American Journal of Molecular Biology
Vol.3 No.1(2013), Article ID:27675,5 pages DOI:10.4236/ajmb.2013.31008
Anti-HBc and HBV-DNA detection in blood donors negative for hepatitis B virus surface antigen
![]()
Department of Clinical Oncological Pathology, South Egypt Cancer Institute, Egypt
Email: rania.bakty@lycos.com
Received 28 June 2012; revised 28 July 2012; accepted 17 August 2012
Keywords: HBC IgM; HBV DNA; Occult HBV
ABSTRACT
Occult HBV infection is defined as the presence of hepatitis B virus (HBV) DNA in blood or liver tissues in patients negative for Hepatitis B surface Antigen (HBsAg). Those patients may or may not be positive for HBV antibodies. The objective of this study is to determine the presence or absence of HBV DNA in the serum samples from HBsAg negative blood donors. In addition we aimed to assess the magnitude of occult HBV infection and to reduce the risk of HBV infection. Over a period of one year a total of 7340 blood units were collected at blood transfusion center in our locality for the prevalence of HBV infection and 180 HBsAg negative blood specimens were randomly selected for anti-HBcIgM, anti-HBs antibody and HBV DNA. Ninety seven out of 7340 collected blood units were positive for HbsAg (1.3%). The randomly selected 180 tested donors revealed 7 (3.8%) positive for antiHBc IgM and 34 (18.8%) were positive for anti-HBs antibodies. Four out of 7 positive for anti-HBc IgM were also positive for anti-HBs and 2/180 (1.1%) specimens were positive for HBV DNA by PCR. Anti-HBc antibody should be tested routinely at any blood transfusion center and if they were positive regardless of anti-HBs titer, the blood should be discarded. Also HBV DNA is preferable to be performed to all blood donors to present completely safe blood transfusion.
1. INTRODUCTION
Current serological screening for blood-borne hepatitis viruses has reduced the risk of post-transfusion hepatitis dramatically. Among the three viral infections routinely tested in blood; hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV), the residual transmission risk is highest for HBV [1,2]. This is attributed to the interval between initial HBV infection and the detection of HBsAg, resulting in a long window phase during which the virus is transmissible [3]. Detection of HBsAg in blood is a diagnostic marker for infection with HBV and in blood banks screening for HBsAg is carried out routinely to detect HBV infection. Occult HBV infection is defined as the presence of HBV-DNA in blood or liver tissues in patients negative for HBsAg but who may or may not be positive for HBV antibodies [4,5]. It is possible that donors with occult HBV infection, who lack detectable HBsAg whose exposure to HBV infection indicated by positive anti-HBc antibodies against HBV core antigen and HBV DNA are a potential source of HBV infection [6]. Low levels of viraemia have been shown to continue long after clinical recovery from acute self-limiting HBV infection [7]. HBV is also transmitted very frequently when liver is transplanted from HBsAg negative, anti-HBc positive blood donors who shows that liver harbors infectious HBV in some persons negative for HBsAg but positive for anti-HBc [8]. However, some HBsAg negative individuals with positive anti-HBc and/or positive for antibodies against HBsAg (anti-HBs) continue to be positive for HBV DNA. Due to limitations in current blood screening practices in developing countries, donation by such individuals is a potential source of HBV transmission to the recipients [9]. The prevalence of occult heaptitis B found in 15 different studies ranged from 1% to 95%, which might be accounted to differences between endemic and non endemic areas, presence of risk factors and to the sensitivity of the HBV-DNA assay [10]. On the other hand in some areas occult HBV infection has not been detected [11,12].
In this study we are trying to detect the presence of occult hepatitis B virus infection and to predict the incidence of anti-HBc IgM in serum samples from blood donors as a trial to reduce HBV infection after blood transfusion.
2. PATIENTS AND METHODS
2.1. Samples
A total of 7340 blood units had been donated at the blood bank of South Egypt Cancer Institute, Assiut university, Egypt from December 2010 to November 2011. Routine viral detections for all units had been performed. One hundred and eighty HBsAg negative blood specimens were selected randomly for anti-HBcIgM, anti-HBs antibody and HBV DNA testing. A full questionnaire used for each donor according to the American Association of Blood Bank and informed consent were obtained from all donors at the time of blood donation.
Blood specimens were collected from each subject in plain vial; kept in room temperature for 30 minutes and then centrifuged at 3000 rpm for 15 minutes. Serum was separated and then stored at −20˚C until tested.
2.2. Anti-HbcIgM and Anti-Hbs Antibody Detection
The anti-HBc IgM was done using AxsYM core-M: IgM antibody to hepatitis B virus core antigen (ABBOTT Diagnostic Division), which is based on the micro-particle enzyme immunoassay (MEIA) technology. The presence or absence of IgM anti-HBc in the sample was determined by comparing the rate of formation of fluorescent product in the test sample to a mean index calibrator rate determined from a previous AxsYM Core-M index calibration to give an index value. Samples with index values greater than 1.2 were considered reactive for anti Hbc IgM. Also the anti-Hbs antibody was done using AxSYM AUSAB technique which is based on MEIA that has been developed to measure the amount of antibody (anti-HBs) present in human serum or plasma. The concentration of anti-HBs antibody in the sample was determined using a previously generated calibration curve. The sample considered positive if the concentration of the sample was greater than or equal to 10.0 mIU/mL.
2.3. HBV DNA Extraction and PCR Amplification
DNA was extracted using QIAmp blood mini kit (Qiagen, Germany) and PCR was performed to detect the HBV using the primers which are designed to amplify a 500- bp amplicon of the surface antigen, HBV sense, 5’- TCGTGTTACAGGCGGGGTTT-3’ and HBV antisense 5’-CGAACCACTGAACAAATGGC-3’ (mtebion international AG-Lena-Christ-Strasse44/1-D-82152 Martinsried/Deutschland) a 50 µL of reaction mixture containing 3 µL of the DNA sample, 25 µL PCR master mix (Promega, catag # M7505, system Lot # 287246 ), 2.5 µL for upstream and downstream primers (final concentration 0.5 µM) and the final volume completed by nuclease free water. This reaction was amplified in a thermal cycler (PTC-100TM Peltier thermal Cycler, MJ Research). PCR amplification was performed using the touchdown method which include one cycle of 93˚C for 60 sec, 58˚C for 20 sec, and 72˚C for 40 sec then five cycles of 92˚C for 20 sec, 60˚C to 56˚C for sec and 72˚C for 40 sec followed by 30 cycles of 93˚C for 20 sec, 55˚C for 20 sec and 72˚C for 40 sec, according to the Arababadi MK et al. [13]. HBV genomic DNA and a negative patient sample were used as positive and negative controls, respectively. For the analysis of the PCR amplification, 10 μL of the amplified DNA were run on 2% agarose gel after addition of 4 μL of loading buffer using a chemi-docXRS system (Bio-Rad, USA). The presence of a 500-bp fragment indicated a positive result. In parallel with samples, a 100-bp DNA ladder was also run on the gels to estimate the molecular weight of DNA fragments in the gel (Figure 1).
2.4. Statistical Analysis
Using SPSS software version 16.0, qualitative data was expressed as mean ± SD while quantitative data was expressed as number and percentage.
3. RESULTS
The demographic data revealed that the patient ages ranged from 18 to 48 years with the mean age of 26.2 ± 5.7. The maximum donation were observed in age group of 18 - 35 years represented 82.8% from the selected 180 donors for concerned tests, while the rest of the donors aged fro 36 to 48 years revealed a percentage of 17.2%. As regard the sex male to female ratio was 6.4:1. Most of the selected donors were males (N = 152) constituting a percentage of 84.4%, while female numbers and frequency were 28 and 15.6% respectively (Table 1).
The positive donors for HbsAg were 97 (1.3%) from a total of 7430 donated units. Donors positive for antiHBcIgM were 7/180 (3.8%), 6 males versus 1 female,
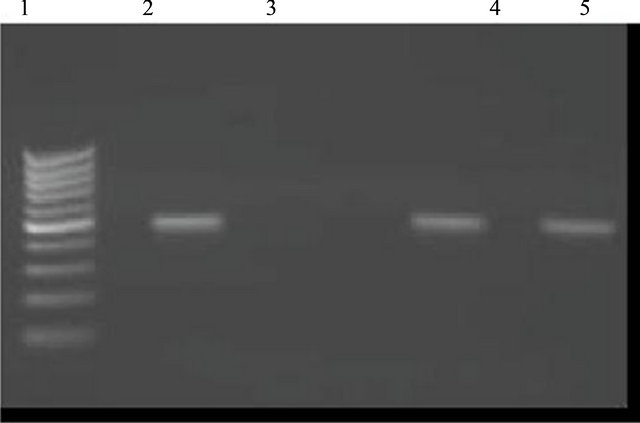
Figure 1. HBV-DNA PCR. An ethidum bromide stained agrose gel showing typical results of HBV-DNA PCR screening of collected sample which were HBsAg negative and anti-HBc positive. Lane 1: DNA ladder marker, lane 2: A positive control showing the expected 500-bp product, lane 3: A negative control; lanes 4 and 5: Positive samples.
while positive donors for anti-HBs antibody were 34/180 (18.8%), 31 males versus 3 females.
Four out of the 7 positive for anti-HBc IgM, were positive for anti-HBs (57.2%). Two specimens were positive for HBV DNA by PCR, representing a percentage of 1.1%, one of these previous 2 specimens was positive for anti-HBs antibodies and the other was negative for anti-Hbs antibodies (Table 2).
4. DISCUSSION
After the introduction of reliable serologic screening of blood donations, post transfusion hepatitis has become rare. However, the identification of blood donors with occult HBV infection has created some concern with regards to the safety of blood supply. It is generally accepted that the diagnosis of infection by HBV is based on the presence of the HBsAg in the bloodstream4. However, screening of blood bank donors for HBsAg does not totally eliminate the risk of HBV infection through blood transfusion [14] since the absence of this marker in the serum does not exclude the presence of HBV DNA [15]. It is possible that, donors with occult HBV infection, who lacked detectable HBsAg but whose exposure to HBV infection was indicated by a positive anti-HBc and HBV DNA, are a potential source of HBV infection [16]. So the study of anti-HBc and HBV DNA are very important to reduce the post transfusion hepatitis. Our 3.8% obtained figure for the studied blood donors with positive anti-HBcIgM and negative HBsAg was comparable to the previous reports which vary from 0.56% in United Kingdom, 0.84% in United States, 1.4% in Germany and 4.85% in Italy [1,17-19]. On the other hand, higher prevalence was recorded as 13.5% in Korean blood donors, 15.03% in Greece, 16.4% in Saudi Arabia and 76% in Ghana [16,20-22]. Our results are less than the previous Egyptian studies which reported the prevalence of anti-HBc as 10.96% and 7.8% [23,24]. The discrepancies between our results and the previous ones may be due to many factors such as different methodologies used in the studies, number of samples and statistical analysis.
Regarding HBV-DNA positive donors, we have recorded a prevalence of 1.1% which is close to a recent Egyptian study with a record of 1.26% [23]. However our figure is still far of another Egyptian study with recorded 6.25% prevalence of concerned occult infection [24]. In other group of population Asim and his coworkers [25] reported a positive HBV DNA in 4.6%, while Behzad-Behbahani [26] found that HBV DNA was positive in 16 out of 131 (12.2%). For the anti HBs antibody, this study recorded a percentage of 18.8% in 180 tested blood units and 50% (titer more than 10 IU/mL) from positive HBV-DNA donors. In Behbahani study positive specimens for both anti HBs and anti HBcIgM antibody were 37.5% [26]. This higher percentage of positive anti HBs antibody out of positive HBV-DNA donors can be explained that these individuals may have recovered from previous infection but have persistent low level of HBV. Symptomatic hepatitis B has never been observed in immunized persons who develop anti-HBs titer more than 10 IU/mL. Some vaccine recipients may develop anti-HBc, which is indicative of HBV infection; but they usually do so in the absence of disease [27]. Moreover, the protective anti-HBs antibody is normally directed against the “a” determinant of HBsAg. In some cases, the antibodies are directed against one of the determinants other than “a” and are unable to neutralize the circulating virion. These cases should therefore be regarded as chronic infection [28]. Detection of HBV-DNA in anti-HBc and anti-HBs positive individuals may be also due to chronic persistent HBV infection.
The exclusion of anti-HBc positive donors is impractical in countries where HBV infection is prevalent and more than 20 per cent of the population is anti-HBc positive [29].
Table 1. Demographic data of blood donors.

Yrs: years; N: number; %: percentage.
Table 2. Summary of the serological profile according to sex distribution of the blood donors.
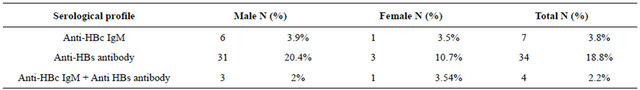
N: number; %: percentage; HBC: hepatitis B virus core antigen; HBs: hepatitis B virus S antigen.
In the study of Manzini et al., they estimated that 2298 units per million donated units could potentially contain HBV-DNA representing a risk 100 times higher than the risk of transfusing HBV-DNA-positive donation collected during the window phase of the infection (estimated to be 15.78 units per million donated units) [19]. Whether HBsAg-negative/anti-HBc-positive units can transmit HBV infection to recipients is debated [30,31]. The low viremic content and often the concomitant presence of neutralizing anti-HBs antibodies have led several authors to conclude that donated blood from anti-HBc/anti-HBspositive is safe. The English experience seems reassuring as no case of hepatitis B transmission was related to transfusion in a survey of 20,000 blood units controlled only for HBsAg [32]. However, the prevalence of HBV is very low in Northern Europe and the number of units traced was relatively small, while the transfusion risk may be different in areas such as Italy, where HBV remains endemic. In four large studies of post-transfusion hepatitis performed in the 1970s in the United States, Australia and The Netherlands, the overall rate of HBV infection after receipt of blood tested for HBsAg only varied from 1% to 2.5%; however in all series, hepatitis B was more frequent after transfusion with anti-HBcpositive than with anti-HBc-negative blood, with the rate of transmission varying between 2% and 8.6% among recipients of anti-HBc-positive blood [31,33-35]. A number of other studies showed cases of HBV transmission after the transfusion of anti-HBc-positive blood [36,37].
5. CONCLUSIONS
Anti-HBc antibody and HBV DNA should be tested routinely in blood donors and if they were Positive regardless of anti-HBs titer, the blood should be discarded to reach a completely safe blood transfusion.
Although this conclusive finding is ethical, however there are many other factors which should be considered. These factors include geographic distribution of HBV infection, primary investigations, available cost-related additional testing and technical methodologies.
REFERENCES
- Soldan, K., Davison, K. and Dow, B. (2005) Estimates of the frequency of HBV, HCV and HIV infectious donations entering the blood supply in the United Kingdom, 1996 to 2003. Eurosurveillance, 10, 9-10.
- Pillonel, J. and Laperche, S. (2003) Trends in risk of transfusion transmitted viral infections (HIV, HCV, HBV) in France between 1992 and 2003 and impact of nucleic acid testing (NAT). Eurosurveillance, 10, 5-6.
- Stroffolini, T., Mele, A., Tosti, M.E., Gallo, G., Balocchino, E. and Ragni, P. (2000) The impact of the hepatitis B mass immunization campaign on the incidence and risk factors of acute hepatitis B in Italy. Journal of Hepatology, 33, 980-985. doi:10.1016/S0168-8278(00)80132-4
- Badur, S. and Akgun, A. (2001) Diagnosis of hepatitis B infections and monitoring of treatment. Journal of Clinical Virology, 21, 229-237. doi:10.1016/S1386-6532(01)00147-0
- Schreiber, G.B., Busch, M.P., Kleinman, S.H. and Korelitz, J.J. (1996) The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. The New England Journal of Medicine, 4, 1685-1690. doi:10.1056/NEJM199606273342601
- Dreier, J., Kroger, M., Diekmann, J., Gotting, C. and Kleesiek, K.L. (2004) Level viraemia of hepatitis B virus in an anti-HBcand anti-HBs-positive blood donor. Transfusion Medicine, 14, 97-103. doi:10.1111/j.0958-7578.2004.0486.x
- Yotsuyanagi, H, Yasuda, K. and Iino, S. (1998) Persistent viremia after recovery from self-limited acute hepatitis B. Hepatology, 27, 1377-1382. doi:10.1002/hep.510270526
- Dickson, R.C., Everhart, J.E. and Lake, J.R. (1997) Transmission of hepatitis B by transplantation of livers from donors positive for antibody to hepatitis B core antigen. The National Institute of Diabetes and digestive and Kidney diseases liver Transplantation Database. Gastroenterology, 113, 1668-1674. doi:10.1053/gast.1997.v113.pm9352871
- Kleinman, S.H., Kuhns, M.C. and Todd, D.S. (2003) Frequency of HBV DNA detection in US blood donors testing positive for the presence of anti-HBc: Implications for transfusion transmission and donor screening. Transfusion, 43, 696-704. doi:10.1046/j.1537-2995.2003.00391.x
- Torbenson, M. and Thomas, D.L. (2002) Occult hepatitis B. The Lancet Infectious Diseases, 2, 479-486. doi:10.1016/S1473-3099(02)00345-6
- Souza, L.O., Pinho, J.R., Carrilho, F.J. and da Silva, L.C. (2004) Absence of hepatitis B virus DNA in patients with hepatitis C and non-A-E hepatitis in the State of São Paulo, Brazil. Brazilian Journal of Medical and Biological Research, 37, 1665-1668. doi:10.1590/S0100-879X2004001100011
- Alencar, R.S., Gomes, M.M. and Sitnik, R. (2008) Low occurrence of occult hepatitis B virus infection and high frequency of hepatitis C virus genotype 3 in hepatocellular carcinoma in Brazil. Brazilian Journal of Medical and Biological Research, 41, 235-240. doi:10.1590/S0100-879X2006005000197
- Arababadi, M.K., Mohammadzadeh, A., Pourfathollah, A.A. and Kennedy, D. (2011) Polymorphisms within Fas gene are not associated with occult hepatitis B virus infection. Hepatitis Monthly, 11, 23-26.
- Conjeevaram, H.S. and Lok, A.S. (2001) Occult hepatitis B virus infection: A hidden menace? Hepatology, 34, 204-206. doi:10.1053/jhep.2001.25225
- Comanor, L. and Holland, P. (2006) Hepatitis B virus blood screening. Vox Sanguinis, 91, 1-12. doi:10.1111/j.1423-0410.2006.00773.x
- Allain, J.P., Candotti, D. and Soldan, K. (2003) The risk of hepatitis B virus infection by transfusion in Kumasi, Ghana. Blood, 101, 2419-2425. doi:10.1182/blood-2002-04-1084
- Kleinman, S.H., Kuhns, M.C. and Todd, D.S. (2003) Frequency of HBV-DNA detection in US blood donors testing positive for the presence of anti-HBc: Implication for transfusion transmission and donor screening. Transfusion, 43, 696-704. doi:10.1046/j.1537-2995.2003.00391.x
- Henning, H., Puchta, I. and Luhm, J. (2002) Frequency and load of hepatitis B virus DNA in first time blood donors with antibodies to hepatitis B core antigen. Blood, 100, 2637-2641. doi:10.1182/blood-2002-03-0798
- Manzini, P., Girotto, M. and Borsotti, R. (2007) Italian blood donors with anti-HBc and occult hepatitis B virus infection. Haematologica, 92, 1664-1670. doi:10.3324/haematol.11224
- Zervou, E.K., Dalekos, G.N., Boumba, D.S. and Tsianos, E.V. (2001) Value of anti-HBc screening of blood donors for prevention of HBV infection: Results of a 3-year prospective study in Northwestern Greece. Transfusion, 41, 652-658. doi:10.1046/j.1537-2995.2001.41050652.x
- Bernvill, S.S., Andrews, V., Kuhns, M.C. and McNamara, A.L. (1997) Hepatitis B core antigen antibody as an indicator of a low grade carrier state for hepatitis B virus in a Saudi Arabian blood donor population. Transfusion Science, 18, 49-53. doi:10.1016/S0955-3886(96)00076-8
- Seo, D.H., Whang, D.H., Song, E.Y., Kim, H.S. and Park, Q. (2011) Prevalence of antibodies to hepatitis B core antigen and occult hepatitis B virus infection in Korean blood donors. Transfusion, 18, 121-128.
- El-Zayadi, A.R., Ibrahim, E.H. and Badran, H.M. (2008) Anti-HBc screening in Egyptian blood donors reduces the risk of hepatitis B virus transmission. Transfusion Medicine, 18, 55-61. doi:10.1111/j.1365-3148.2007.00806.x
- Antar, W., El-Shokry, M.H., Abd El Hamid, W.A. and Helmy, M.F. (2010) Significance of detecting anti-HBc among Egyptian male blood donors negative for HBsAg. Transfusion Medicine, 20, 409-413. doi:10.1111/j.1365-3148.2010.01021.x
- Asim, M., Ali, R., Luqman, A., Khan, S.A. and Singla, R. (2010) Significance of anti-HBc screening of blood donors & its association with occult hepatitis B virus infection: Implications for blood transfusion. Indian Journal of Medical Research, 132, 312-317.
- Behbahani, B., Mafi-Nejad, A., Lankarani, K.B., Torab, A. and Moaddeb, A. (2006) Anti-HBc & HBV-DNA detection in blood donors negative for hepatitis B virus surface antigen in reducing risk of transfusion associated HBV infection. Indian Journal of Medical Research, 123, 37-42.
- Roche, B., Feray, C. and Gigou, M. (2003) HBV DNA persistence 10 years after liver transplantation despite successful anti-HBS passive immunoprophylaxis. Hepatology, 38, 86-95. doi:10.1053/jhep.2003.50294
- Tonekaboni, S.S., Waters, J.A., Jeffers, S., Gehrke, R., Ofenloch, B., Horsch, A., et al. (2000) Effect of variation in the common “a” determinant on the antigenicity of hepatitis B surface antigen. Journal of Medical Virology, 60, 113-121. doi:10.1002/(SICI)1096-9071(200002)60:2<113::AID-JMV2>3.0.CO;2-0
- Nandi, J. and Benerjee, K. (1992) Detection of hepatitis B virus DNA in donor blood by the polymerase chain reaction. The National Medical Journal of India, 5, 5-7
- Matsumoto, C., Tadokoro, K. and Fujimura, K. (2001) Analysis of HBV infection after blood transfusion in Japan through investigation of a comprehensive donor specimen repository. Transfusion, 41, 878-884. doi:10.1046/j.1537-2995.2001.41070878.x
- Cossart, Y.E., Kirsch, S. and Ismay, S.L. (1982) Post transfusion hepatitis in Australia. Report of the Australian Red Cross study. Lancet, 1, 208-213. doi:10.1016/S0140-6736(82)90770-X
- Regan, F., Hewitt, P., Barbara, J. and Contreras, M. (2000) Prospective investigation of transfusion transmitted infection in recipients of over 20,000 units of blood. British Medical Journal, 320, 403-406. doi:10.1136/bmj.320.7232.403
- Rakela, J., Mosley, J.W., Aach, G.L., Gitnick, G.L., Hollinger, F.B., Stevens, C.E., et al. (1980) Viral hepatitis after transfusion with blood containing antibody to heaptitis B core antigen. Gastroenterology, 78, 1318-1323.
- Katchaki, J.N., Siem, T.H., Brouwer, R., Brandt, K.H. and van der Waart, M. (1980) Detection and significance of anti-HBc in the blood bank; Preliminary results of a controlled prospective study. Journal of Virological Methods, 2, 119-125. doi:10.1016/0166-0934(80)90045-2
- Koziol, D.E., Holland, P.V. and Alling, D.W. (1986) Antibody to hepatitis B core antigen as a paradoxical marker for non-A, non-B hepatitis agents in donated blood. Annals of Internal Medicine, 104, 488-495.
- Mosley, J.W., Stevens, C.E. and Aach, R.D. (1995) Donor screening for antibody to hepatitis B core antigen and hepatitis B virus infection in transfusion recipients. Transfusion, 35, 5-12. doi:10.1046/j.1537-2995.1995.35195090661.x
- Soldan, K., Ramsay, M. and Collins, M. (1999) Acute hepatitis B infection associated with blood transfusion in England and Wales. British Medical Journal, 318, 95-99. doi:10.1136/bmj.318.7176.95

