and through observation the Pinang River catchments do not have mining activities.
Geo-accumulation Index (Igeo) is used to assess the quality of sediments [34] and is calculated by the following formula:
Where, Cn is concentrations of a heavy metal element in the sample, Bn is background value, 1.5 is the correction factor.
An Igeo value of ≥1 is considered unpolluted to moderate level. Pollution level of Cr (Igeo = 2.1) showed moderately to strongly polluted and Igeo Cd was found to be 1.54. The calculated Igeo values followed the order of Cr > Cd > Zn > Pb > Cu > Mn.
Pollution load index (PLI) gives better understanding for the level of contamination of heavy metals [35] in coastal and estuarine surface sediments.
PLI is calculated as:
where, CF is contamination factor which (C)sample is concentration of metal in sample, (C)background is background value of the metal. “n” is the number of metals measured.
The PLI varied from 0.60 to 3.09 (average ~1.44). A PLI value above 1 indicates contamination. Sampling station with PLI value above 1 are in the order of P12 > P17 > P18 > P20 > P14 > P19 > P21 > P16 > P22 > P13 > P24 > P23 were mostly found at the downstream of river. This might be caused by the higher anthropogenic input at downstream than the upstream and due to the accumulative build up in sediments (i.e. sink) of metals. Fishing activities were found at P12 and there are high possibilities of major sources of metals were derived from boat activities. Retention of metals in sediments is due to the multi factors including grain size, TOC and pH-redox conditions and when conditions change metals can become mobile (i.e. source) in the aquatic ecosystem.
5. Conclusions
Among the elements measured in surface sediments of Penang River, Cd, Cr, Zn and Pb concentrations were found to be significantly elevated and, therefore, might pose a threat to the aquatic ecosystem. A multivariate assessment indicated moderately to highly polluted levels of metals which are in agreement with overall river quality previously classified as degraded but without reporting of the elemental data. Serious issues of pollution are related to the site near jetty which possibly serves as a point source for metal enrichment to the sediment. Sources of rare earth elements are natural and no significant environmental issue was observed owing to the low anthropogenic inputs (i.e. mining).
Most of the metals, TOC, fine grain size fraction and ƩREE concentration showed a trend of increasing values from upstream towards the river mouth. It is most likely due to the hydraulic conditions caused by intense tropical rain events that remove and transport fine sediment particles from upper catchments (dominantly sand) to the downstream locations (silty sand texture).
Chondrite-normalized rare-earth elements (REE) patterns showed higher LREE as compared to HREE. Fine particles, TOC and high pH-redox conditions favor enrichment of metals close to the estuary as compared to the upstream river waters. Longer contact time of metal ions in the water column under low flow rate (downstream locations) retains metals on surface sediments resulting in elevated levels. Fine sediment size has higher ratio of surface area to volume than the coarser sediments and therefore, provides larger area of binding site. This work contributed by documenting the current levels of 22 elements so that any changes in future can be monitored and managed considering the Penang River catchment areas undergoing rapid urban development.
Acknowledgements
Authors would like to thank the Oceanography and Biodiversity Laboratory, School of Marine and Environmental Science and Institute of Oceanography and Environment for providing the facilities to carry out the research work. Thanks also to our friends for their help in sampling and continued support during the laboratory work. We appreciate valuable time of Mr Yuzwan bin Mohammad spent in preparing maps using GIS.
Cite this paper
M. C.Ong,F. M.Fok,K.Sultan,B.Joseph,11, (2016) Distribution of Heavy Metals and Rare Earth Elements in the Surface Sediments of Penang River Estuary, Malaysia. Open Journal of Marine Science,06,79-92. doi: 10.4236/ojms.2016.61008
References
- 1. Zhou, J., Ma, D., Pan, J., Nie, W. and Wu, K. (2008) Application of Multivariate Statistical Approach to Identify Heavy Metal Sources in Sediment and Waters: A Case Study in Yangzhong, China. Environmental Geology, 54, 373-380. http://dx.doi.org/10.1007/s00254-007-0824-5
- 2. He, Z., Song, J., Zhang, N., Zhang, P. and Xu, Y. (2009) Variation Characteristics and Ecological Risk of Heavy Metals in the South Yellow Sea Surface Sediments. Environment Monitor Assessment, 157, 515-528. http://dx.doi.org/10.1007/s10661-008-0552-7
- 3. Mulligan, C., Fukue, M. and Sato, Y. (2010) Sediment Contamination and Sustainable Remediation. CRC Press, London.
- 4. Dhanakumar, S., Murthy, K.R., Solaraj, G. and Mohanraj, R. (2013) Heavy-Metal Fractionation in Surface Sediments of the Cauvery River Estuarine Region, Southeastern Coast of India. Archives of Environmental Contamination and Toxicology, 65, 14-23. http://dx.doi.org/10.1007/s00244-013-9886-4
- 5. Lin, C., He, M., Zhou, Y., Guo, W. and Yang, Z. (2008) Distribution and Contamination Assessment of Heavy Metals in Sediment of the Second Songhua River, China. Environment Monitor Assessment, 137, 329-342. http://dx.doi.org/10.1007/s10661-007-9768-1
- 6. Ochieng, E.Z., Lalah, J.O. and Wandiga, S.O. (2007) Analysis of Heavy Metals in Water and Surface Sediment in Five Rift Valley Lakes in Kenya for Assessment of Recent Increase in Anthropogenic Activities. Bulletin of Environmental Contamination and Toxicology, 79, 570-576.
http://dx.doi.org/10.1007/s00128-007-9286-4 - 7. Ong, M.C., Menier, D., Shazili, N. and Kamaruzzaman, B.Y. (2013) Geochemical Characteristics of Heavy Metals Concentration in Sediments of Quiberon Bay Waters, South Brittany, France. Oriental Journal of Chemistry, 29, 39-45. http://dx.doi.org/10.13005/ojc/290106
- 8. The Geological Society (2011) Rare Earth Elements: A Briefing Note by the Geological Society of London. http://www.geolsoc.org.uk
- 9. Burton, G.A. (2002) Sediment Quality Criteria in Use around the World. Limnology, 3, 65-75.
http://dx.doi.org/10.1007/s102010200008 - 10. Francaa, S., Vinagrea, C., Cacadora, I. and Cabrala, H.N. (2005) Heavy Metal Concentrations in Sediment, Benthic Invertebrates and Fish in Three Salt Marsh Areas Subjected to Different Pollution Loads in the Tagus Estuary (Portugal). Marine Pollution Bulletin, 50, 998-1003.
http://dx.doi.org/10.1016/j.marpolbul.2005.06.040 - 11. Mei, J., Li, Z., Sun, L., Gui, H. and Wang, X. (2011) Assessment of Heavy Metals in the Urban River Sediments in Suzhou City, Northern Anhui Province, China. Procedia Environmental Sciences, 10, 2547-2553. http://dx.doi.org/10.1016/j.proenv.2011.09.396
- 12. Drainage and Irrigation Department (2000) Quality Monitoring Report for Sungai Pinang River Basin. Drainage and Irrigation Department, Penang, Malaysia
- 13. Usali, N., and Ismail, M.H. (2010) Use of Remote Sensing and GIS in Monitoring Water Quality. Journal of Sustainable Development, 3. http://dx.doi.org/10.5539/jsd.v3n3p228
- 14. Maznah, W.O. and Mansor, M. (2002) Aquatic Pollution Assessment Based on Attached Diatom Communities in the Pinang River Basin, Malaysia. Hydrobiologia, 487, 229-241.
http://dx.doi.org/10.1023/A:1022942200740 - 15. Saad, F.N., Rahman, N.N., Kadir, M.O. and Omar, F.M. (2008) Identification of Pollution Sources within the Sungai Pinang River Basin. Universiti Sains Malaysia, 478-485.
- 16. Liu, B., Hu, K., Jiang, Z., Yang, J., Luo, X. and Liu, A. (2011) Distribution and Enrichment of Heavy Metals in a Sediment Core from the Pearl River Estuary. Environment Earth Science, 62, 265-275. http://dx.doi.org/10.1007/s12665-010-0520-8
- 17. Thomas, K.V., McHugh, M. and Waldock, M. (2002) Antifouling Paint Booster Biocides in UK Coastal Waters: Inputs, Occurrence, and Environmental Fate. Science of Total Environment, 293,117-127. http://dx.doi.org/10.1016/S0048-9697(01)01153-6
- 18. Gibert, O., Martínez-Lladó, X., Martí, V., Díez, S., Romo, J., Bayona, J.M. and Pablo, J. (2009) Changes of Heavy Metal and PCB Contents in Surficial Sediments of the Barcelona Harbour after the Opening of a New Entrance. Water, Air, and Soil Pollution, 204, 271-284. http://dx.doi.org/10.1007/s11270-009-0044-6
- 19. Wang, G.-P., Yu, X.-F., Wang, J., Zhao, H.-M., Bao, K.-S. and Lu, X.-G. (2011) Dominants and Accumulation of Rare Earth Elements in Sediments Derived from Riparian and Depressional Marshes. Environmental Earth Sciences, 62, 207-216. http://dx.doi.org/10.1007/s12665-010-0515-5
- 20. Sany, S.B., Salleh, A., Sulaiman, A.H., A. Sasekumar, M.R. and Tehrani, G.M. (2013) Heavy Metal Contamination in Water and Sediment of the Port Klang Coastal Area, Selangor, Malaysia. Environmental Earth Sciences, 69, 2013-2025. http://dx.doi.org/10.1007/s12665-012-2038-8
- 21. Erdem, E., Karapinar, N. and Donat, R. (2004) The Removal of Heavy Metal Cations by Natural Zeolites. Journal of Colloid and Interface Science, 280, 309-314. http://dx.doi.org/10.1016/j.jcis.2004.08.028
- 22. Li, C., Kang, S., Zhang, Q. and Wang, F. (2009) Rare Earth Elements in the Surface Sediments of the Yarlung Tsangbo (Upper Brahmaputra River) Sediments, Southern Tibetan Plateau. Quaternary International, 208, 151-157. http://dx.doi.org/10.1016/j.quaint.2009.05.003
- 23. Lee, S., Kim, J.K., Yang, D.Y. and Kim, J.Y. (2008) Rare Earth Element Geochemistry and Nd Isotope Composition of Stream Sediments, South Han River Drainage Basin, Korea. Quaternary International, 176-177, 121-134.
- 24. Morgan, B., Rate, A.W., Burton, E.D. and Smirk, M.N. (2012) Enrichment and Fractionation of Rare Earth Elements in FeS- and Organic-Rich Estuarine Sediments Receiving Drainage from Acid-Sulfate Soils. Chemical Geology, 308-309, 60-73. http://dx.doi.org/10.1016/j.chemgeo.2012.03.012
- 25. Dar, M.A. (2012) Distribution Patterns of Some Heavy Metals in the Surface Sediment Fractions at Northern Safaga Bay, Red Sea, Egypt. Arabian Journal of Geosciences, 7, 1-13.
- 26. Fu, J., Zhao, C., Luo, Y., Liu, C., Kyzas, G.Z., Luo, Y., Zhao, D., An, S. and Zhu, H. (2014) Heavy Metals in Surface Sediments of the Jialu River, China: Their Relations to Environmental Factors. Journal of Hazardous Materials, 270, 102-109. http://dx.doi.org/10.1016/j.jhazmat.2014.01.044
- 27. Wedepohl, K.H. (1995) The Composition of the Continental Crust. Geochemicaet Cosmochimica Acta, 59, 1217-1239. http://dx.doi.org/10.1016/0016-7037(95)00038-2
- 28. Cuong, D.T., Karuppiah, S. and Obbard, J.P. (2008) Distribution of Heavy Metals in the Dissolved and Suspended Phase of the Sea Surface Microlayer, Seawater Column and in the Sediments of Singapore’s Coastal Environment. Environmental Monitoring Assessment, 138, 255-272.
http://dx.doi.org/10.1007/s10661-007-9795-y - 29. Sultan, K. and Shazili, N.M. (209) Rare Earth Elements in Tropical Surface Water, Soil and Sediments of the Terengganu River Basin, Malaysia. Journal of Rare Earths, 27, 1072-1078.
http://dx.doi.org/10.1016/S1002-0721(08)60391-9 - 30. Khadijeh, R.E., Elias, S.B., Wood, A.K. and Reza, A.M. (2009) Rare Earth Elements Distribution in Marine Sediments of Malaysia Coasts. Journal of Rare Earths, 27, 1066-1076.
http://dx.doi.org/10.1016/S1002-0721(08)60390-7 - 31. Anders, E. and Grevesse, N. (1989) Abundances of the Elements: Meteoritic and Solar. Geochemicaet Cosmochimica Acta, 53, 197-214. http://dx.doi.org/10.1016/0016-7037(89)90286-X
- 32. Kanazawa, Y. and Kamitani, M. (2006) Rare Earth Minerals and Resources in the World. Journal of Alloys and Compounds, 408-412, 1339-1343. http://dx.doi.org/10.1016/j.jallcom.2005.04.033
- 33. Buat-Menard, P. and Chesselet, R. (1979) Variable Influence of Atmospheric Flux on the Trace Metal Chemistry of Oceanic Suspended Matter. Earth and Planetary Science Letters, 42, 398-411.
http://dx.doi.org/10.1016/0012-821X(79)90049-9 - 34. Nobi, E., Dilipan, E., Thangaradjou, T., Sivakumar, K. and Kannan, L. (2010) Geochemical and Geo-Statistical Assessment of Heavy Metal Concentration in the Sediments of Different Coastal Ecosystems of Andaman Islands, India. Estuarine, Coastal and Shelf Science, 87, 253-264.
http://dx.doi.org/10.1016/j.ecss.2009.12.019 - 35. Tomlinson, D.L., Wilson, J.G., Harris, C.R. and Jeffrey, D.W. (1980) Problems in the Assessment of Heavy-Metal Levels in Estuaries and the Formation of a Pollution Index. Helgoländer Meeresuntersuchungen, 33, 566-575.
http://dx.doi.org/10.1007/BF02414780
















