International Journal of Organic Chemistry
Vol.3 No.1(2013), Article ID:29154,10 pages DOI:10.4236/ijoc.2013.31008
Evaluation of Some Insulated Greases Prepared from Rubber and Bitumen Thickeners
1Egyption Petroleum Research Institute, Cairo, Egypt
2Faculty of Science, Al-Azhar University, Cairo, Egypt
Email: *azza_mazroua2005@yahoo.com
Received February 10, 2013; revised March 11, 2013; accepted March 22, 2013
Keywords: Wax Gel; Butyl Rubber Grease; Isoprene Rubber Grease; Bitumen Grease and Dielectric Properties
ABSTRACT
The wax gel grease (S0) was prepared from a mixture of 2.3:1 base oil blend (base oil grade 260/290, transformer oil), and microcrystalline wax in the presence of 0.1% - 2% of polyoxyethylene sorption nano-palmitate as antioxidant and 2,2 methylene bis (4-methyle-6-tertiary butyl phenol) as anticorrosion. It was found that the prepared Wax gel grease has inconvenient physico-chemical and dielectric properties, so in order to improve its physico-chemical properties (viscosity, penetration, dropping point and water resistance) and dielectric Properties (dielectric constant, dielectric loss and volume resistivity), Butyl rubber, isoprene rubber and bitumen were added separately as thickening agents to the prepared wax gel in certain proportion at certain frequency range 1 - 1000 KHz at 35˚C. The best dielectric properties were achieved by adding butyl rubber to the prepared wax gel.
1. Introduction
It is well known that true grease consists of oil or other similar fluid lubricants or both together mixed with another thicker substance [1-4]. Choosing the type of cohesive greases to be applied to particular lubrication should be done carefully. When rubber parts come in contact with lubricating greases, a change in dimensions, shape and the mechanical characteristics of such parts may take place. This change is most frequently attributed to an increase in rubber’s weight (swelling) in the grease dispersion medium, or to a weight loss due to migration of rubber parts in frictional component (plasticizers) to grease. In practice, weight loss of rubber parts in frictional components is not virtually observed [5]. The effects of greases on rubbers are determined mainly by the nature and viscosity of the grease dispersion medium, where rubbers of none or low polarity are strongly swollen in low polarity petroleum oil [6]. Lubricating greases from polyisoprene rubber (100) parts by weight, about (15 - 45) parts by weight of aliphatic amides and (5 - 30) parts by weight of polyethylene wax, paraffinic wax and microcrystalline wax were mixed. A bull joint is disclosed to contain this lubricant composition [7]. Rubber component is added to grease for use as a cable filling materials as well as to improve bleed resistance. Adding dielectric grease to switch contacts and potentiometer tracks improves performance and operating life [8-10].
Bitumen has been widely used in the electrical Industry because of its excellent electrical properties, resistance to water and chemicals and its thermoplastic properties which make it easily workable for manufacture. Its applications include filling and sealing compounds for dry batteries, capacitors, cable junctions, terminal boxes, insulation tape and the insulation of cables, motors, transformers, etc. Bitumen has a high resistance (low conductivity) and is therefore used as an excellent insulating material. Harder grades have slightly higher resistance than soft grades although the difference is not important in practice. The resistance decreases with increasing temperature, the influence of fillers on resistance is negligible; provided that the filler is not conductive [11].
In this study, we attempt to obtain greases with enhanced physico-chemical and dielectric properties than wax gel alone, which allows their use as insulating materials in cables and other electrical equipments.
2. Expermental Work
2.1. Materials
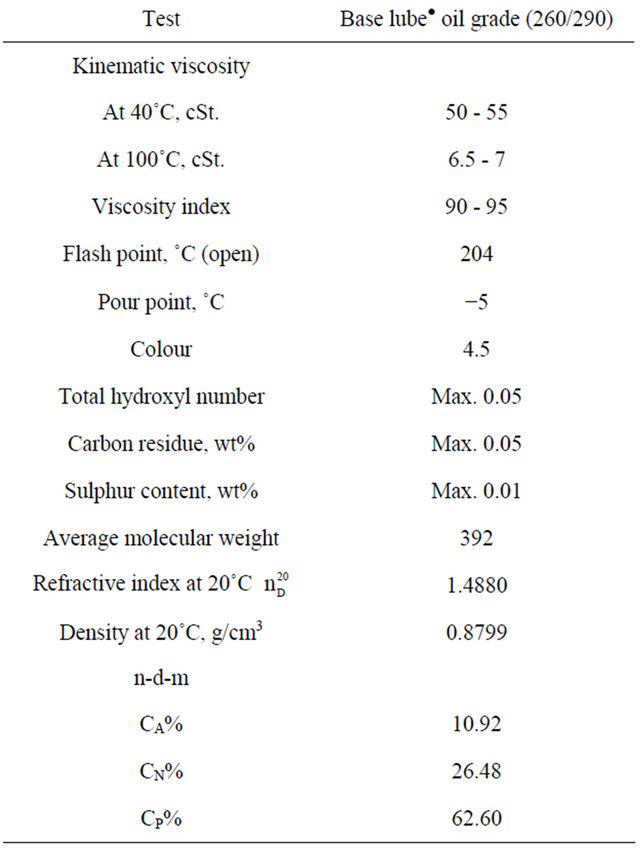
Base lube oil grade (260/290):
Flash point (open): 204˚C, Viscosity index: 90 - 95, Pour point: (−5˚C). Supplier from Misr petroleum company and the grade 260/290.
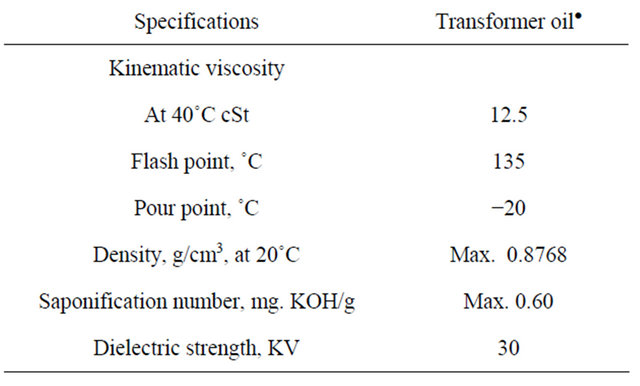
Transformer oil:
Flash point: 135˚C, Viscosity at 40˚C: 12.5 cSt, Pour point: (−20˚C).
Microcrystalline wax:
Melting range: 80˚C - 81˚C, Oil content %: 0.5, Flash point, open: 288˚C; Density at 20˚C: 0.9002 g/Cm3. Wax supplier from Alex Petroleum Company.
Atactic polypropylene:
Density: 0.92 g/cm3, Melting point: 170˚C, Volume resistivity: 1016 ohm∙cm.
Bitumen:
Viscosity at 135˚C: 390 cSt, Softening point: 52˚C, Flash point (open): 250˚C.
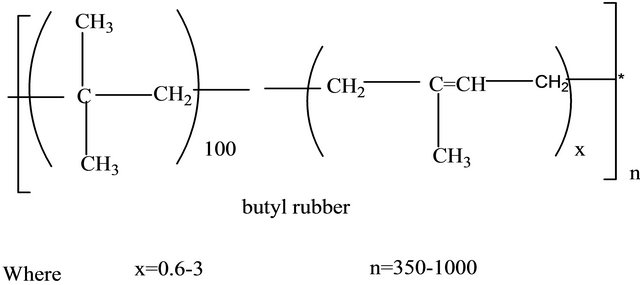
Butyl rubber (isobutylene-isoprene copolymer).
Polyisoprene rubber
Polyoxyethylene sorbiton-nano-palmitate, Tween (20):
Refractive index: 1.4685 Specific gravity: 1.1 Boiling Point: >100˚C; Specification number: 43 - 49 pH of 1% Aqueous solution: 5-7.
2, 2'-methylen-bis(4-methyl-6-tertiary butyl phenol)
Melting point: 125˚C, Specific gravity: 1.08, Molecular weight: 340.4989.
2.2. Methods
2.2.1. Dielectric and Resistivity Measurement
The computerized LRC (Hioki model 3531 Z Hi Tester) was used to conduct the electrical properties of the investigated samples. The bridge measures the capacitance from 19 pF up to 370 mf, the resistance from 100 m Ω up to 200 MΩ and the dielectric loss (ε''), tan (δ) from 10−5 up to 101.
The relative dielectric permittivity was calculated using the following equations:
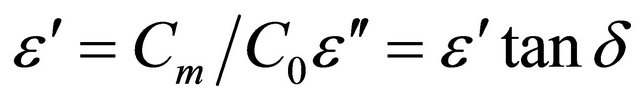
where Cm: the measured capacitance of the used material; C0: the capacity of the empty condenser. ε'': the dielectric loss, tanδ: the loss tangent.
The resistivity was calculating using the equation

where ρ is the resistivity ohm∙cm−1. L is the length of the sample in mm, A is the cross sectional area, and σ is the electrical conductivity [12].
2.2.2. Dynamic Viscosity
The apparent viscosities of the prepared greases were determined using a digital Rheometer LVDV-Ш-Ultra AST.
2.2.3. Infrared Spectroscopy (IR)
FTIR spectra were obtained using (Fourier transform infrared, ATI Maston Gensis series (USA) infrared spectrophotometer adopting KBr technique. For all samples, the KBr technique was carried out approximately in a quantitative manner since the weight of sample and that of KBr, were always kept constant. At wave number from 500 - 4000 cm−1, transmittance from 0% to 100% and resolution was 4 mm. Number of scans used during the collection of FTIR is one scan.
2.2.4. Dropping Point Test
It’s used for determining the dropping point of lubricating grease. This point is the temperature at which the first drop of material falls from the cup. In general, the dropping point is the temperature at which the grease passes from a semisolid to a liquid state under conditions of test. This change in state is typical for greases containing a thickener soap of conventional types. Greases containing a different thickener material may be without change in state and separate oil. The method is usually used identifying the grease as to type and/or establishing and maintaining bench marks for quality control ASTM D-56.
2.2.5. Penetration
Penetration was determined by using ASTM D-217.
2.2.6. Flash Point
Flash point was determined by using open system of ASTM D-92.
2.3. Preparation
2.3.1. Preparation of Wax Gel (Wax-Oil Mixture)
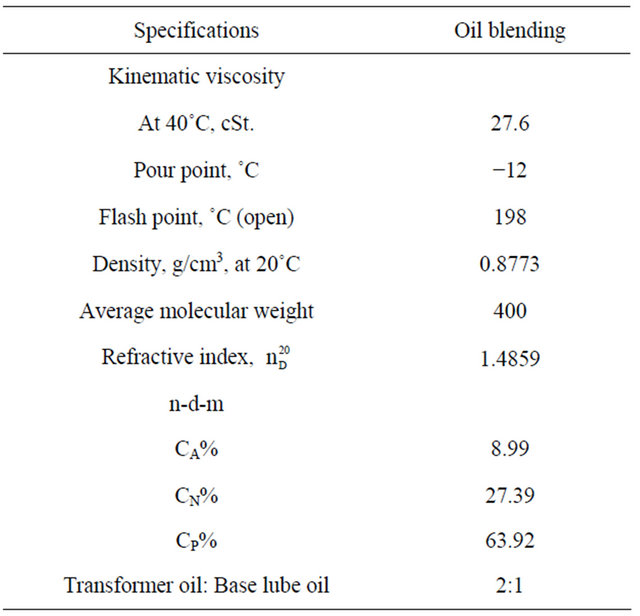
In this experiment two types of oils had been used, the first was a base lube oil grade (260/290) and the second was transformer oil.
Lube base oil and transformer oil in the ratio 2:1 by weight were mixed under stirring at 110˚C for 30 minutes to produce the lube oil blend. The physico-chemical characteristics of these oils were carried out using ASTM standard methods.
The lube oil blend was mixed with microcrystalline wax (Alexandria for petroleum company), under stirring at 100˚C - 120˚C for 30 minutes with different ratios 1:1, 1.85:1, 2.3:1 and 4:1 respectively [13]. After cooling, the mixture turn to thicker gel. The obtained samples are denoted by WG1, WG2, WG3 and WG4 respectively. The formulation and specification were given in Tables 1 and 2.
2.3.2. Preparation of Greases Containing Wax Gel (S0)
Oil blend 250.9 g (base lube oil 167.27 g and transformer oil 83.63 g) was heated to 110˚C - 120˚C and the microcrystalline wax 109 g was added portionwise under stirring for 30 minutes, followed by adding of 2,2'-methylene-bis-(4-methyle-6-tertiary butyl phenol) 1.4 g as anti-
Table 1. Formulation of the prepared samples WG1, WG2, WG3 and WG4 wax gel.
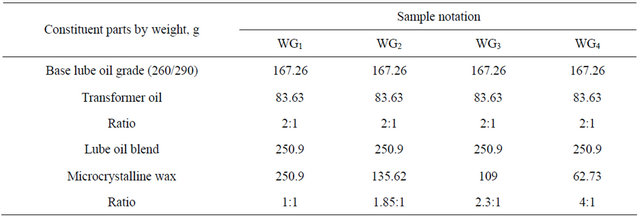
Table 2. Specification of the prepared wax gels WG1, WG2, WG3 and WG4.
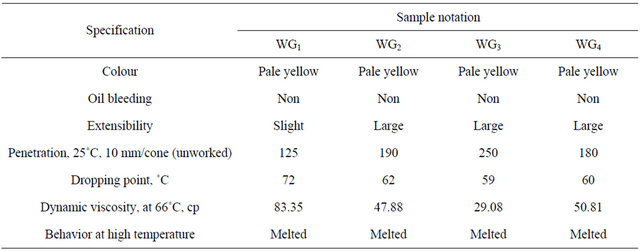
oxidant and Polyoxyethylene sorbiton-nano-palmitate 1.4 g as anticorrosion additives and stirring was continued to disperse the additives. After cooling the mixture was thickened to grease S0 [13]. The formulation of sample S0 grease was shown in Table 3. The specification of the resulted S0 grease is given in Table 4.
2.3.3. Preparation of Grease S1
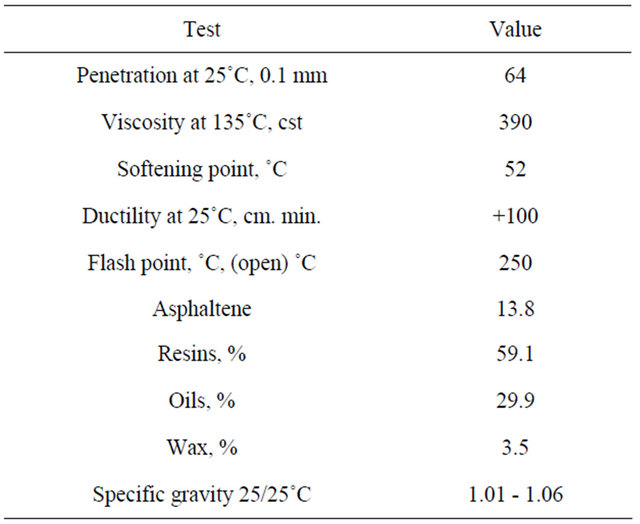
A glass container was charged with 250.9 g of lube oil blend and 1.4 g of both antioxidation and anticorrosion were added then the content, was heated to the temperature of 50˚C with stirring for 15 minutes. Heating was raised to 70˚C followed by adding of 16.4 g bitumen under stirring, when the content was melted and become homogenous; the temperature was raised to 100˚C - 120˚C for 30 minutes. 92.6 g of microcrystalline wax were added and stirring was continued to obtain a viscous smooth rubbery grease, followed by cooling to the normal temperature [13], its formulation and specification are shown in Tables 3 and 4. The phsico-chemical properties of bitumen included in S1 grease was observed in Table 5.
2.3.4. Preparation of Grease S2
A lubricating grease S2 consisting essentially of about 99 g microcrystalline wax, 250.9 lube oil blend, 10 g butyl rubber (isobutylene-isoprene copolymer) together with 1.4 g of both antioxidant and anticorrosion were mixed under stirring for 1/2 hour at 85˚C - 90˚C until it became homogenous. On cooling coherent and homogenous grease S2 was obtained [13], its formulation and specification are shown in Tables 3 and 4.
2.3.5. Preparation of Greases S3
10 g of polyisoprene rubber, 250.9 g lube oil blend, were mixed with 99 g of microcrystalline wax, 1.4 g of antioxidant and 1.4 g of anticorrosion, the mixture being kept at 100˚C - 120˚C for 1/2 hour during stirring, and then allowed to cool to ambient temperature to produce coherent and homogenous rubbery greases S3 [13]. Formulation and specification of the finished grease were shown in Tables 3 and 4.
3. Results and Discussion
Organic hydrocarbon greases including rubber, resin or waxes are very widely distributed at industrial branches. They are used in electrical systems and electronic equipments to seal connectors, plug and sockets, to pot cob transformers and capacitors, cables and to prevent electrical leakage [14-17]. The known greases used in
Table 3. Formulation of the prepared greases S0, S1, S2 and S3.
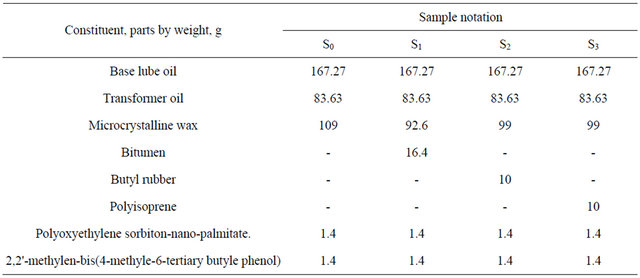
Table 4. Specification of the prepared greases S0, S1, S2 and S3.

Table 5. Physico-chemical properties of bitumen.
insulators [13] service are classified on the basis of the formulations into hydrocarbon type (thickened by paraffin, petrolatum, and ceresin), polymer type (thickened by high molecular weight compounds as polymers, rubbers, resins and copolymers), and inorganic type.
Such types of greases should have particular characteristics such as: high flash point, high dielectric properties, high resistance to water, and high dropping point to overcome the problem of grease sliding in insulation in hot weather. They should adhere to insulation and in the same time have mobility at either ambient or discharge temperature, proper formulation which provides resistance to oxidation and corrosion and which also allows stability on storage.
The grease produced, is properly thickened in order to remain in contact with the surface and does not leak out, or be squeezed out. A grease is a two-phase dispersed system of oil gelled with wax.
3.1. Characterization of Oils (Dispersion Medium)
3.1.1. Physico-Chemical Characterization
Tables 6-8 show the physico-chemical characteristics of the used oils, it’s clear that the viscosity of base lube oil grade 260/290-transformer oil blend is suitable to be used as fluid part in the preparation of grease 27.6 cSt, where 50 - 55 cSt for base lube oil grade 260/290 at 40˚C which was very large compared with the required value for the insulating oils [transformer oils which are mainly used today ~20 cSt]. The chief point of difference between the types of greases is the viscosity of the oil used as an ingredient of the grease. A base lube oil grade 260/290 and transformer oil, were mixed and used for this purpose.
The suitable flash point of the oils blend is (198˚C) according to ASTM D-93, where the flash point for good insulating oil is not less than 135˚C. The pour point for the base lube oil grade 260/290 (−5˚C) is not suitable according to ASTM D-97 where it is high, but after treating with transformer oil (−20˚C) it became (12˚C). Studying the distribution of %CA, %CP, %CN was
Table 6. Physico-chemical properties of base lube oil grade (260/290).
●Misr petroleum company, Cairo.
Table 7. Specification of transformer oil.
●Misr petroleum company, Cairo.
Table 8. Specification of base lube oil (260/290) and transformer oil after blending
shown. It was deduced that as the %CP is greater than 50%, this means that the base lube oil grade (260/290) and base lube oil-transformer oil blend are considered as paraffinic oils.
As the naphthenic percentage of CN for the oil blend is high, the dielectric properties for this oil are better than the first oil grade (260/290) [18]. This means that the base lube oil grade (260/290) is not suitable as insulating oil, but after blending it with transformer oil (12.5 cst) it improved i.e. became suitable as insulating medium.
From the above discussion, it may be pointed out that the first oil under study is not suitable for using as fluid part as insulating before carrying out blending (with transformer oil) to overcome the high aromatic contents and to decrease the pour point.
3.1.2. FTIR Characterization
Infrared absorption spectrometry has been applied to determine the functional groups of base lube oil (first oil), transformer oil (second oil) and base lube oil-transformer oil blend. The measurement of IR spectra in the range from (4000 - 500 cm−1). Figures 1-3 show that the above oils have low intensity bands in the region (3431 - 3436 cm−1), indicating low concentrations of –OH and –NH groups which have important role in the polarity of oils. The spectra also show strong two bands at (2850 - 3000 cm−1) resulting from C-H bending vibrations asymmetric of methyl and methylene groups, the strong band at (1459 cm−1), which due to CH2 asymmetric bending vibration. In addition, the weak band obtained at (1374

Figure 1. IR spectrum for base lube oil grade (260/290).
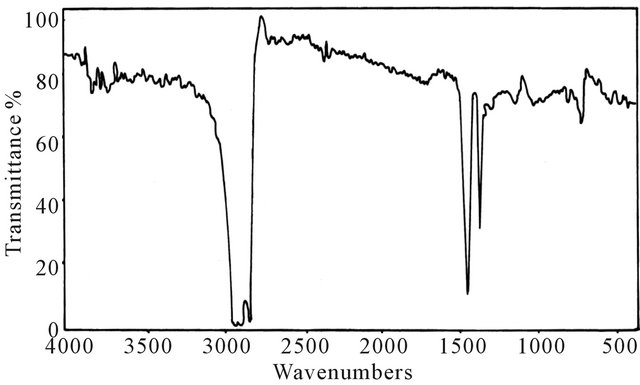
Figure 2. IR spectrum for transformer oil.
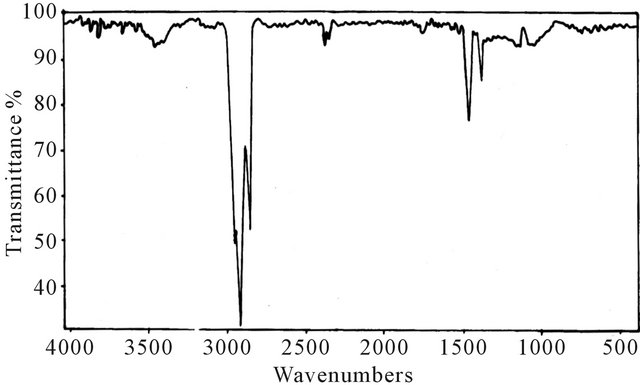
Figure 3. IR spectrum for base lube oil grade (260/290) and transformer oil after blending.
cm−1) may be attributed to C-N stretching vibration for aromatic amines or CH3 bending vibration symmetric for methyl groups and a weak band at (1605 cm−1) is due to the stretching vibration of C=C aromatic rings.
3.2. Characteristics of Microcrystalline Wax
3.2.1. Physico-Chemical Characterization
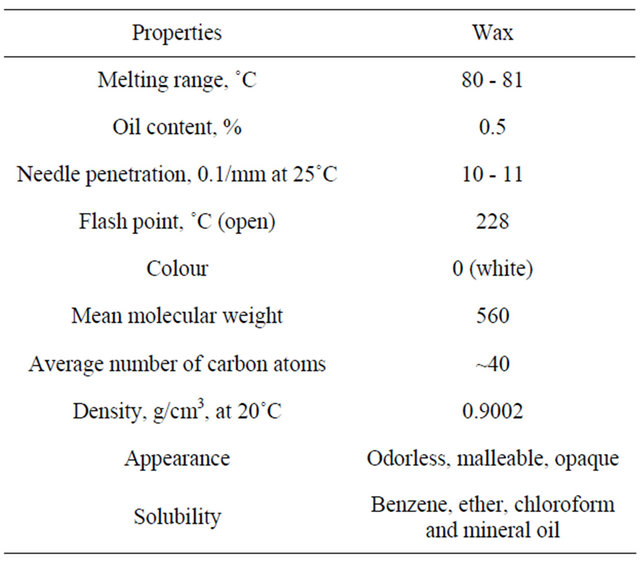
The data in Table 9 are presenting the physico-chemical properties of microcrystalline wax shows that having high molecular weight (large hydrocarbon chain ~40) 560 and have melting point of range of 80 - 81 which give rise for it use in coating applications, also the fine crystal structure enables microcrystalline wax to bind solvent or oil and prevent the sweating-out of compositions.
3.2.2. FTIR Charactrization
The FTIR spectra of the microcrystalline wax Figure 4 indicates sharp band in the region of (3000 - 2850 cm−1) for stretching vibration of C-H of aliphatic and band in the region (1450 - 1375 cm−1) for stretching vibration of methyl and methylene groups.
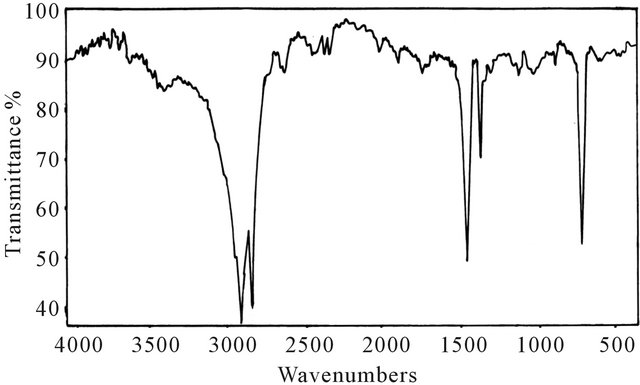
Figure 4. IR spectrum for microcrystalline wax.
Table 9. Physical properties of microcrystalline wax.
3.3. Comparison of Wax-Oil Mixture (Wax Gel)
The chief points of difference between the types of waxoil mixtures Tables 1 and 2 WG1, WG2, WG3 and WG4 are the viscosity, penetration and dropping point.
The data in the Table 2 reveal that the sample WG3 (comprising about 250.9 g of lube oil blend and 109 g of microcrystalline wax i.e. 2.3:1) proved to be advantageous over the other samples WG1, WG2 and WG4 because of that the It has apparent viscosity equals to 47.88 cP at 66˚C, penetration 190 and dropping point was 50˚C.
This means that the ratios of the lube oil blend and wax used in the formulations of the prepared wax gel proved that it is the best, since other attempt to mix these ingredient in other ratios failed to produce good quality wax gel (wax-oil blend), where higher amount of oil defected them (caused oil bleeding, lowering dropping point and viscosity), i.e. excessive oil separation.
Moreover, higher ratios of wax lead to a decrease in the cohesive force of the components (resistance to motion, more difficult to handle). For this, wax-oil mixture WG3 is suitable for formulation greases S0 (wax gel).
In this investigation, a base lube oil blend (base lube oil 260/290 and transformer oil 2:1), microcrystalline wax (2.3:1) and 2,2'-methylen-bis(4-methyle-6-tertiary butyl phenol) as antioxidant, and Polyoxyethelene sorbiton-nano-palmitate as anticorrosion additives were formulated together to form wax gel (S0 greases) that is found to have inconvenient.
In an attempt to improve these properties of sample S0 and directing it to have a good dropping point, viscosity, penetration, water resistance, flash point and electrical insulation properties, any of the following: butyl rubber, polyisoprene rubber, bitumen ultramarine was added.
In general, each of these greases was made from the prepared hydrocarbon wax gel with one of the above mentioned polymers or rubbers as well as bitumen, inorganic compounds and antioxidant as well as anticorrosion additives.
The Physico-Chemical Characterization of the Prepared Grease
It is clear from Table 10 that all samples proved to be enhanced or improved physico chemical character of the prepared wax gel (S0).
It has been established that the addition of butyl rubber to grease S0 improved the penetration of S2 (including butyl rubber) because butyl rubber forms viscous liquids [19]. The penetration test for S1 - S3 greases shows the difference of penetration values between these greases in the order S2 > S1 > S3 > S0 respectively. The increase of penetration of S1 (including bitumen) reveals that the compatibility of S0 greases (including wax gel) with the thickening agent bitumen.
Table 10 indicated that samples can be arranged according to the best flash point (highest flash point as follows: S1 > S2 > S3 > S0. It is clear that (S1 - S3) proved to exhibit flash point than the prepared sample S0 which containing wax gel only. These greases can stand high flash points as much as possible about (184˚C - 250˚C) without ignition but carbonization may be allowed.
Data in Table 10 showed that greases (S1 - S3) have the highest value of water repel test i.e. it should not be washed off by heavy rain and wind, Table 10 showed that the formulated greases (S1 - S3) exhibited a marked improvement in appearance viscosity when compared
Table 10. Specification of the prepared greases S0, S8, S9, and S10.
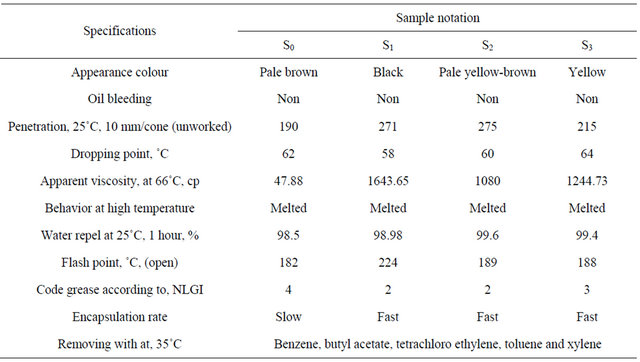
with S0 only and the difference of viscosity was in the order S1 > S2 > S3 > S0. Rubber component is added to grease for use as a cable filling materials as well as to improve bleed resistance.
Adding dielectric grease to switch contacts and potentiometer tracks improves performance and operating life [8-10]. Bitumen has been widely used in the electrical industry because of its excellent electrical properties, resistance to water and chemicals besides its thermoplastic properties which make it easily workable for manufacture. Its application include filling and sealing compounds for dry batteries, capacitors, cable junctions, terminal boxes, insulation tape and the insulation of cables, motors, transformers, etc. Bitumen has a high resistance (low conductivity) and is therefore used as an excellent insulating material. Harder grades have slightly higher resistance than soft grades although the difference is not important in practice. The resistance decrease with increasing temperature, the influence of fillers on resistance is negligible; provided that the filler is not conductive [11]. All these results go according to reference [12,13].
3.4. The Dielectrical Properties of the Prepared Greases
The dielectric constant ε', dielectric loss ε'' and volume resistivity for the prepared samples over the frequency range from 1 KHz to 1000 KHz at 35˚C were studied.
Dielectric loss depends on the chemical constitution of the repeating unit in the chain, the nature and number of polar groups, substitute size, size radical isomerism and steric hindrance. Good insulating greases have low dielectric constant, low dielectric loss and high volume resistivity [20].
The presenting data given in Table 11 and Figures 5 and 6 show that the ε', and ε" of the samples S0, S1, S2
and S3 decrease with increasing frequency from 1 KHz to 1000 KHz at 35˚C.
It is shown that the ε' and ε" of the sample as following: S2 has more dielectric properties than S1, S2 and S3. Since the sample S2 (containing isobutylene-isoprene copolymer) butyl rubber has good electrical insulation i.e. least value of ε' and ε".
Also, the volume resistivity of these samples which are represented in Table 12 and Figure 7 showed that the values of volume resistivity decrease with increasing frequency (1 KHz to 1000 KHz). It is in the order S2 > S1 > S3 > S0. Since S2 has the highest value of volume resistivity.
This means that the dielectric properties (electrical insulation) of these samples are in the order S2 > S1 > S3 > S0. This indicates that the presence of methyl groups as branched groups and presence of saturated bonds (σ bond) in sample S2, decreases the moving of units of butyl rubber and leading to the decreasing of ε' and ε" and increasing volume resistivity (despite of the presence of isoprene with small proportions) [21], i.e. improving dielectric properties of sample S2.
It is also clear that from Tables 11 and 12 and Figures 5-7 that sample S1 (containing bitumen) has the highest dielectric properties than sample S3 (containing polyiso-
Table 11. Dielectric measurement of the prepared greases S0, S1, S2 and S3.
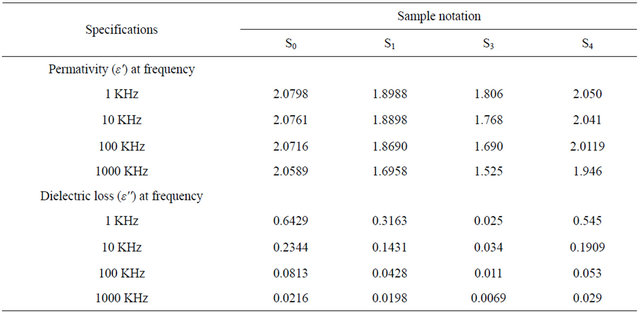
Table 12. Volume resistivity measurements of the prepared greases S0, S1, S2, and S3.
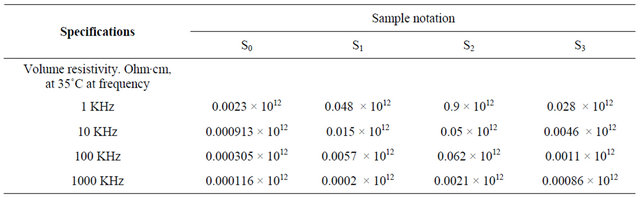

Figure 5. The dielectric constant (ε') vs. frequency at temperature 35˚C for S0, S1, S2 and S3 greases.
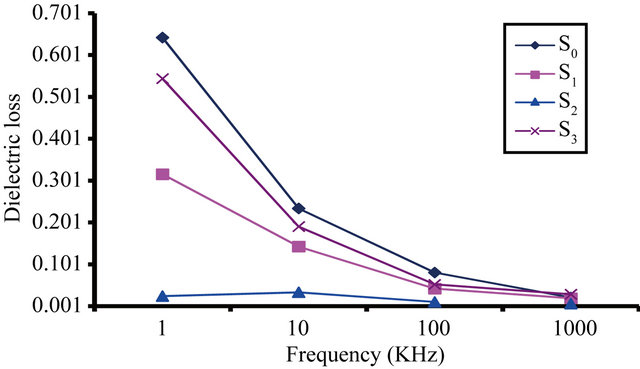
Figure 6. The dielectric loss (ε") vs. frequency at temperature 35˚C for S0, S1, S2 and S3 greases.

Figure 7. The relation between volume resistivity and frequency at temperature 35˚C for S0, S1, S2 and S3 greases.
prene) this may be due to that sample S1 has (83% carbon, 10% hydrogen, 7% oxygen, nitrogen, sulfure) i.e. bitumen contains a much higher proportions of relatively high molecular weight paraffin, naphthenic hydrocarbon and their derivatives (having carbon number greater than C25 with a high carbon to hydrogen ratio) [19].
On the other hand sample S3 (containing polyisoprene), has unsaturated bond in its structure (π bond). Due to π bonds are usually weaker than sigma bonds because of their (negatively charged) electron density farther far from the positive charge of the atomic nucleus and thus it requires less energy to be free and cause rupture of the double bond.

• 4. Conclusions
• Greases that have high insulation properties was prepared and modified by adding different quantities of thickener that can be used as a good insulating lubricant to many of equipment and telephone cables.
• The prepared wax gel( base oil blend; and microcrystalline wax) has inconvenient physicochemical and dielectric properties
• Butyl rubber, isoprene rubber and bitumen are added to the prepared wax gel separately as thickening agent to the prepared wax gel in certain proportion to improve its physicochemical, and its dielectric properties.
• Each of formulated greases has better physicochemical properties (dropping point) flash point, viscosity, penetration, dropping point, and water resistance) and dielectric properties (dielectric constant, dielectric loss, and volume resistivity) than the prepared wax gel alone.
• Butyl rubber and isoprene rubber have a good abilities to improve these properties by decreasing the degree of electrical conductivity, Bitumen also improve the dielectric properties of wax gel.
• The dielectric properties of these sample are in the order butyl rubber > bitumen > isoprene rubber > wax gel alone. These mean that the best dielectric properties were achieved by adding butyl rubber to the prepared wax gel.
REFERENCES
- Malakule, “Grease Technology, Part I,” Tsikot, Philippines, 2003.
- V. P. Sukhanov, “Petroleum Processing,” Mir Publishers, Moscow, 1982, p. 378.
- W. Artemas, “Domain 1911 Edition of the Grocers,” Encyclopedia, New York, 2008.
- C. J. Donahue, “Lubricating Grease: A Chemical Primer” Journal of Chemical Education, Vol. 83, No. 6, 2006, p. 862.
- Y. Toomas, “Oil or Greases Lubrication,” 2001.
- V. V. Sinitsyn, N. N. Grishin, G. F. Ogorodnikova and S. M. Utekhina, “Structural Compatibility of Rubbers and Lubricating Greases,” Chemistry and Technology of Fuels and Oils, Vol. 21, No. 4, 1985, pp. 26-27. doi:10.1007/BF00723298
- K. Tamakas and Y. Mansumori, “Yoshibisa,” Canadian Patent No. 5110489, 2000.
- M. Brauer and Y. C. Chu, “Cable Grease Composition and Articles Incorporating Same,” US Patent No. 5348669, 1994.
- M. Braver, “Polypropylene Compatible Grease Compositions for Optical Fiber Cable,” US Patent No. 5672640, 1997.
- B. De Vivo, L. Egiziano, P. Lamberti, V. Tucci and CEIDP “Electrical Insulation and Dielectric Phenomena,” Annual Report, 14-17 October 2007, pp. 45-48.
- R. John and W. David, “Shell Bitumen, the Shell Bitumen Industrial Hand Book,” Thomas Telford, London, 1995, p. 339.
- M. A. E. Youssif, “Evaluation of Some Prepared Greases Based on Petroleum Wax Gel,” M.Sc. Thesis, Al-Azhar University, Cairo, 2009.
- A. M. Hassan, “Greases from Some Petroleum and Coal Tar Products,” M.Sc. Thesis, Faculty of Science, Al-Azhar University, Cairo, 1978.
- L. Meals, “Reinhold Plastic Applications Series, Silicon,” Reinhold Pub. Corp., New York, 1961, pp. 84, 204-205.
- Gunderson and Hart, “Gunderson and Hart, Synthetic Lubricants,” Reinhold Puble. Corp., New York, 1962, pp. 259-263, 317-319, 355-356, 470-473.
- L. Jan and B. Seven, “Hand Book of Grease Applications,” Industrial Lubrication and Tribology, Vol. 52, No. 5, 2000, p. 221.
- V. P. Sukhanov, “Petroleum Processing,” Mir Publishers, Moscow, 1982, p. 17.
- A. R. El-Adly and A. S. Ward, “Evaluation of Different Grades of Lube Base Oils as Insulating Oil,” Egyptian Journal of Petroleum, Vol. 13, No. 1, 2004, p. 69.
- I. G. Fuks, M. B. Bakaleinikov and V. V. Samgina, “Structurizing Effect of Polyethylene and Polyisobutylenes in Oils and Greases,” Chemistry and Technology of Fuels and Oils Article, Vol. 10, No. 7, 1974, p. 559.
- M. A. Youssif, M. Y. Abed and I. M. EL-Anwar, “Preparation and Dielectric Properties of MethylemethacryLate-Parabanic Acid Polymer Using Some Additives,” Egyptian Journal of Petroleum, Vol. 2, 1993, p. 1-8.
- D. Feldman and A. Barbalate, “Synthetic Polymers, Tech-nology, Properties, Applications,” Chapman & Hall, Lon-don, 1995, p. 46.
NOTES
*Corresponding author.

