New Journal of Glass and Ceramics
Vol.2 No.1(2012), Article ID:16982,8 pages DOI:10.4236/njgc.2012.21008
Electron Paramagnetic Resonance Studies of Cu2+ and VO2+ Spin Probes in RO-Li2O-Na2O-K2O-B2O3 (R = Zn, Mg, Sr and Ba) Glass Systems
![]()
1Department of Physics, Osmania University, Hyderabad, India; 2Department of Physics, Vasavi College of Engineering, Hyderabad, India; 3Department of Physics, Bhavan’s New Science College, Hyderabad, India.
Email: {hameed138, dr.ramdev}@gmail.com
Received September 9th, 2011; revised October 13th, 2011; accepted October 23rd, 2011
Keywords: Borate Glasses; Electron Paramagnetic Resonance (EPR); Optical Absorption; Spin-Hamiltonian Parameters
ABSTRACT
Mixed alkali and alkaline earth oxide glasses of RO-Li2O-Na2O-K2O-B2O3 (R = Zn, Mg, Sr and Ba) system were studied by electron paramagnetic resonance (EPR) and optical absorption spectroscopy. Cu2+ and VO2+ ions were used as the spin probes. The glasses containing 1 mole% of Cu2+ and 2 mole% of VO2+ were prepared by the melt quenching method. X-ray diffraction studies of the samples did not reveal crystalline phases. EPR measurements were made at X-band frequencies with 100 kHz field modulation, at 310 K. Optical spectra were recorded in the wavelength range 300 nm - 800 nm. From the EPR spectra the spin-Hamiltonian parameters were evaluated. The spin-Hamiltonian parameter values in the case of Cu2+ indicated that the ground state of Cu2+ was  orbital (2B1g) and the site symmetry around Cu2+ is tetragonally distorted octahedral. The variation of g// and A// with the alkaline earth oxide (RO) composition was found to be non-linear which may be due to the change in the ligand field strength at the site of Cu2+ ions. From the spin-Hamiltonian parameters of VO2+, it was observed that the vanadyl ions exist as VO2+ ions in octahedral coordination with tetragonal compression and have C4V symmetry with ground state dxy. Tetragonality (Dg///Dg^) of V4+ ion sites exhibited non-linear variation with RO content, which indicated change in the ligand field at the site of V4+. A broad optical absorption band was observed in the glass containing Cu2+ ions corresponding to the 2B1g à 2B2g transition. From the EPR and optical data the bond parameters were evaluated. In the case of VO2+ ions, the covalency rates were estimated.
orbital (2B1g) and the site symmetry around Cu2+ is tetragonally distorted octahedral. The variation of g// and A// with the alkaline earth oxide (RO) composition was found to be non-linear which may be due to the change in the ligand field strength at the site of Cu2+ ions. From the spin-Hamiltonian parameters of VO2+, it was observed that the vanadyl ions exist as VO2+ ions in octahedral coordination with tetragonal compression and have C4V symmetry with ground state dxy. Tetragonality (Dg///Dg^) of V4+ ion sites exhibited non-linear variation with RO content, which indicated change in the ligand field at the site of V4+. A broad optical absorption band was observed in the glass containing Cu2+ ions corresponding to the 2B1g à 2B2g transition. From the EPR and optical data the bond parameters were evaluated. In the case of VO2+ ions, the covalency rates were estimated.
1. Introduction
Electron Paramagnetic Resonance (EPR) spectroscopy is an experimental technique to obtain information on some of the structural and dynamic phenomenon of a material and to identify the site symmetry around the transition metal (TM) ions in glasses. Glasses doped with TM ions have attracted a great deal of attention because of their potential applications in the development of new tunable solid-state lasers, solar energy converters. It has been well established [1-5] that alkali borate glasses can be used as solid electrolytes, in solid state batteries.
Electrical conductivity, EPR and optical studies were carried on double alkali (mixed alkali) borate glasses by several wsorkers [6-8]. However less work is carried out on glasses containing three alkali oxides [9].Therefore, in this paper we report the EPR and optical absorption studies on ternary alkali oxide glasses containing alkaline earth oxides (ZnO, MgO, SrO, and BaO) using Cu2+ and VO2+ as the spin probes. It is interesting to study the effect of the two paramagnetic probes (Cu2+ and VO2+) on the structural aspects of the glasses.
2. Experimental
The starting materials used in the present study were analytical grade lithium carbonate (Li2CO3), sodium carbonate (Na2CO3), potassium carbonate (K2CO3), zinc oxide (ZnO), magnesium oxide (MgO), strontium oxide (SrO), barium oxide (BaO) and boric acid (H3BO3). These materials were weighed to get the required composition and grounded in a mortar with pestle for half an hour to obtain homogeneous mixtures.
The base glass compositions taken were 1) 10RO-10 Li2O-10Na2O-10K2O-59B2O3-1CuO and 2)10RO-10Li2O- 10Na2O-10K2O-58B2O3-2V2O5 (where R = Zn, Mg, Sr and Ba). Each batch was melted in a porcelain crucible in an electric furnace at 1173 K for about 30 minutes. The homogeneous melt was rapidly quenched on to a stainless steel plates maintained at temperature of 373 K. The glasses were annealed for 24 hours at 373 K to relieve the mechanical stresses. For all the glass samples X-ray diffractograms (XRD) were recorded. The featureless, peak free XRD spectra revealed the glassy nature of the samples prepared. Table 1 presents the different glass compositions prepared for the present investigation.
EPR spectra were recorded on dry and perfectly powdered glass samples at room temperature (310 K) using EPR spectrometer (JEOL FEIX) operating at X-band frequency (9.205 GHz) with a modulation frequency of 100 kHz. Uncertainty in the measurement of “g” and “A” were about ±0.002 and ±2 × 10–4 cm–1 respectively.
The optical absorption spectra of the glasses containing copper were recorded at room temperature (310 K) using UV-VIS spectrophotometer (Shimadzu) in the wavelength region 300 nm to 1100 nm. The uncertainty in the measurement was about ±1 nm. However no optical absorption spectra were observed for the vanadyl glasses.
3. Results and Discussion
3.1. EPR Spectra of Cu2+ Ions
The EPR spectra of Cu2+ in 10RO-10Li2O-10Na2O-10 K2O-59B2O3-1CuO are shown in Figure 1. The Cu2+ ion, with effective spin S = 1/2, has a nuclear spin I = 3/2 for both 63Cu (natural abundance 69%) and 65Cu (natural abundance 31%). Hence, (2I + 1) i.e. four parallel and four perpendicular hyperfine (hf) components were expected. In the present work, three weak parallel components were observed in the lower field region and fourth parallel component was overlapped with the perpendicular component. The perpendicular components in the high field region were not resolved. It was found that high field side of the spectra was more intense than the low field side. The EPR spectra of all the glass samples containing Cu2+ ions is similar to those reported for Cu2+ ions in other glass systems [7,10-15]. An axial spinHamiltonian was employed in the analysis of EPR spectra [16,17] which is given as
 (1)
(1)
where z is the symmetry axis, b the Bohr magneton, S and I the electron and nuclear spin operators, HX, HY and HZ the static magnetic field components, g// and g^ the parallel and perpendicular components of “g” tensor
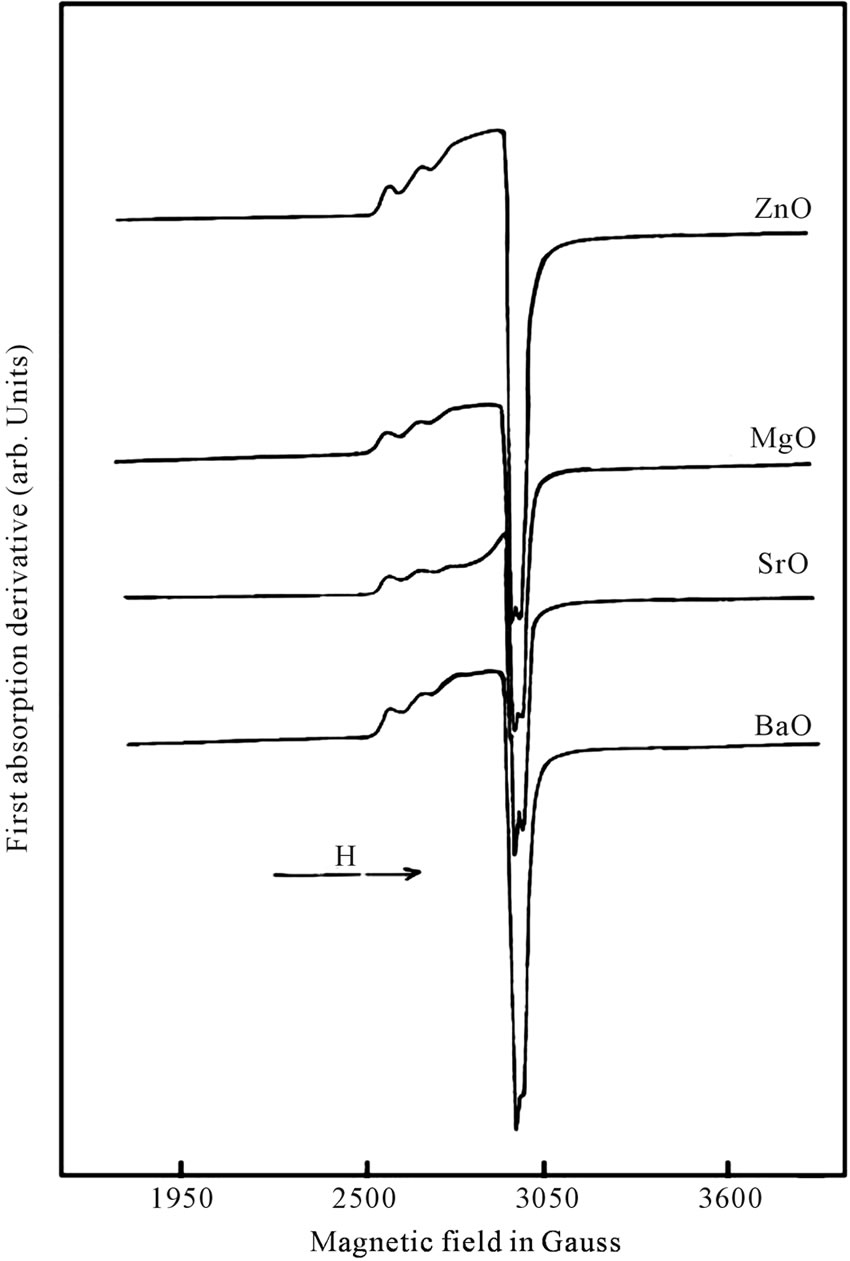
Figure 1. EPR spectra of Cu2+ ions in RO-Li2O-Na2O-K2OB2O3 glasses.
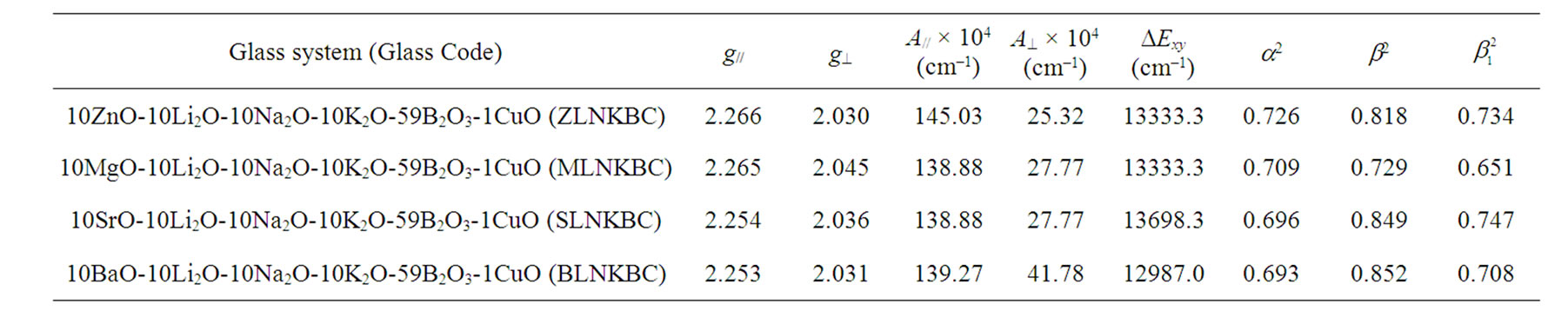
Table 1. Spin-Hamiltonian parameters, optical absorption bands and bonding parameters of Cu2+ ions in the glass systems.
while A// and A^ are parallel and perpendicular components of the hyperfine tensor A. The nuclear quadrupole contribution is neglected [18].
The solution to the spin-Hamiltonian gives the following expressions for the peak position related to the principal values of g and A tensors [19], for the parallel and perpendicular hyperfine peaks respectively.
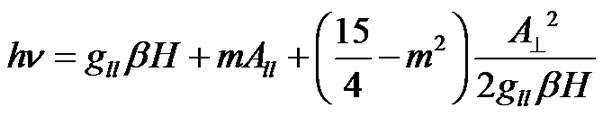 (2)
(2)
and
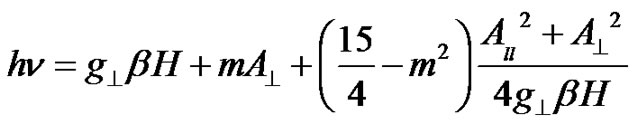 (3)
(3)
Here m is the nuclear magnetic quantum number of the copper nucleus with the values +3/2, +1/2, –1/2 and –3/2 and n is the microwave frequency. The spin-Hamiltonian parameters have been evaluated and are presented in Table 1.
It was observed that, g// > g^> ge = 2.0023. From the “g” values and the shape of the EPR spectra it can be concluded that the ground state of Cu2+ ions is 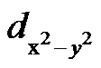
orbital (2B1g state), the Cu2+ ions being located in tetragonally distorted octahedral sites [20-24]. The high “g” values indicate the presence of a CuO6 chromophore [25, 26]. The line width of the parallel hyperfine components was found to increase with increasing values of the nuclear spin quantum number mI (Figure 1), which may be due to fluctuation in both the ligand fields and bond covalencies from one copper(II) complex to the next, giving rise to a narrow distribution in g [24,27]. It can be observed from Figure 2 that the variation of g// and A// for different ZnO, MgO, SrO and BaO composition is non linear. This may be due to change in the tetragonal distortion. Variation in g and A values may be associated with the change in the environment of Cu2+, i.e. in the ligand field strength at the site of Cu2+ which may be attributed to the structural changes in the glass. In the B2O3 glasses the addition of the network modifiers will lead to an increase in the coordination number of some portion of the boron atoms from 3 to 4. The resulting glass may be composed of both triangular and tetrahedral units which form a relatively open network with holes between the oxygen atoms of sufficient size to accommodate the alkali and alkaline earth ions [28]. As a doubly charged cation, R2+ is sufficiently strong to split the network. Therefore, sufficient non-bridging oxygen’s will be available for coordination in the broken network. The alkali oxides make available additional weakly bonded O2– for each R2+, i.e. R2+ captures the O2– from alkali oxide which happens at the expense of alkali oxide coordination. The solubility of the Cu2+ ions increases with the addition of the alkali and alkaline earth oxides presumably due to the coordination of the metal ion by the extra oxygen ions. Therefore incorporation of RO in the glass will influence the field at the site of Cu2+, which in turn may reflect in the non-linear variation of the spin Hamiltonian parameters as observed in the present case.
3.1.1. Optical Absorption Spectra
The optical absorption spectra of all the glasses containing Cu2+ ions resulted in a broad absorption band. The observed peak positions of the optical absorption spectra of the glasses are listed in Table 1. The observed broad band was assigned to the 2B1g à 2B2g transition of Cu2+ ions [12]. The variation of peak position of the optical absorption band with composition (ZnO, MgO, SrO and BaO) is shown in Figure 3. The variation is found to be non-linear. It is observed that SLNKBC glass has the lowest value of absorption peak wavelength, which may be due to increase in the ligand field strength around
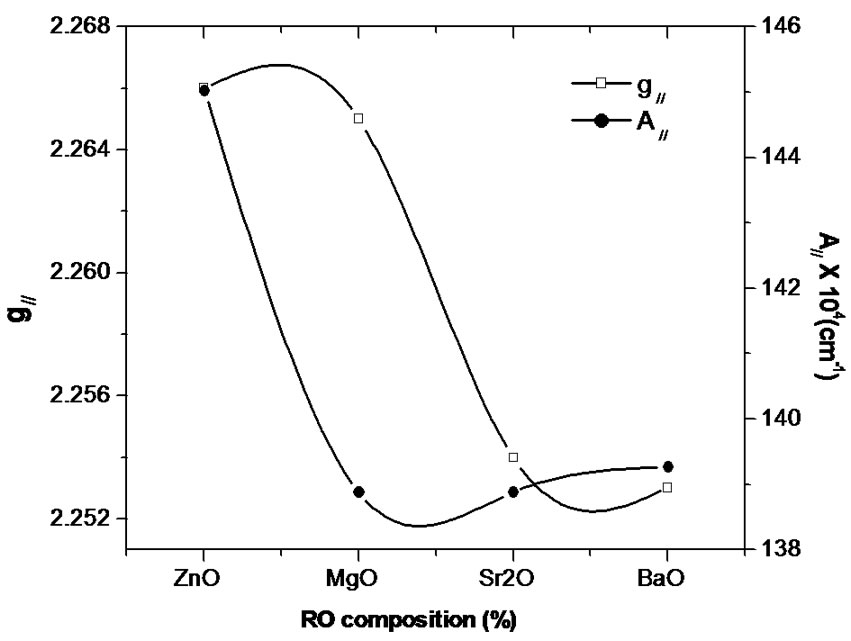
Figure 2. Variation of g// and A// with RO composition.

Figure 3. Variation of optical absorption maximum (l) with RO composition.
Cu2+ ion. The optical absorption spectrum is influenced by the host structure into which the TM ions are incorporated. In oxide glasses, the TM ions mostly form coordination complexes with doubly charged oxygen as the ligands. However Cu2+, being as d9 ion, experiences a strong Jahn-Teller distortion, which leads to the splitting of energy levels [29,30] and causes predominantly an elongated octahedral coordination with four short inplane bond lengths and longer axial bond lengths. Accordingly three transitions, viz, 2B1g à 2A1g, 2B1gà 2B2g and 2B1g à 2Eg are expected. However only a single optical absorption maximum was observed in most of the cases [31,32]. Various authors [33-35,37] have placed the 2B1g à 2B2g and 2B1g à 2A1g transitions under the observed band while 2B1g à 2Eg transitions is considered to be hidden under the intense charge transfer absorption in the UV region. Most of the authors [33,35,38,39-44] assigned the observed optical peak to the 2B1g à 2B2g transition (DExy) and have used this value in the evaluation of the bond parameters. Therefore in the present case also the optical absorption band was assigned to 2B1g à 2B2g (DExy) transition.
3.1.2. Cu2+ Ligand Bond Nature
The EPR and optical absorption spectra data can be correlated to evaluate the bonding coefficients of Cu2+ [24, 34,35]. The bonding parameters were evaluated using the equations given below [24,33,44].
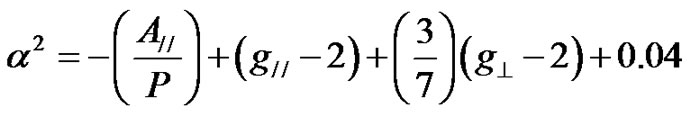 (4)
(4)
 (5)
(5)
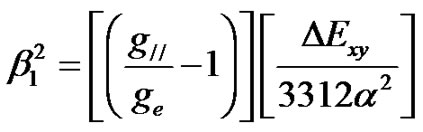 (6)
(6)
where P is the dipolar hyperfine coupling parameter (= 0.036 cm–1), DExy, DExz, yz are the heights of dxy, and dxz,yz
molecular orbital levels above the ground state 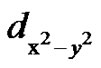
respectively [33,38]. Here a2 describes the in-plane sbonding with copper 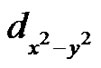 orbital, b2 describes the out-of-plane p-bonding with the
orbital, b2 describes the out-of-plane p-bonding with the  and
and 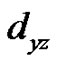 orbital and
orbital and  is a measure of in-plane p-bonding with
is a measure of in-plane p-bonding with 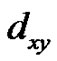 orbital. The positions of optical peak indicate the value of DExy [34,35,39,41]. The corresponding value of DExz, yz was calculated using the approximate relation [40]
orbital. The positions of optical peak indicate the value of DExy [34,35,39,41]. The corresponding value of DExz, yz was calculated using the approximate relation [40]
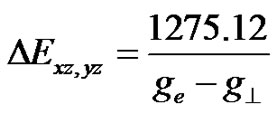 (7)
(7)
The calculated values (Table 1) of a2, 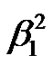 indicate moderate covalency for the in-plane sand pbonding respectively while the b2 value indicated that the out-ofplane p-bonding is slightly ionic in nature.
indicate moderate covalency for the in-plane sand pbonding respectively while the b2 value indicated that the out-ofplane p-bonding is slightly ionic in nature.
3.2. EPR Spectra of VO2+ Ions
The EPR Spectra of VO2+ ions in 10RO-10Li2O- 10Na2O-10K2O-58B2O3-2V2O5 are shown in Figure 4. The spectra have structures that are characteristic of hyperfine interactions arising from an unpaired electron with 51V nucleus, whose spin is 1/2 and present in 99.75% abundance. These spectra were analyzed by assuming [45-47] that vanadium is present as vanadyl ion in a ligand field of C4V symmetry. The EPR spectra were analyzed by using an axial spin-Hamiltonian (Equation (1)). The solutions of the spin-Hamiltonian [20], for parallel and perpendicular hyperfine lines are given respectively as:
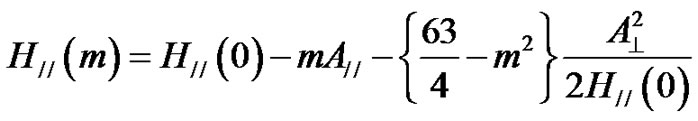 (8)
(8)
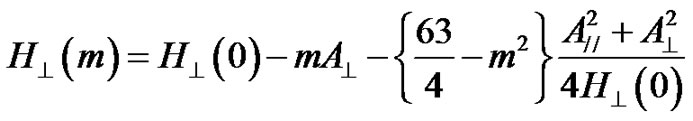 (9)
(9)
where m is the magnetic quantum number of the vanadium nucleus having the values of ±7/2, ±5/2, ±3/2, ±1/2, H//(0) = (hn/g//b) and H^(0) = (hn/g^b). The spin-Hamiltonian parameters for various compositions were calculated using Equations (8) and (9) and are listed in Table 2. The paramagnetism of the vanadyl ion (V4+) arises from a single unpaired electron, as the crystalline fields quench the orbital angular momentum. The crystal fields of V4+ ions in glasses can be described either by threefold or fourfold symmetries [48]. The variation of g// and g^ depend critically on the local symmetry of this field. Although the V4+ ion usually in six-fold coordination in complexes containing vanadyl, its local symmetry is generally a distorted octahedron of oxygen ions.
An octahedral site with tetragonal compression would give values of g// < g^ < ge and A// > A^. In the present investigation, it is observed that of g// < g^ < ge and A// > A^. It is therefore concluded that V4+ in the present glass samples exist as VO2+ ions in octahedral coordination with tetragonal compression. The symmetry of vanadyl complex is C4V, and the ground state of 3d1 ion is dxy. The measure of tetragonality of the VO2+ site is given by Dg///Dg^ [Dg// = g// – ge and Dg^ = g^ – ge] values (Table 3). The glass (ZLNKBV) has high Dg///Dg^ value compared to other glasses (MLNKBV, SLNKBV, and BLNKBV). The high (Dg///Dg^) value for (ZLNKBV) glass indicates that the vanadyl ions in the glass (ZLNKBV) are more tetragonally distorted. The low value of Dg^ for the glass (ZLNKBV) also support that vanadyl ions in this glass
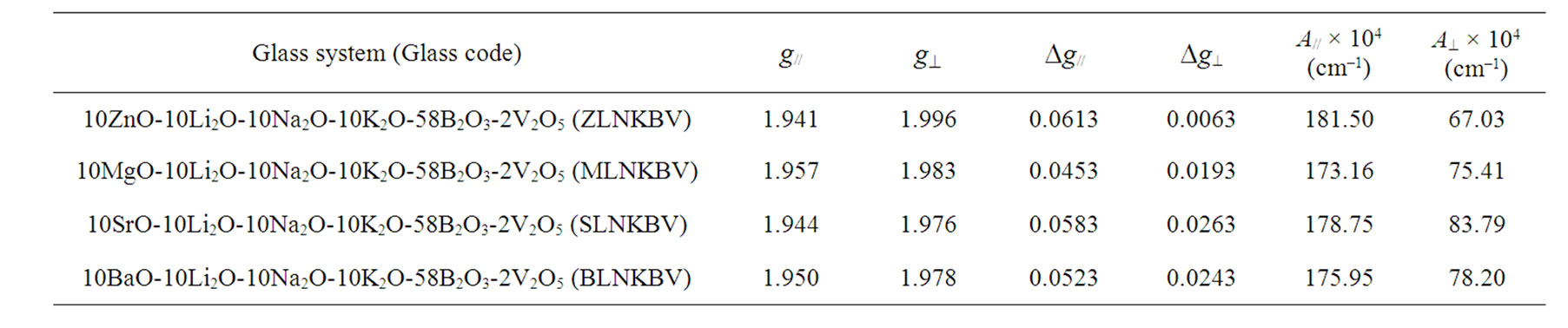
Table 2. Spin-Hamiltonian parameters of VO2+ ion the glass systems.

Figure 4. EPR spectra of VO2+ ions in RO-Li2O-Na2O-K2OB2O3 glasses.
are more tetragonally distorted. Figure 5 shows the variation of Dg///Dg^ with different compositions (ZnO, MgO, SrO, and BaO). The variation is non-linear which may due to change of ligand field at the transition metal (TM) ion site. For the glasses containing (MgO, SrO, BaO) the (Dg///Dg^) values decrease in the order (MgO, SrO, BaO) as shown in Table 3. The decrease in the values Dg///Dg^ suggests that the octahedral symmetry in these glasses is improved [49].
The Fermi contact interaction term K, the dipolar hyperfine coupling parameter P and the covalency rates (1 – a2) and (1 – n2) were calculated using the following equations [50].

Figure 5. Variation of Dg///Dg^ with RO composition.
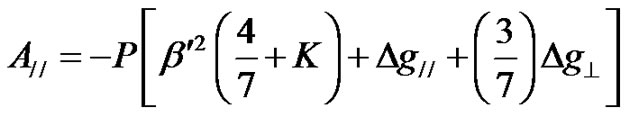 (10)
(10)
 (11)
(11)
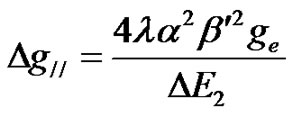 (12)
(12)
 (13)
(13)
The parameter  which is measure of out-of-plane p-bonding with the equitorial ligands is assumed to be unity for many oxide glasses [50]. (1 – a2) and (1 – n2) represent the covalency rates. (1 – n2) provides an estimate of covalency of the p-bonding between the V4+ ion and the vanadyl oxygen, while (1 – a2) gives an estimation of the s-bonding with the equitorial ligands. The covalency rates were estimated by taking the values of DE1 = 12500 cm–1 and DE1 = 16000 cm–1 [51]. The spin orbit coupling constant (l) is taken as 249 cm–1 [50-52]. The covalency rates (1 – a2) and (1 – n2) are given in Table 3. The values of (1 – a2) and (1 – n2) indicate a moderate covalency for s and p-bonds. These values indicate only the trends in the variation of magnitude of bonding parameters. The Equations (10) and (11) can be
which is measure of out-of-plane p-bonding with the equitorial ligands is assumed to be unity for many oxide glasses [50]. (1 – a2) and (1 – n2) represent the covalency rates. (1 – n2) provides an estimate of covalency of the p-bonding between the V4+ ion and the vanadyl oxygen, while (1 – a2) gives an estimation of the s-bonding with the equitorial ligands. The covalency rates were estimated by taking the values of DE1 = 12500 cm–1 and DE1 = 16000 cm–1 [51]. The spin orbit coupling constant (l) is taken as 249 cm–1 [50-52]. The covalency rates (1 – a2) and (1 – n2) are given in Table 3. The values of (1 – a2) and (1 – n2) indicate a moderate covalency for s and p-bonds. These values indicate only the trends in the variation of magnitude of bonding parameters. The Equations (10) and (11) can be

Table 3. Tetragonality and covalency rates of V4+ ion.
rewritten as,
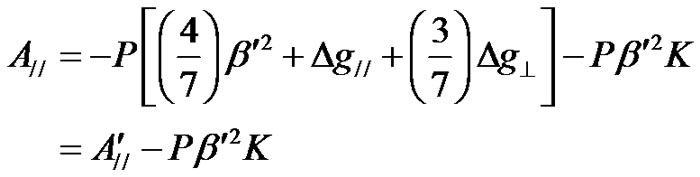 (14)
(14)
 (15)
(15)
From the molecular orbital theory, it was observed [50] that the components A// and A^ consists of the contributions A// and A^ of the 3dxy electron to the hyperfine structure. P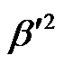 K term arises due to the anomalous contribution of the s-electrons. The values of P, K,
K term arises due to the anomalous contribution of the s-electrons. The values of P, K, 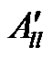 and
and 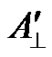 were calculated and are given in Table 3.
were calculated and are given in Table 3.
The decrease of the anisotropic contribution 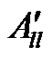 and
and 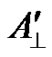 of the 3dxy electron to the hyperfine splitting for the glasses containing MgO, BaO and SrO is brought about by the increase of screening of the 3dxy orbital from its nucleus through the overlap of the electron orbits of the surrounding ligands of oxygen [49]. This is also supported by the decrease in the value of P, and increase in the value of K. The high values of K indicate a large contribution to the hyperfine constant by the “s” electron.
of the 3dxy electron to the hyperfine splitting for the glasses containing MgO, BaO and SrO is brought about by the increase of screening of the 3dxy orbital from its nucleus through the overlap of the electron orbits of the surrounding ligands of oxygen [49]. This is also supported by the decrease in the value of P, and increase in the value of K. The high values of K indicate a large contribution to the hyperfine constant by the “s” electron.
4. Conclusions
The EPR studies revealed that the Cu2+ ions are is present in all the glass systems investigated and they exist in tetragonally distorted octahedral sites with 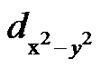 (2B1g)
(2B1g)
ground state. The spin-Hamiltonian parameters are influenced by the change in the glass composition. The bond parameter values indicated that in plane s and pbodings are moderately covalent. The out-of-plane pbonding is slightly ionic in nature.
In all the glass systems, the vanadium ions (V4+) exist as VO2+ ions in octahedral coordination with tetragonal compression and belong to C4V symmetry. The ground state of V4+ ion is dxy. The variation of Dg///Dg^ with different compositions (ZnO, MgO, SrO and BaO) is nonlinear which may be due to change of electric field at the transition metal (TM) ion site. The (1 – a2) and (1 – n2) values indicated moderate covalency for s and p-bonds respectively.
REFERENCES
- M. D. Ingram, “Ionic Conductivity in Glasses,” Physics and Chemistry of Glasses, Vol. 28, 1987, pp. 215-234.
- M. Dawy and A. H. Salama, “Electrical and Optical Properties of Some Sodium Borates,” Materials Chemistry and Physics, Vol. 71, No. 2, 2001, pp. 137-147. doi:10.1016/S0254-0584(01)00280-2
- Md Jamal, G. Venugopal, Md Shareefuddin and M. N. Chary, “Sodium Ion Conducting Glasses with Mixed Glass Formers NaI-Na2O-V2O5-B2O3: Application to Solid State Battery,” Materials Letters, Vol. 39, No. 1, 1999, pp. 28-35.
- Md Jamal, Md Shareefuddin and M. N Chary, “Ionic Transport and Battery Characterization Studies on NaINa2O-B2O3 Ternary Glassy System,” Journal of Power Sources, Vol. 58, No. 2, 1996, pp. 217-219. doi:10.1016/S0378-7753(96)02379-8
- Md Shareefuddin, Md Jamal and M. N Chary, “Ionic Transport and Battery Characterization Studies on 15NaF- 15Na2O-70 [(1-x)B2O3-xCr2O3] Quaternary Glassy Systems,” Materials Letters, Vol. 24, No. 5, 1995, pp 291- 296. doi:10.1016/0167-577X(95)00113-1
- R. P. S. Chakradhar, B. Yasoda, J. L. Rao and N. O. Gopal, “EPR and Optical Studies of Mn2+ ions in Li2ONa2O-B2O3 Glasses—An Evidence of Mixed Alkali Effect,” Journal of Non-Crystalline Solids, Vol. 353, No. 24-25, 2007, pp. 2355-2362
- R. P. S. Chakradhar, B. Yasoda, J. L. Rao and N. O. Gopal, “Mixed Alkali Effect in Li2O-Na2O-B2O3 Glasses Containing CuO—An EPR and Optical Study,” Journal of Non-Crystalline Solids, Vol. 352, No. 36-37, 2006, pp. 3864-3871. doi:10.1016/j.jnoncrysol.2006.06.033
- N. Nagaraja, T. Sankarappa and M. P. Kumar, “Electrical Conductivity Studies in Single and Mixed Alkali Doped Cobalt-Borate Glasses,” Journal of Non-Crystalline Solids, Vol. 354, No. 14, 2008, pp. 1503-1508, doi:10.1016/j.jnoncrysol.2007.08.042
- Y. Gao and C. Cramer, “Mixed Cation Effects in Glasses with Three Types of Alkali Ions,” Solid State Ionics, Vol. 176, No. 29-30, 2005, pp. 2279-2284. doi:10.1016/j.ssi.2005.06.010
- V. R. Kumar, J. L. Rao and N. O. Gopal, “EPR and Optical Absorption Studies of Cu2+ Ions in Alkaline Earth Alumino Borate Glasses,” Materials Research Bulletin, Vol. 40, No. 8, 2005, pp. 1256-1269. doi:10.1016/j.materresbull.2005.04.010
- Md Shareefuddin, Md Jamal and M. N Chary, “Electron Spin Resonance and Optical Absorption Spectra of Cu2+ Ions in xNaI-(30-x)Na2O-70B2O3 Glasses,” Journal of Non-Crystalline Solids, Vol. 201, No. 1-2, 1996, pp. 95- 101. doi:10.1016/0022-3093(95)00627-3
- G. Ramadevudu, Md Shareefuddin, N. S Bai, M. L. Rao, and M. N Chary, “Electron Paramagnetic Resonance and Optical Absorption Studies of Cu2+ Spin Probe in MgONa2O-B2O3 Ternary Glasses,” Journal of Non-Crystalline Solids, Vol. 278, No. 1-3, 2000, pp. 205-212. doi:10.1016/S0022-3093(00)00255-6
- I. Ardelean, O. Cozar, S. Filip, V. Pop and I. Cenan, “EPR and Magnetic Susceptibility Studies of Cu2+ Ions in Bi2O3·GeO2 Glasses,” Solid State Communications, Vol. 100, No. 8, 1996, pp. 609-613. doi:10.1016/0038-1098(96)00472-3
- I. Ardelean, M. Peteanu, S. Filip, V. Simon and G. Györffy, “EPR and Magnetic Susceptibility Studies of Iron Ions in 70TeO2·25B2O3·5PbO Glass Matrix,” Solid State Communications, Vol. 102, No. 4, 1997, pp. 341-346. doi:10.1016/S0038-1098(96)00772-7
- F. Ciorcas, S. K. Mendiratta, I. Ardelean and M. A. Valente, “Structural and Magnetic Studies of CuO-TeO2 and CuO-TeO2-B2O3 Glasses,” The European Physical Journal B—Condensed Matter and Complex Systems, Vol. 20, No. 2, 2001, pp. 235-240.
- Md Jamal, Md Shareefuddin and M. N Chary, “Ionic Transport and Spectroscopic Studies on xNaI-(30-x)Na2O- 70B2O3 and 15NaI-(15-x)Na2O-xLi2O-70B2O3 Glasses,” Bulletin of Electrochemistry, Vol. 12, No. 11, 1996, pp. 686-692.
- Md Shareefuddin, K. Vanaja, P. M. Rao, Md Jamal and M. N. Chary, “Electron Paramagnetic Resonance and Optical Absorption Spectra of Cu2+ Ions in xKI·(30-x) K2O·70B2O3 Glasses,” Physics and Chemistry of Glasses, Vol. 39, No. 3, 1998, pp. 184-187.
- A. Abragam and B. Bleany, “EPR of Transition Metal Ions,” Clarendon, Oxford, 1970.
- B. D. Bleany, K. D. Bowers and D. J. E. Ingram, “Paramagnetic Resonance in Diluted Copper Salts. I. Hyperfine Structure in Diluted Copper Tutton Salts,” Proceedings of the Royal Society A, Vol. 228, No. 1173, 1955, pp. 147- 157. doi:10.1098/rspa.1955.0039
- T. Bates and J. D. Mackenzie, “Modern Aspects of the Vitreous State,” 2nd Edition, Butterworth, London, 1962.
- I. Taoufik, M. Haddad, A. Nadiri, R. Brochu and R. Berger, “X and Q Band EPR Studies of CuO·5Zr2(PO4)3 Phosphates,” Journal of Physics and Chemistry of Solids, Vol. 60, 1999, pp. 701-707. doi:10.1016/S0022-3697(98)00067-5
- L. D. Bogomolova, Yu. G. Tepliakov and F. Caccavale, “EPR of Some Oxide Glasses Implanted with Mn+ and Cu+ Ions,” Journal of Non-Crystalline Solids, Vol. 194, No. 3, 1996, pp. 291-296. doi:10.1016/0022-3093(95)00496-3
- A. Abragam and M. H. L. Pryce, “The Theory of the Nuclear Hyperfine Structure of Paramagnetic Resonance Spectra in the Copper Tutton Salts,” Proceedings of the Royal Society A, Vol. 206, No. 1085, 1955, pp. 164-172. doi:10.1098/rspa.1951.0062
- H. Imagawa, “ESR Studies of Cupric Ion in Various Oxide Glasses,” Physica Status Solidi B, Vol. 30, No. 2, 1968, pp. 469-478, doi:10.1002/pssb.19680300207
- M. A. Hitchman and T. D. Waite, “Electronic Spectrum of the Hexaaquocopper(2+) Ion,” Inorganic Chemistry, Vol. 15, No. 9, 1976, pp. 2150-2154. doi:10.1021/ic50163a030
- M. D. Joesten, R. C. Koch, T. W. Martin and J. H. Venable, “Electron Paramagnetic Resonance Studies of Some Copper(II) Complexes with Organophosphorus Chelates,” Journal of the American Chemical Society, Vol. 93, No. 5, 1971, pp. 1138-1140. doi:10.1021/ja00734a018
- J. G. Darab and R. K. Maccrone, “Electron Paramagnetic Resonances of Copper(II) Complexes Incorporated in Silica Sol-Gels,” Physics and Chemistry of Glasses, Vol. 32, 1991, pp. 91-102.
- A. H. Dietzel, “On the So-Called Mixed Alkali Effect,” Physics and Chemistry of Glasses, Vol. 24, 1983, p. 172.
- P. W. France, S. F. Carter and J. M. Parker, “Oxidation States of 3d Transition Metals in ZrF4 Glasses,” Physics and Chemistry of Glasses, Vol. 27, 1986, pp. 32-41.
- Y. Ohishi, S. Mitachi, T. Kanamori and T. Manabe, “Optical Absorption of 3d Transition Metal and Rare Earth Elements in Zirconium Fluoride Glasses,” Physics and Chemistry of Glasses, Vol. 24, 1983, pp. 135-140.
- L. E. Orgel, “Spectra of Transition-Metal Complexes,” Journal of Chemical Physics, Vol. 23, No. 6, 1955, pp. 1004-1014. doi:10.1063/1.1742182
- C. J. Ballhausen, “Introduction to Ligand Field Theory,” McGraw-Hill, New York, 1962.
- I. Siegel and J. A. Lorenc, “Paramagnetic Resonance of Copper in Amorphous and Polycrystalline GeO2,” Journal of Chemical Physics, Vol. 45, No. 6, 1966, pp. 2315- 2320. doi:10.1063/1.1727927
- D. Kivelson and R. Neiman, “ESR Studies on the Bonding in Copper Complexes,” Journal of Chemical Physics, Vol. 35, No. 1, 1961, pp. 149-145. doi:10.1063/1.1731880
- A. H. Maki and B. R. McGarvey, “Electron Spin Resonance in Transition Metal Chelates. I. Copper(II) BisAcetylacetonate,” Journal of Chemical Physics, Vol. 29, No. 1, 1958, pp. 31-35. doi:10.1063/1.1744456
- J. Bjerrum, C. J. Ballahausen and C. K. Jorgensen, “Studies on Absorption Spectra. I. Results of Calculations on the Spectra and Configuration of Copper(II) Ions,” Acta Chemica Scandinavica, Vol. 8, 1954, pp. 1275-1289. doi:10.3891/acta.chem.scand.08-1275
- D. P. Graddon, “The Absorption Spectra of Complex SaltsIII Cupric Ethylacetoacetate,” Journal of Inorganic and Nuclear Chemistry, Vol. 14, No. 3-4, 1960, pp. 161-168. doi:10.1016/0022-1902(60)80253-9
- H. Hosono, H. Kawazoe and T. Kanazawa, “ESR and Optical Absorption of Cu2+ in Na2O-SiO2 Glasses,” Journal of Non-Crystalline Solids, Vol. 33, No. 1, 1979, pp. 103-115. doi:10.1016/0022-3093(79)90099-1
- A. Klonkowski, G. H. Frischat and T. Richter, “Properties of Mixed Alkali Glasses in the System Li2O-Na2OA12O3-SiO2,” Physics and Chemistry of Glasses, Vol. 24, 1983, pp. 47-53.
- B. Sreedhar, J. L. Rao and S. V. J. Lakshman, “Electron Spin Resonance and Optical Absorption Spectra of Cu2+ Ions in Alkali Zinc Borosulphate Glasses,” Journal of Non- Crystalline Solids, Vol. 124, No. 2-3, 1990, pp. 216-220. doi:10.1016/0022-3093(90)90265-N
- A. Klonkowski, “Bond Characteristics of Phosphate Glasses of the M(II)O-P2O5 Type. Part 1. The Extreme Position of Strontium Phosphate Glasses,” Physics and Chemistry of Glasses, Vol. 26, No. 1, 1985, pp. 11-16.
- N. C. Biswas, R. Dayal and P. Chand, “Electron Paramagnetic Resonance and Optical Absorption of Cu2+ in NaFB2O3 and NaF-Na2O-B2O3 Glasses,” Physics and Chemistry of Glasses, Vol. 37, No. 1, 1996, pp. 31-35.
- A. Klonkowski, “The Structure of Sodium Aluminosilicate Glass,” Physics and Chemistry of Glasses, Vol. 24, 1983, pp. 166-171.
- H. R. Gersmann and J. D. Swalen, “Electron Paramagnetic Resonance Spectra of Copper Complexes,” Journal of Chemical Physics, Vol. 36, No. 12, 1962, pp. 3221- 3233. doi:10.1063/1.1732450
- V. P. Seth, S. Yadav and S. K. Gupta, “EPR in CoO·BaO·B2O3 Glasses Containing Two Transition Elements,” Radiation Effects and Defects in Solids, Vol. 132, No. 2, 1994, pp. 187-191. doi:10.1080/10420159408224308
- G. Hochstrasser, “Detection of VO2+ in Glass by Electron Spin Resonanc,” Physics and Chemistry of Glasses, Vol. 7, 1966, pp. 178-182.
- L. D. Bogomolva and V. A. Jachkin, “ Electron Paramagnetic Resonance of Cu2+ and V4+ Ions in Borate Glasses,” Journal of Non-Crystalline Solids, Vol. 58, No. 2-3, 1983, pp. 165-178. doi:10.1016/0022-3093(83)90021-2
- H. G. Hecht and T. S. Johnston, “Study of the Structure of Vanadium in Soda—Boric Oxide Glasses,” Journal of Chemical Physics, Vol. 46, No. 1, 1967, pp. 23-34. doi:10.1063/1.1840378
- A. K. Bandyopadhyay, “Optical and ESR Investigation of Borate Glasses Containing Single and Mixed Transition Metal Oxides,” Journal of Materials Science, Vol. 16, No. 1, 1981, pp. 189-203. doi:10.1007/BF00552072
- D. Kivelson and S.-K. Lee, “ESR Studies and the Electronic Structure of Vanadyl Ion Complexes,” Journal of Chemical Physics, Vol. 41, No. 7, 1964, pp. 1896-1903. doi:10.1063/1.1726180
- B. Sreedhar, P. Indira, A. K. Bhatnagar and K. Kojima, “Electron Paramagnetic Resonance Spectra of VO2+ Ions in Alkali Fluoroborate Glasses,” Journal of Non-Crystalline Solids, Vol. 167, No. 1-3, 1994, pp. 106-111. doi:10.1016/0022-3093(94)90373-5
- G. R. Devudu, Md Shareefuddin, M. N. Chary and M. L. Rao, “EPR and DSC Studies on MgO-R2OB2O3-V2O5 Alkali Glasses,” Modern Physics Letters B, Vol. 22, No. 16, 2008, pp. 1579-1588. doi:10.1142/S0217984908016236

