 Vol.1, No.2, 15-26 (2011) doi:10.4236/oji.2011.12003 C opyright © 2011 SciRes. Openly accessible at http://www.scirp.org/journal/OJI/ Open Journal of Immunology Molecular and cellular pathways involved in the therapeutic functions of MHC molecules; a novel approach for mitigation of chronic rejection Thomas S. Skelton1, Malgorzata Kloc*, Rafik M. Ghobrial* 1Department of Surgery, Methodist Hospital and Methodist Hospital Research Institute, Houston, USA. *Department of Surgery, Methodist Hospital, Fannin St., Houston, USA; RMGhobrial@tmhs.org; mkloc@tmhs.org Received 24 June 2011; revised 20 July 2011; accepted 28 July 2011. ABSTRACT The mutated major histocompatibility complex (MHC) class I that contains donor-type epitopes displayed on recipient-type molecule was show- n to inhibit acute and chronic rejection and in- duce indefinite survival of heterotopic cardiac allografts when administered in combination with a sub-therapeutic dose of cyclosporine (CsA) in a rat transplantation model. To eluci- date the molecular pathways involved in the immunosuppressive effects of the mut a ted MHC molecule, we analyzed gene and protein ex- pression profile during early and late phase following post-transplantation. Cytoskeletal str- ucture analysis and expression status of Rho GTPase proteins, vacuolar transport and cy- toskeleton regulatory pathways involved in im- mune response in T and dendritic cells demon- strated the nov el mechanism fo r the ab rogati on of chronic rejection. Our studies confirm a new role of Rho GTPase pathway in the modification of T cell motility and infiltration of the graft. We dis- cuss these results within the framework of the most recent literature on MHC and molecular machinery controlling T cell functions and den- dritic cell an tigen p resentation. Keywords: MHC; Transplantation; Allograft; Chronic Reje cti on; Rat 1. INTRODUCTION The immune system plays a critical role in maintaining an individual’s autonomy, and essential to its function is the ability to differentiate self from potentially harmful agents, such as microbial or viral proteins, foreign lipids and polysaccharides, which are viewed as non-self and are capable of eliciting an immune response. The trans- plantation of genetically incongruous organs produces an immunological response through allorecognition of non- self histocompatibility antigens. These antigens are lo- cated on cell surfaces and are capable of inducing an immune response in genetically dissimilar (allogeneic) recipients, resulting in graft injury and acute rejection of tissue or cells bearing non-compatible antigens. Solid organ transplantation had been unsuccessful for many decades until the discovery in 1967 of the human major histocompatibility complex (MHC), which led to the introduction of human leukocyte antigens (HLA)- matching method [1,2]. However, in spite of that dis- covery, patient and graft survival remained poor. Not until the 1980s, when the immunosuppressant cyc- losporine became available for clinical use, did a sig- nificant improvement in transplant success rates appear [3,4]. Despite this discovery and the development of other novel immunosuppressants, better organ preserva- tion, refined surgical techniques, and post-operative care, all of which help to eliminate acute rejection, chronic rejection continues to plague the majority of allografts and is a major obstacle for the long-term success of transplants. For instance, the one-year kidney allograft survival from cadaveric and living related donors has increased from 50% to roughly 90% and 95%, respect- tively, when compared with the allograft survival out- comes of 20 years ago, but the 10-year graft survival rates have fallen below 60%, due to chronic allograft dysfunction [5,6]. 2. MHC AND THE IMMUNE RESPONSE The rejection response elicited by transplantation be- tween members of the same species is regulated through T and B cell recognition of tissues expressing genetically encoded polymorphisms within the MHC and the pep- tide fragments that they carry [6]. In humans, the MHC region resides on the short arm of chromosome 6 and contains more than 200 genes of which more than 40 known as the HLA genes, encode codominantly expressed  T. S. Skelton et al. / Open Journal of Immunology 1 (2011) 15-26 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/OJI/ 16 cell surface proteins or components of the complement system. These genes and their translational products have been further grouped into three MHC classes, which are termed I, II, and III, based on their tissue dis- tribution, structure, and function [7-9]. An important discovery in 1974 revealed that MHC molecules are functionally responsible for presenting peptide antigens to immune cells, thereby allowing the T cell receptor (TCR) to interact specifically with peptide fragments of a foreign protein bound within the peptide-binding groove of a MHC molecule [10]. Each MHC molecule consists of an extracellular peptide-binding cleft an- chored to the cell by transmembrane and cytoplasmic domains. There is a high degree of allelic variation asso- ciated with the HLA system. For instance, there are 200 known variants of HLA-B, 500 variants of HLA-A, and over 1000 variants of HLA-DR [11,12]. Polymorphisms in the amino-acid sequence encoded by these alleles typically reside within or adjacent to the peptide cleft and facilitate antigen binding and presentation to T cells. Because of the amino acid variability in this region, each MHC binds an individual repertoire of protein antigens that are recognized by distinctly different T cells. The ability to potentially present a wide range of peptides on MHC molecules is the primary cause of HLA polymor- phism and may provide a balance between maximizing the immunological response against invading antigens while preventing self-recognition and autoimmunity [13]. MHC class I and class II molecules both use very similar peptide binding domains, and peptide binding plays an integral role in the proper folding of the final pep- tide-MHC complex [14]. Peptide antigens are held in place by non-covalent interactions between the peptide and binding cleft, which determines peptide specificity, and a network of hydrogen bonds that determine the length of bound peptide [15]. Given the role of MHC molecules in antigen presentation, in the case of trans- plantation, allogeneic MHC molecules can act as both antigen-presenting molecules and as foreign antigens capable of eliciting an immune response [13]. Class I MHC is present on all nucleated cells and is composed of a 45-kd α heavy chain encoded by genes of the HLA-A, HLA-B, and HLA-C loci on chromosome 6 in association with a 12-kd protein, β2-microglobulin, which is encoded by a gene on chromosome 15 [2,9]. Class II MHC is expressed by professional antigen pre- senting cells (APC), namely, dendritic cells (DCs) and by B lymphocytes, macrophages, endothelial cells, and thymic epithelial cells [15]. Class II MHC is a het- erodimer composed of non-covalently associated α and β chains of approximately 230 amino acids, each encoded by the HLA-DR, HLA-DP, and HLA-DQ genes’ loci [2]. Class III MHC genes encode components of the com- plement system and will not be discussed in this review. CD8+ cytotoxic T cells bind preferentially to class I MHC molecules and CD4+ helper T cells bind to class II MHC molecules. 3. MHC AND ANTIGEN PRESENTATION PATHWAYS Three unique, but not mutually exclusive, pathways of antigen presentation, including the direct, indirect, and semi-direct pathways, are used by MHC class I and class II molecules for the presentation of peptide antigens to CD8+ and CD4+ T cells, respectively, in the presence of transplanted tissue (Figures 1-3). Transplantation of a solid organ from a genetically identical (syngeneic) individual does not lead to an im- munological response against the graft. Disparities be- tween self and non-self major histocompatibility com- plex (MHC) molecules, as in the context of genetically disparate (allogeneic) individuals, result in the activation of the innate immune response, as well as the initiation of an adaptive immune response, which subsequently leads to graft rejection and possible patient death [6,15]. Adaptive immunity involves allorecognition of alloge- neic or foreign MHC antigens. An alloresponse to the graft occurs through the presentation of donor-specific antigens to recipient T cells by engagement of a recep- tor-ligand system between the T cell receptor (TCR) and a foreign peptide-MHC complex. Following TCR liga- tion, additional co-stimulatory signals lead to T cell ac- tivation, proliferation of allospecific T cells, and re- cruitment of effector leukocytes to the graft itself [13,16]. This specificity depends on the recognition of short pep- tide antigens bound to polymorphic MHC-encoded gly- coproteins on the cell surface of APCs and the antigenic nature of the MHC molecules themselves [6,17]. Addi- tionally, B lymphocytes participate in the humoral re- sponse by presenting antigen to CD4+ T cells, which subsequently help B cells differentiate into all- anti- body-producing plasma cells. 4. DIRECT PATHWAY The direct antigen-presentation pathway, or the en- dogenous class I pathway (Figure 1(a)), is active in nearly all cells and consists of mechanisms for display- ing peptides produced within cells in the context of class I MHC. This method of antigen presentation allows for the internal proteome to be sampled and immunologi- cally surveyed by cytolytic CD8+ T cells, which have the ability to kill cells expressing viral proteins or tumor antigens [15]. During class I presentation, the MHC class I heavy chains assemble with the β2-microglobulin fol- 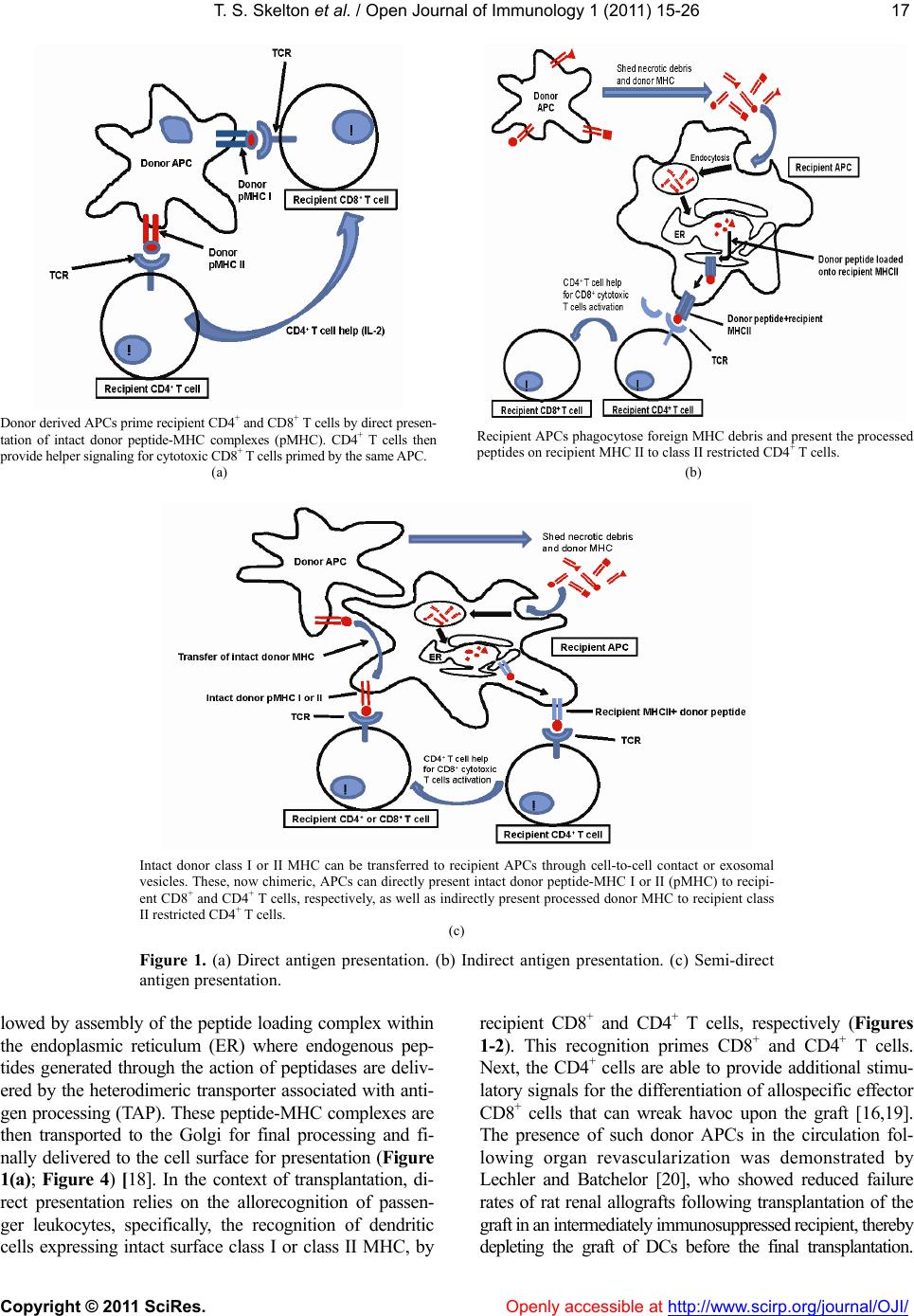 T. S. Skelton et al. / Open Journal of Immunology 1 (2011) 15-26 Copyright © 2011 SciRes. http://www.scirp. org/journal/OJI/ 1717 Donor derived APCs prime recipient CD4+ and CD8+ T cells by direct presen- tation of intact donor peptide-MHC complexes (pMHC). CD4+ T cells then provide helper signaling for cytotoxic CD8+ T cells primed by the same APC. Recipient APCs phagocytose foreign MHC debris and present the processed peptides on recipient MHC II to class II restricted CD4+ T cells. (a) (b) Intact donor class I or II MHC can be transferred to recipient APCs through cell-to-cell contact or exosomal vesicles. These, now chimeric, APCs can directly present intact donor peptide-MHC I or II (pMHC) to recipi- ent CD8+ and CD4+ T cells, respectively, as well as indirectly present processed donor MHC to recipient class II restricted CD4+ T cells. (c) Figure 1. (a) Direct antigen presentation. (b) Indirect antigen presentation. (c) Semi-direct antigen presentation. lowed by assembly of the peptide loading complex within the endoplasmic reticulum (ER) where endogenous pep- tides generated through the action of peptidases are deliv- ered by the heterodimeric transporter associated with anti- gen processing (TAP). These peptide-MHC complexes are then transported to the Golgi for final processing and fi- nally delivered to the cell surface for presentation (Figur e 1(a); Figure 4) [18]. In the context of transplantation, di- rect presentation relies on the allorecognition of passen- ger leukocytes, specifically, the recognition of dendritic cells expressing intact surface class I or class II MHC, by recipient CD8+ and CD4+ T cells, respectively (Fi gures 1-2). This recognition primes CD8+ and CD4+ T cells. Next, the CD4+ cells are able to provide additional stimu- latory signals for the differentiation of allospecific effector CD8+ cells that can wreak havoc upon the graft [16,19]. The presence of such donor APCs in the circulation fol- lowing organ revascularization was demonstrated by Lechler and Batchelor [20], who showed reduced failure rates of rat renal allografts following transplantation of the graft in an intermediately immunosuppressed recipient, thereby depleting the graft of DCs before the final transplantation. Openly accessible at 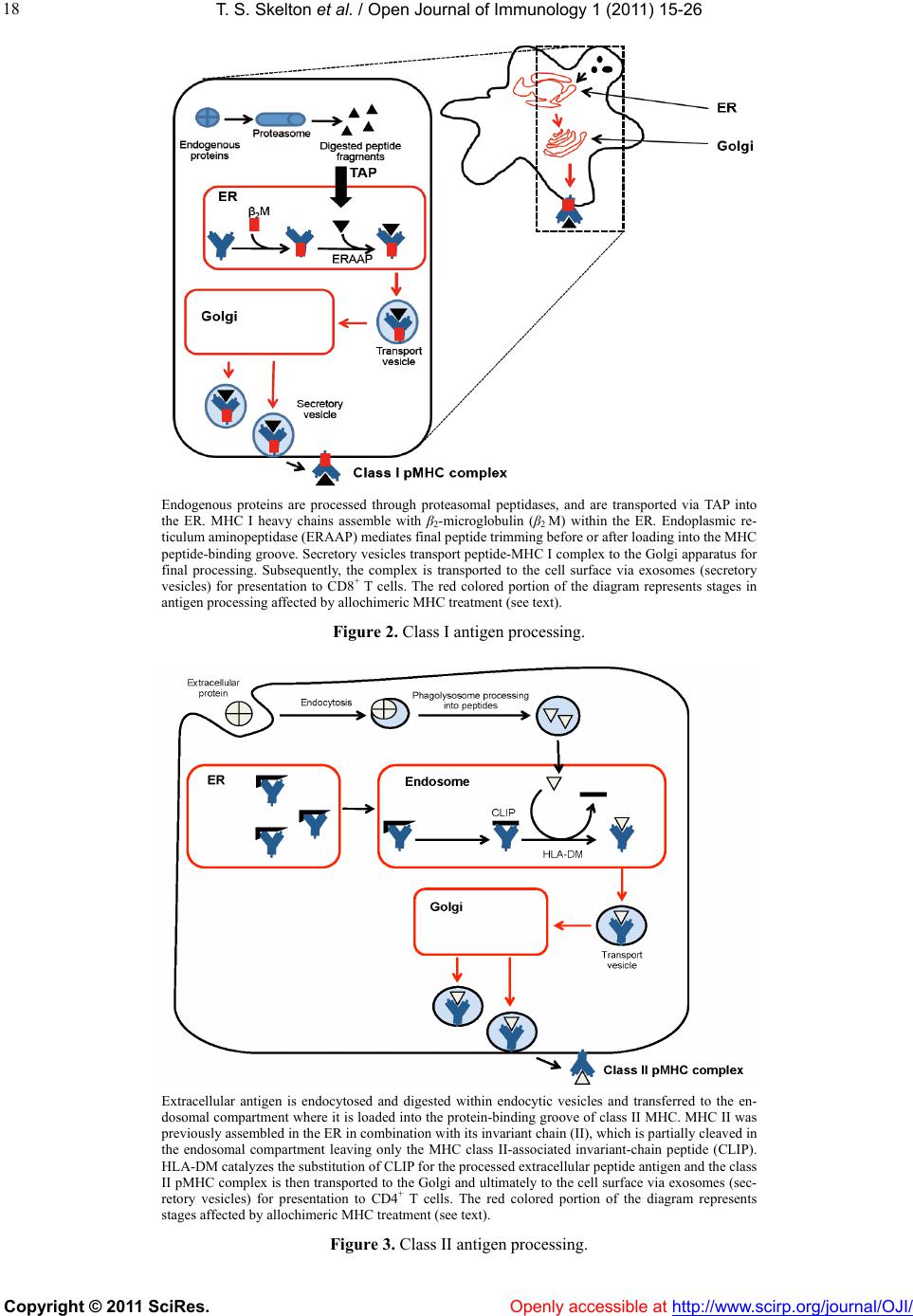 T. S. Skelton et al. / Open Journal of Immunology 1 (2011) 15-26 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/OJI/ 18 Endogenous proteins are processed through proteasomal peptidases, and are transported via TAP into the ER. MHC I heavy chains assemble with β2-microglobulin (β2 M) within the ER. Endoplasmic re- ticulum aminopeptidase (ERAAP) mediates final peptide trimming before or after loading into the MHC peptide-binding groove. Secretory vesicles transport peptide-MHC I complex to the Golgi apparatus for final processing. Subsequently, the complex is transported to the cell surface via exosomes (secretory vesicles) for presentation to CD8+ T cells. The red colored portion of the diagram represents stages in antigen processing affected by allochimeric MHC treatment (see text). Figure 2. Class I antigen processing. Extracellular antigen is endocytosed and digested within endocytic vesicles and transferred to the en- dosomal compartment where it is loaded into the protein-binding groove of class II MHC. MHC II was previously assembled in the ER in combination with its invariant chain (II), which is partially cleaved in the endosomal compartment leaving only the MHC class II-associated invariant-chain peptide (CLIP). HLA-DM catalyzes the substitution of CLIP for the processed extracellular peptide antigen and the class II pMHC complex is then transported to the Golgi and ultimately to the cell surface via exosomes (sec- retory vesicles) for presentation to CD4+ T cells. The red colored portion of the diagram represents stages affected by allochimeric MHC treatment (see text). Figure 3. Class II antigen processing. 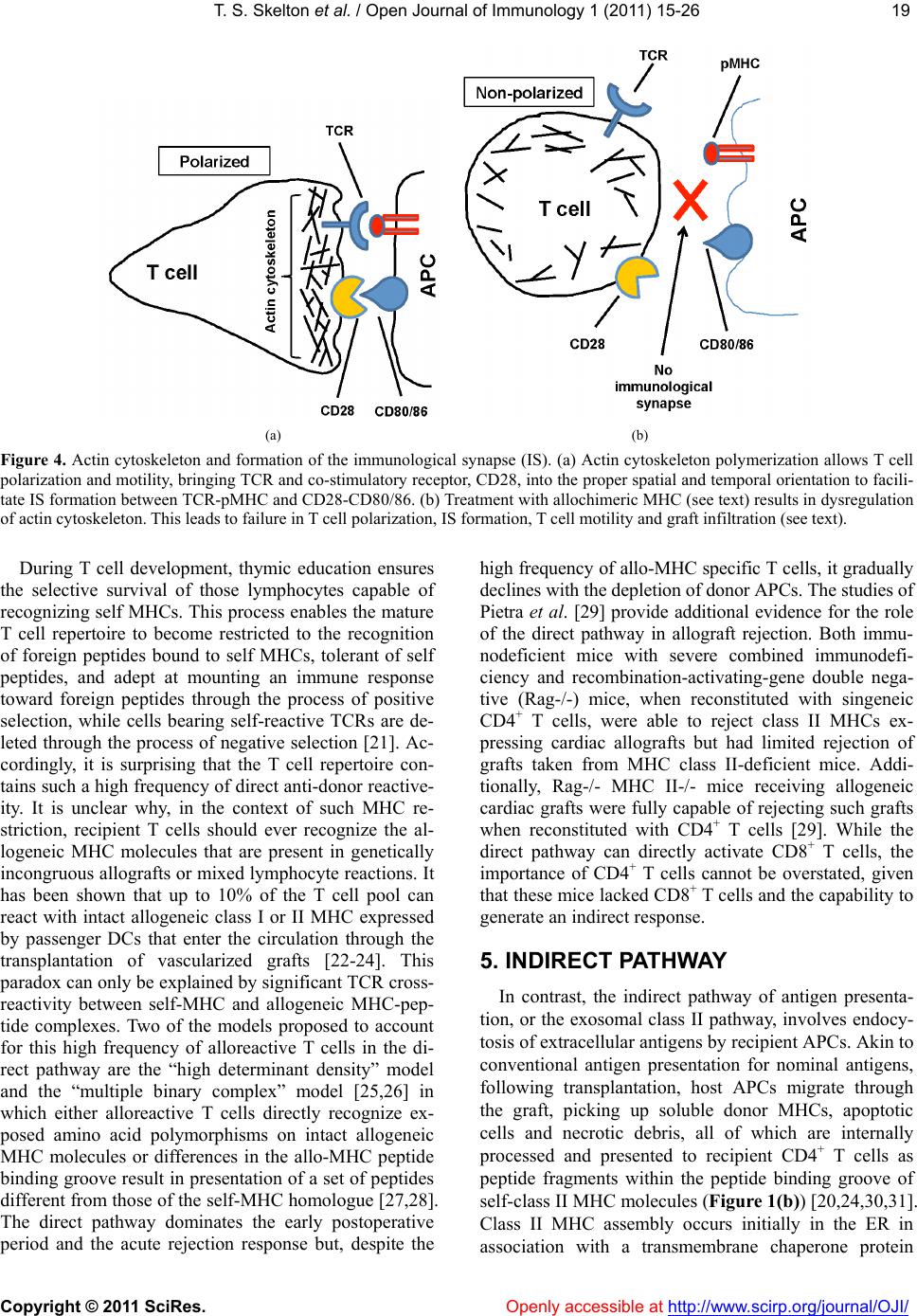 T. S. Skelton et al. / Open Journal of Immunology 1 (2011) 15-26 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/OJI/ 1919 (a) (b) Figure 4. Actin cytoskeleton and formation of the immunological synapse (IS). (a) Actin cytoskeleton polymerization allows T cell polarization and motility, bringing TCR and co-stimulatory receptor, CD28, into the proper spatial and temporal orientation to facili- tate IS formation between TCR-pMHC and CD28-CD80/86. (b) Treatment with allochimeric MHC (see text) results in dysregulation of actin cytoskeleton. This leads to failure in T cell polarization, IS formation, T cell motility and graft infiltration (see text). During T cell development, thymic education ensures the selective survival of those lymphocytes capable of recognizing self MHCs. This process enables the mature T cell repertoire to become restricted to the recognition of foreign peptides bound to self MHCs, tolerant of self peptides, and adept at mounting an immune response toward foreign peptides through the process of positive selection, while cells bearing self-reactive TCRs are de- leted through the process of negative selection [21]. Ac- cordingly, it is surprising that the T cell repertoire con- tains such a high frequency of direct anti-donor reactive- ity. It is unclear why, in the context of such MHC re- striction, recipient T cells should ever recognize the al- logeneic MHC molecules that are present in genetically incongruous allografts or mixed lymphocyte reactions. It has been shown that up to 10% of the T cell pool can react with intact allogeneic class I or II MHC expressed by passenger DCs that enter the circulation through the transplantation of vascularized grafts [22-24]. This paradox can only be explained by significant TCR cross- reactivity between self-MHC and allogeneic MHC-pep- tide complexes. Two of the models proposed to account for this high frequency of alloreactive T cells in the di- rect pathway are the “high determinant density” model and the “multiple binary complex” model [25,26] in which either alloreactive T cells directly recognize ex- posed amino acid polymorphisms on intact allogeneic MHC molecules or differences in the allo-MHC peptide binding groove result in presentation of a set of peptides different from those of the self-MHC homologue [27,28]. The direct pathway dominates the early postoperative period and the acute rejection response but, despite the high frequency of allo-MHC specific T cells, it gradually declines with the depletion of donor APCs. The studies of Pietra et al. [29] provide additional evidence for the role of the direct pathway in allograft rejection. Both immu- nodeficient mice with severe combined immunodefi- ciency and recombination-activating-gene double nega- tive (Rag-/-) mice, when reconstituted with singeneic CD4+ T cells, were able to reject class II MHCs ex- pressing cardiac allografts but had limited rejection of grafts taken from MHC class II-deficient mice. Addi- tionally, Rag-/- MHC II-/- mice receiving allogeneic cardiac grafts were fully capable of rejecting such grafts when reconstituted with CD4+ T cells [29]. While the direct pathway can directly activate CD8+ T cells, the importance of CD4+ T cells cannot be overstated, given that these mice lacked CD8+ T cells and the capability to generate an indirect response. 5. INDIRECT PATHWAY In contrast, the indirect pathway of antigen presenta- tion, or the exosomal class II pathway, involves endocy- tosis of extracellular antigens by recipient APCs. Akin to conventional antigen presentation for nominal antigens, following transplantation, host APCs migrate through the graft, picking up soluble donor MHCs, apoptotic cells and necrotic debris, all of which are internally processed and presented to recipient CD4+ T cells as peptide fragments within the peptide binding groove of self-class II MHC molecules (Figure 1(b)) [20,24,30,31]. Class II MHC assembly occurs initially in the ER in association with a transmembrane chaperone protein  T. S. Skelton et al. / Open Journal of Immunology 1 (2011) 15-26 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/OJI/ 20 invariant chain. Within the endosomal compartment a series of protease cleave the invariant chain, leaving an MHC class II-associated invariant chain peptide (CLIP). Subsequently, the intracellular protein HLA-DM cata- lyzes the removal of CLIP and the loading of endocyto- sed peptides, or the peptide exchange, before their trans- port to the cell surface for immunologic presentation (Figure 1(b); Figure 3) [11,17,32,33]. Studies in mice suggested a role for the indirect antigen presentation pathway in transplant rejection through the presentation of allogeneic MHC by self-class II MHC H. DCs from H-2Ab recipients, which lack the H-2E antigen, stained positive for H-2E antigen after recipient injection with H-2E expressing H-2k cells [34]. Furthermore, CD8+- depleted or MHC class I-deficient mice receiving class II MHC deficient skin grafts rejected their grafts through the presentation of donor class I MHC on re- cipient class II molecules [35]. The pathways for cellular pep- tide antigen processing and their subsequent presen- tation involve an elaborate multistep process consisting of numerous cytosolic vesicles and organelles as well as a number of catalytic and degradation enzymes that function in peptide cleavage and loading into the protein binding groove of the MHC [36]. The gradual decline in direct alloreactivity, as donor APCs are depleted, is evi- dent in grafts both with and without signs of chronic rejection, indicating that direct presentation does not play a major role in the development of chronic rejection. In contrast, indirect antigen presentation, by virtue of requiring the processing and presentation of endocytosed proteins, is a slower process and persists for as long as the graft is present, highly contributing to the develop- ment of chronic rejection [37]. The indirect antigen presentation pathway has been shown to be sufficient for the development of chronic allograft vasculopathy and arteriosclerosis in heart transplant models [38]. The re- quirement of antigen processing and presentation by recipient APCs in the context of self-class II MHC signi- fies that the indirect pathway of antigen presentation is largely dominated by CD4+ T cells [6]. However, some overlap exists, as alloreactive CD4+ T cells can directly respond to intact class II MHC, and alloreactive CD8+ T cells can be indirectly activated through “cross priming” which involves recipient APCs presenting donor anti- gens shed by surrounding cells in the context of self-class I MHC molecules [39]. The significance of the role that these indirectly responding CD8+ T cells play in facilitating chronic rejection remains unclear, but the importance of the indirect pathway as a whole has been well documented. While the majority of studies focus on the interaction between APCs and responding T cells, evidence also exist for the ability of recipient-derived endothelial cells to utilize indirect antigen presentation to promote further the rejection cascade. The endothe- lium of a transplanted graft is gradually replaced with recipient cells capable of presenting peptide in the con- text of class II MHC [34]. Targeting of these replaced cells by allo-reactive T cells provides further insight into the role of the indirect pathway in the development of the characteristic vascular lesions associated with chronic rejection [40]. 6. SEMI-DIRECT PATHWAY Recently, an additional mechanism of antigen presen- tation, known as semi-direct allorecognition has been uncovered (Figure 1(c)) [41]. The traditional model of cross talk between CD4+ and CD8+ T cells involves a linked “three cell” system in which the generation of antigen-specific CD8+ effector T cells by APCs requires additional stimulatory signaling from helper CD4+ T cells activated by the same APC [42]. Transplantation reveals limitations in this “three cell” model and sug- gests the presence of an unlinked “four cell” model in which crosstalk between directly activated CD8+ T cells relies on amplifying signals from indirectly activated CD4+ T cells stimulated by completely different APCs [43,44]. Immunological cells have the capability to ex- change surface molecules through either cell-cell con- tact or through exosomes [45,46]. Semi-direct allorec- ognition therefore resolves the “four cell” conundrum by stipulating that recipient DCs can acquire and present intact donor class I or II MHC directly to CD8+ or CD4+ T cells while maintaining the ability to internalize, proc- ess and present donor MHC as peptides indirectly to CD4+ T cells (Figure 3). In this manner, both direct and indirect antigen presentation occurs in a three cell man- ner involving the DC, CD4+ T cells, and CD8+ T cells [6,41]. The transfer of intact donor MHC could be a means of continual direct antigen presentation in the face of diminishing donor APCs. Although no evidence has been identified for the in vivo occurrence of this mode of antigen presentation regarding allograft rejection, the semi-direct pathway could provide an alternative expla- nation for several scenarios that cannot be explained by only considering direct and indirect pathways. One ex- ample of these scenarios is the acute rejection of injected embryonic thymic epithelium in the absence of indirect presentation. Devoid of DCs, this response presumably occurs through the acquisition of donor thymic antigens presented directly to recipient CD8+ T cells [47]. 7. ALLOGRAFT TOLERANCE Large-scale efforts in clinical research and basic sci- ence have been devoted to unraveling the molecular ba- sis of T-cell allorecognition, allograft rejection, and the  T. S. Skelton et al. / Open Journal of Immunology 1 (2011) 15-26 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/OJI/ 2121 development of allograft tolerance without the need for chronic immune suppression. Allorecognition by any of the previously mentioned pathways can ultimately lead to the activation and recruitment of allospecific T cell clones capable of producing aninflammatory response in the graft. However, the outcome of allorecognition is not as clear-cut as once thought. Allorecognition, in trans- plantation is also capable of inducing a state of graft acceptance, or tolerance. Allograft tolerance has been observed in numerous allogeneic animal transplant mod- els across class I/II MHC barriers as well as in humans, especially following liver transplantation [48] in which the liver has an inherent ability to resist rejection through the expression of class I and II MHCs. This type of protective allorecognition is also evident during pregnancy where semi-allogeneic fetal tissue, consisting of both paternal and maternal antigens, is present yet the fetus is not rejected. These disparities allow a distinction to be made between antigenicity and immunogenicity, or the ability to recognize a foreign substrate and the ability to produce an immune response to eliminate it. The development of immune tolerance involves the deletion of a large proportion of T cells with direct al- lospecificity and the continual suppression of remaining direct and indirect alloreactive cells that continue to be primed for the life of the graft. Many cells with such suppression or regulatory activity have been described in both mice and humans. The most studied of them are the CD4+ CD25+ T regulatory cells (Tregs), which express high levels of the transcription factor Foxp3 [49]. The development of Tregs occurs through the indirect path- way after recipient dendritic cells have migrated to the peripheral lymphoid tissue. The dependence on this pathway has been recently shown in rats in which deple- tion of host APCs caused the abrogation of tolerance in Lewis liver allografts in Brown Norway recipients [50]. The role of indirect antigen presentation in antibody- mediated rejection is also influenced by Tregs. The re- jection of class I disparate cardiac allografts in rats has been shown to be antibody-mediated and driven by indi- rect T cell help, which was abrogated following toleriz- ing protocols that induced specific CD4+ regulatory cells [51]. 8. MHC AS THERAPEUTIC AGENTS Studies looking at the potential for MHC to serve as immunomodulating agents have been underway for years, sparked by the work of Billingham, Brent and Medewar, who showed donor-specific tolerance to skin grafts produced by exposing recipients to donor antigens during fetal life [52]. Since then numerous studies inves- tigating the therapeutic value of MHC molecules have been performed. Immunization with allogeneic peptides, eliciting only an indirect response is sufficient to propagate transplant rejection. However, hyporesponsiveness of indi- rectly allo-specific T cells can be achieved through intra- thymic injection of MHC peptides [35,53]. Synthetic peptides corresponding to specific HLA sequences, spe- cifically those within the α1 helix of HLA-A have been shown to inhibit cytotoxic T cell proliferation, produce immunological tolerance and prolong survival in animal transplant models. This peptide therapy was licensed under the brand name Allotrap and showed inhibition of cell-mediated immune responses to kidney allografts in phase II trials [54,55]. Synthetic peptides based on the α1 helix of HLA class II molecules similarly blocked T cell proliferation [55]. Although all of these studies in- dicate the potential therapeutic value of MHC molecules, the mechanisms underlying their immunomodulatory effects remain largely elusive. 9. MOLECULAR AND CELLULAR PATHWAYS INVOLVED IN INHIBITION OF CHRONIC REJECTION BY MHC We have previously produced donor-specific trans- plantation tolerance in a rat heterotopic cardiac trans- plant model through the pre- and peri-operative admini- stration of an allochimeric class I MHC. Dominant amino-acid epitopes identified on the α1 helix of the do- nor MHC were inserted into the α1 helical region of the recipient class I MHC, creating the allochimeric con- struct [α1 l/u]-RT1.Aa [56]. By delivering this protein, in combination with sub therapeutic doses of cyclosporine (CsA) through the portal vein at the time of transplanta- tion, we achieved donor specific tolerance, the attenua- tion of acute and chronic rejection, and prolongation of graft survival [56]. These observations have led our laboratory to investigate the molecular and cellular mechanism underlying the immunosuppressive effects of soluble allochimeric class I MHC. Heterotopic cardiac transplantation was performed between donor Wistar Furth (WF) rats and ACI recipe- ents. Recipient animals were either untreated, treated for 7 days with therapeutic CsA, or treated with 3 days of sub-therapeutic CsA plus a single intraoperative dose of allochimeric class I MHC molecule delivered through the portal vein. Animals that received no immunosup- pressant or received only sub-th e rap euti c CsA rejected their grafts in 5.4 or 16.2 days, respectively, whereas grafts from animals treated with combined low dose CsA and allochimeric peptide survived indefinitely [56]. Mi- croarray analysis of the gene expression profile of heart allograft tissue and splenic T cells, demonstrated that allochimeric class I MHC therapy caused increase in expression of genes involved in the structural integrity of the heart muscle, Nexilin and Myocardin, at day 1 and 3  T. S. Skelton et al. / Open Journal of Immunology 1 (2011) 15-26 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/OJI/ 22 post-transplantation compared with acutely rejecting controls, as well as downregulation in the pro-inflammatory cytokine IL-1β within the cardiac al- lograft [57]. There was also decreased expression of the partitioning defective 6 homologue beta (PAR6) gene, which functions in cell polarity and motility [57]. In addition, genes that play a role in actin filament polym- erization, including RhoA and Rac1, cell adhesion, in- cluding Vcam, vacuolar transport, including RhoB and the MAPK pathway, including Spred1, were shown to be downregulated in splenic T cells isolated from animals treated with allochimeric MHC compared with rejecting controls [58]. Since all these genes play a role in cell polarity and motility we proposed that the early immu- nosuppressive effects attributed to allochimeric MHC treatment was related to the impairment of T cell motility, immunological synapse formation, and ability of T cells to infiltrate the allograft (Figures 4-5) [57,58]. We further investigated the role of RhoA, a member of GTPase protein family, in splenic T cells and its con- tribution to the attenuation of chronic rejection. Using a combination of RT-PCR and Western blot analysis, we found that T cell expression of RhoA was significantly reduced following allochimeric MHC treatment com- pared with untreated and CsA-treated controls [59]. This finding suggested that the early post-transplantation in- hibition of RhoA played a role in diminishing the early immune response against a foreign graft. In fact, immu- nostaining for RhoA showed aberrant RhoA distribution within splenic T cells in the allochimeric MHC-treated animals isolated at day 1 and 3 post-transplantation compared with CsA-treated controls [59]. Given the role dysregulation.of RhoA in cytoskeleton organization and actin filament polymerization, we further showed that distribution of actin was highly disorganized in T cells from the allochimeric MHC-treated cohort (Figure 6) [59]. In addition, actin binding partner, protein Hip55 was partially dislocated from the actin in allochimeric treated T cells (Figure 7) [59]. We suggested that dis- ruption of the T cell cytoskeleton led to the impairment of T cell migration toward its target, whether it is an APC or foreign protein antigen (Figures 4-5) [59]. In fact, histology of allografts from animals treated with allochimeric MHC showed significantly reduced T cell infiltration into allografts compared to rejecting controls [59]. Another possible mechanism underlying the immu- nosuppressive properties of soluble MHC is the way that this protein molecule is processed by antigen-presenting cells, such as the DCs. Through an elaborate multistep process involving vesicular trafficking between the en- doplasmic reticulum (ER) and the Golgi apparatus, pro- tein antigens are displayed to peripheral T cells, and an immune response is executed. However, nothing is known about how allochimeric class I MHC is processed Rho GTPase pathway related cell functions and events (marked in red) potentially inhibited by allochimeric MHC I treatment in T cells and den- dritic cells in rat cardiac allograft model system (see text). Figur e 5 . Molecular and cellular targets of Rho pathway. within the DC and presented to T cells. Looking at the vesicular trafficking pathway and the morphological appearance of the ER and Golgi apparatus, we discov- ered stark differences in DCs isolated from allochimeric MHC treated recipients compared with untreated and CsA-treated controls [36]. We also found that there was decreased expression and abnormal distribution of RhoB in DCs from animals treated with allochimeric MHC [36]. Endocytic/intracellular membrane trafficking relies on RhoB [60] and its decreased expression compared with controls would indicate that the earliest step in antigen processing has been dysregulated [36]. Furthermore, looking at the ER and Golgi resident proteins KDEL and GM130, we found distinctly abnormal morphology of these two organelles in DCs from allochimeric MHC treated animals. We found that the ER lost its vesicular appearance and dissociated from its normal paranuclear localization. The Golgi apparatus also lost much of its ve- sicular appearance and became markedly reduced in size compared to controls [36]. These changes suggest that in our model system, the intracellular processing of soluble antigen, such as allochimeric MHC, has been highly dis- regulated (Figur es 2-3). Our previous and recent studies indicate that in- tra-operative treatment with allochimeric class I MHC attenuates chronic rejection. Chronic rejection is charac- terized by perivascular graft inflammation, graft vascular intimal hyperplasia and progressive luminal narrowing, as well as necrotizing arteritis. These lesions were shown by Singer et al. [38] to be diminished in long-term graft-surviving ACI recipients of WF grafts compared with 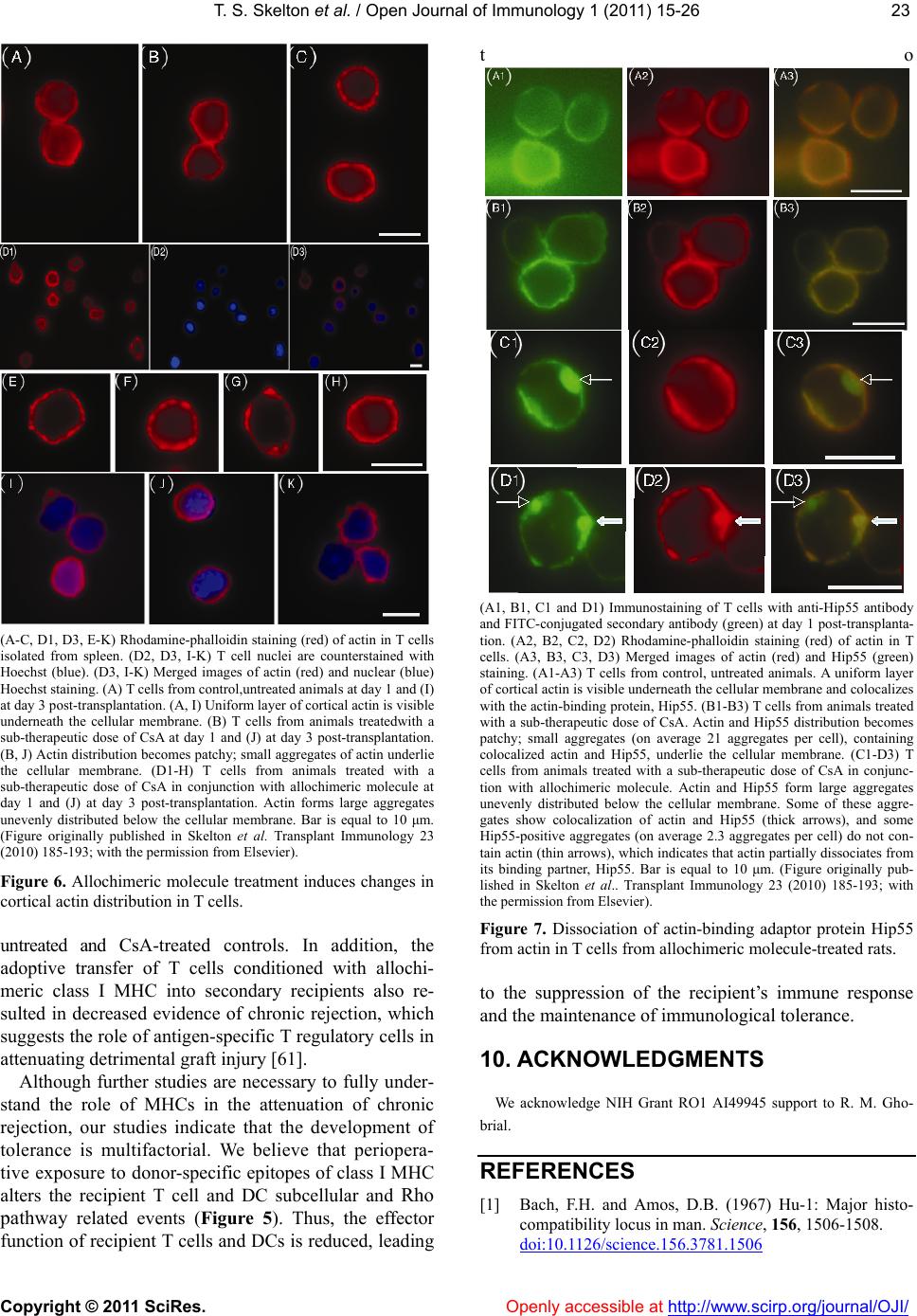 T. S. Skelton et al. / Open Journal of Immunology 1 (2011) 15-26 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/OJI/ 2323 (A-C, D1, D3, E-K) Rhodamine-phalloidin staining (red) of actin in T cells isolated from spleen. (D2, D3, I-K) T cell nuclei are counterstained with Hoechst (blue). (D3, I-K) Merged images of actin (red) and nuclear (blue) Hoechst staining. (A) T cells from control,untreated animals at day 1 and (I) at day 3 post-transplantation. (A, I) Uniform layer of cortical actin is visible underneath the cellular membrane. (B) T cells from animals treatedwith a sub-therapeutic dose of CsA at day 1 and (J) at day 3 post-transplantation. (B, J) Actin distribution becomes patchy; small aggregates of actin underlie the cellular membrane. (D1-H) T cells from animals treated with a sub-therapeutic dose of CsA in conjunction with allochimeric molecule at day 1 and (J) at day 3 post-transplantation. Actin forms large aggregates unevenly distributed below the cellular membrane. Bar is equal to 10 μm. (Figure originally published in Skelton et al. Transplant Immunology 23 (2010) 185-193; with the permission from Elsevier). Figure 6. Allochimeric molecule treatment induces changes in cortical actin distribution in T cells. untreated and CsA-treated controls. In addition, the adoptive transfer of T cells conditioned with allochi- meric class I MHC into secondary recipients also re- sulted in decreased evidence of chronic rejection, which suggests the role of antigen-specific T regulatory cells in attenuating detrimental graft injury [61]. Although further studies are necessary to fully under- stand the role of MHCs in the attenuation of chronic rejection, our studies indicate that the development of tolerance is multifactorial. We believe that periopera- tive exposure to donor-specific epitopes of class I MHC alters the recipient T cell and DC subcellular and Rho pathway related events (Figure 5). Thus, the effector function of recipient T cells and DCs is reduced, leading to (A1, B1, C1 and D1) Immunostaining of T cells with anti-Hip55 antibody and FITC-conjugated secondary antibody (green) at day 1 post-transplanta- tion. (A2, B2, C2, D2) Rhodamine-phalloidin staining (red) of actin in T cells. (A3, B3, C3, D3) Merged images of actin (red) and Hip55 (green) staining. (A1-A3) T cells from control, untreated animals. A uniform layer of cortical actin is visible underneath the cellular membrane and colocalizes with the actin-binding protein, Hip55. (B1-B3) T cells from animals treated with a sub-therapeutic dose of CsA. Actin and Hip55 distribution becomes patchy; small aggregates (on average 21 aggregates per cell), containing colocalized actin and Hip55, underlie the cellular membrane. (C1-D3) T cells from animals treated with a sub-therapeutic dose of CsA in conjunc- tion with allochimeric molecule. Actin and Hip55 form large aggregates unevenly distributed below the cellular membrane. Some of these aggre- gates show colocalization of actin and Hip55 (thick arrows), and some Hip55-positive aggregates (on average 2.3 aggregates per cell) do not con- tain actin (thin arrows), which indicates that actin partially dissociates from its binding partner, Hip55. Bar is equal to 10 μm. (Figure originally pub- lished in Skelton et al.. Transplant Immunology 23 (2010) 185-193; with the permission from Elsevier). Figure 7. Dissociation of actin-binding adaptor protein Hip55 from actin in T cells from allochimeric molecule-treated rats. to the suppression of the recipient’s immune response and the maintenance of immunological tolerance. 10. ACKNOWLEDGMENTS We acknowledge NIH Grant RO1 AI49945 support to R. M. Gho- brial. REFERENCES [1] Bach, F.H. and Amos, D.B. (1967) Hu-1: Major histo- compatibility locus in man. Science, 156, 1506-1508. doi:10.1126/science.156.3781.1506  T. S. Skelton et al. / Open Journal of Immunology 1 (2011) 15-26 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/OJI/ 24 [2] Chinen, J. and Buckley, R.H. (2010) Transplantation immunology: Solid organ and bone marrow. Journal of Allergy and Clinical Immunology, 125, S324-S335. doi:10.1016/j.jaci.2009.11.014 [3] Cohen, D.J., Loertscher, R., Rubin, M.F., Tilney, N.L., Carpenter, C.B. and Strom, T.B. (1984) Cyclosporin: A new immunosuppressive agent for organ transplantation. Annals of Internal Medicine, 10, 667-682. [4] Hariharan, S., Johnson, C.P., Bresnahan, B.A., et al. (2000) Improved graft survival after renal transplantation in the United States, 1988 to 1996. The New England Journal of Medicine, 342, 605-612. doi:10.1056/NEJM200003023420901 [5] Cecka, J.M. (2002) The UNOS Renal Transplant Registry. Clinical Transplantation, 1-20. [6] Safinia, N., Afzail, B., Atalar, K., et al. (2010) T-cell alloimmuntiy and chronic allograft dysfunction. Kidney international, 78 (suppl 119), S2-S12. doi:10.1038/ki.2010.416 [7] Klein, J. and Sato, A. (2000) The HLA system: Second of two parts. The New England Journal of Medicine, 343, 782-786. [8] Marsh, S.G. (2009) WHO nomenclature committee for factors of the HLA system. Nomenclature for factors of the HLA system, update. Tissue Antigens, 74, 364-366. doi:10.1111/ j.1399-0039.2009.01330.x [9] Klein, J. and Sato, A. (2000) The HLA system: First two parts. The New England Journal of Medicine, 343, 702-709. [10] Zinkernagel, R.M. and Doherty, P.C. (1974) Restriction of invitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature, 248, 701-702. doi:10.1038/248701a0 [11] Scott, A., Sant, L. and Sant, A. (2010) Generation of MHC class II-peptide ligands for CD4 T cell allorecogni- tion of MHC class II molecules. CurrOpin Organ Trans- plant, 15, 505-511. doi:10.1097/MOT.0b013e32833bfc5c [12] Li, X. and Raghavan, M. (2010) Structure and function of major histocompatability complex class I antigens. CurrOpin Organ Transplant, 15, 499-504. doi:10.1097/MOT.0b013e32833bfb33 [13] Trivedi, H.L. (2007) Immunobiology of rejection and adaptation. Transplantation Proceedings, 39, 647-652. doi:10.1016/j.transproceed.2007.01.047 [14] Stern, L.J. and Wiley, D.C. (1994) Anitgenic peptide binding by class I and class II histocompatibility proteins. Structure, 2, 245-251. doi:10.1016/S0969-2126(00)00026-5 [15] Jensen, P.E. (2007) Recent advances in antigen process- ing and presentation. Nature Immunology, 8, 1041-1048. doi:10.1038/ni1516 [16] Afzail, B., Lombardi, G. and Lechler, R. (2008) Pathways of major histocompatability complex allorecognition. Current Opinion in Organ Transplantation, 13, 438-444. doi:10.1097/MOT.0b013e328309ee31 [17] Jensen, P.E. (1999) Mechanisms of antigen presentation. Clinical Chemistry and Laboratory Medicine, 37, 179-186. doi:10.1515/CCLM.1999.034 [18] Elliot, T. and Williams, A. (2005) The optimization of peptide cargo bound to MHC class I molecules by the peptide-loading complex. Immunological Reviews, 207, 89-99. doi:10.1111/j.0105-2896.2005.00311.x [19] Warrens, A.N., Lombardi, G., Lechler, R.I., et al. (1994) Presentation and recognition of major and minor histo- compatability antigens. Transplant Immunology, 2, 103-107. doi:10.1016/0966-3274(94)90036-1 [20] Lechler, R.I. and Batchelor, J.R. (1982) Restoration of immunogenicity to passenger cell-depleted kidney al- lografts by the addition of donor strain dendritic cells. The Journal of Experimental Medicine, 155, 31-41. doi:10.1084/jem.155.1.31 [21] Ely, L.K., Burrows, S.R., Purcell, A.W., et al. (2008) T cells behaving badly: Structural insights into alloreactiv- ity and autoimmunity. Current Opinion in Organ Trans- plantation, 20, 575-580. doi:10.1016/j.coi.2008.07.006 [22] Baker, R.J., Hernandez-Fuentes, M.P., Brookes, P.A., et al. (2001) The role of the allograft in the induction of donor-specific T cell hyporesponsiveness. Transplanta- tion, 72, 480-485. doi:10.1097/00007890-200108150-00020 [23] Suchin, E.J., Langmuir, P.B., Palmer, E., et al. (2001) Qunatifying the frequency of allreactive T cells in vivo: new answeres to an old question. The Journal of Immu- nology, 166, 973-981. [24] Gras, S., Kjer-Nelson, L., Chen, Z., et al. (2011) The structural bases of direct T-cell allorecognition: Implica- tion for T-cell mediated transplant rejection. Immunology and Cell Biology, 5, 1-8. [25] Crispe, I.N., Husmann, L.A. and Bevan, M.J. (1986) T cell receptor expression and receptor-mediated induction of clonal growth in the developing mouse thymus. High surface beta-chain density is a requirement for functional maturity. European Journal of Immunology, 16, 1283-1288. doi:10.1002/eji.1830161016 [26] Matzinger, P. and Bevan, M.J. (1977) Hypothesis: Why do so many lymphocytes respond to major histocom- patability antigens? Cell Immunology, 29, 1-5. doi:10.1016/0008-8749(77)90269-6 [27] Archbold, J.K., Macdonald, W.A., Miles, J.J., et al. (2006) Alloreactivity between disparate cognate and allogeneic pMHC-I complexes is the result of highly focused, pep- tide-dependent structural mimicry. The Journal of Bio- logical Chemistry, 281, 34324-34332. doi:10.1074/jbc.M606755200 [28] Turner, S.J., Doherty, P.C., McClusky, J., et al. (2006) Structural derminants of T-cell receptor bias in immunity. Nature Reviews Immunology, 6, 883-894. doi:10.1038/nri1977 [29] Pietra, B.A., Wiseman, A., Bolwerk, A., et al. (2000) CD4 T cell-mediated cardiac allograft rejection requires donor but not host MHC class II. The Journal of Clinical Investigation, 106, 1003-1010. [30] Shoskes, D.A. and Wood, K.J. (1994) Indirect presenta-  T. S. Skelton et al. / Open Journal of Immunology 1 (2011) 15-26 Copyright © 2011 SciRes. Openl y accessible at http://www.scirp.org/journal/OJI/ 2525 tion of MHC antigens in transplantation. Immunology Today, 15, 32-38. doi:10.1016/0167-5699(94)90023-X [31] Liu, Z., Braunstein, N.S. and Suciu, F.N. (1992) T cell recognition of allopeptides in context of self MHC. The Journal of Immunology, 148, 35-40. [32] Watts, C. (2004) The exogenous pathway for antigen presentation on major histocompatibility complex class II and CD1 molecules. Nature Immunology, 5, 670-677. doi:10.1038/ni1088 [33] Cresswell, P. (1996) Invariant chain structure and MHC class II function. Cell, 84, 505-507. doi:10.1016/S0092-8674(00)81025-9 [34] Inaba, K., Turley, S., Yamaide, F., et al. (1998) Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. The Journal of Experimental Medicine, 188, 2163-2173. doi:10.1084/jem.188.11.2163 [35] Fangmann, J., Dalchau, R. and Fabre, J.W. (1992) Rejec- tion of skin allografts by indirect allorecognition of do- nor class I major histocompatabiltiy complex peptides. The Journal of Experimental Medicine, 175, 1521-1529. doi:10.1084/jem.175.6.1521 [36] Skelton, T.S., Tejpal, N., Gong, Y., et al. (2011) Allochi- merich molecules and mechanisms in the abrogation of cardiac allograft rejection. The Journal of Heart and Lung Transplantation, Epub, ahead of print. [37] Gokmen, M.R., Giovanna, L. and Lechler, R.I. (2008) The importance of the indirect pathway of allorecogni- tion in clinical transplantation. Current Opinion in Im- munology, 20, 568-574. doi:10.1016/j.coi.2008.06.009 [38] Singer, J.S., Mhoyan, A., Fishbein, M., et al. (2001) Al- lochimeric class I MHC molecules prevent chronic rejec- tion and attenuate alloantibody response. Transplantation, 72, 1408-1416. doi:10.1097/00007890-200110270-00014 [39] Valujskikh, A., Lantz, O., Celli, S., et al. (2002) Cross primed CD8+ T cells mediate graft rejection via a distinct effector pathway. Nature Immunology, 3, 844-851. doi:10.1038/ni831 [40] Kapessidou, Y., Habran, C., Buonocore, S., et al. (2006) The replacement of graft endothelium by recipient-type cells conditions allograft rejection mediated by indirect pathway CD4+ T cells. Transplantation, 82, 582-591. doi:10.1097/01.tp.0000184444.93108.d1 [41] Herrera, O.B., Golshayan, D., Tibbott, R., et al. (2004) A novel pathway of alloantigen presentation by dendritic cells. The Journal of Immunology, 173, 4828-4837. [42] Ridge, J.P., Di, R.F. and Matzinger, P.A. (1998) A condi- tioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature, 393, 474-478. doi:10.1038/30989 [43] Lee, R.S., Grusby, M.J., Glimcher, L.H., et al. (1994) Indirect recognition by helper cells can induce do- nor-specific cytotoxic T lymphocytes in vivo. The Jour- nal of Experimental Medicine, 179, 865-872. doi:10.1084/jem.179.3.865 [44] Wise, M.P., Bemelman, F., Cobbold, S.P., et al. (1998) Linked suppression of skin graft rejection can operate through indirect recognition. The Journal of Immunology, 161, 5813-5816. [45] Games, D.S., Rogers, N.J. and Lechler, R.I. (2005) Ac- quisition of HLA-DR and costimulatory molecules by T cells from allogeneic antigen presenting cells. American Journal of Transplantation, 5, 1614-1625. doi:10.1111/ j.1600-6143.2005.00916.x [46] Morelli, A.E., Larregina, A.T., Shufesky, W.J., et al. (2004) Endocytosis, intracellular sorting and processing of exosomes by dendritic cells. Blood, 104, 3257-3266. doi:10.1182/blood-2004-03-0824 [47] Pimenta-Araujo, R., Mascarell, L., Huesca, M., et al. (2001) Embryonic thymic epithelium naturally devoid of APCs is acutely rejected in the absence of indirect rec- ognition. The Journal of Immunology, 167, 5034-5041. [48] Calne, R.Y., Sells, R.A., Pena, J.R., et al. (1969) Induc- tion of immunologic tolerance by porcine liver allografts. Nature, 223, 472-476. doi:10.1038/223472a0 [49] Zeigler, S.F. (2006) Foxp3: Of mice and men. Annual Review of Immunology, 24, 209-226. doi:10.1146/annurev.immunol.24.021605.090547 [50] Toyokawa, H., Nakao, A., Bailey, R.J., Nalesnik, M.A., et al. (2008) Relative contribution of direct and indirect allorecognition in developing tolerance after liver trans- plantation. Liver Transplantation, 14, 346-357. doi:10.1002/lt.21378 [51] Callaghan, C.J., Rouhani, F.J., Negus, M.C., Curry, A.J., Bolton, E.M., Bradley, J.A. and Pettigrew, G.J. (2007) Abrogation of antibody-mediated allograft rejection by regulatory CD4 T cells with indirect allospecificity. The Journal of Immunology, 178, 2221-2228. [52] Billingham, R.E., Brent, L. and Medawar, P.B. (2010) Actively acquired tolerance of foreign cell. 1953. The Journal of Immunology, 184, 5-8. doi:10.4049/jimmunol.0990109 [53] Sayegh, M.H., Perico, N., Gallon, L., et al. (1994) Mechanism of acquired thymichyporesponsiveness to renal allografts. Thymic recognition of immunodomi- nantallo-MHC peptides induces peripheral T cell anergy. Transplantation, 58, 125-132. doi:10.1097/00007890-199407270-00001 [54] Zhou, C., Lu, R., Lin, G., et al. (2011) The latest devel- opments in synthetic peptides with immunoregulatory activities. Peptides, 32, 408-414. doi:10.1016/j.peptides.2010.10.019 [55] Krensky, A.M. and Clayberger, C. (1997) HLA-derived peptides as novel immunosuppressives. Nephrology Di- alysis Transplantation, 12, 865-878. doi:10.4049/jimmunol.0990109 [56] Gobrial, R.M., Hamashima, W., Wang, M., et al. (1996) Induction of transplantion tolerance by chimeric do- nor/recipient class I RT1.Aa molecules. Transplantation, 62, 1002-1010. doi:10.1097/00007890-199610150-00020 [57] Lisik, W., Gong, Y., Tejpal, N., et al. (2010) Intragraft gene expression profile associated with the induction of tolerance by allochimeric MHC I in the rat heart trans- plant model. Genesis, 1, 8-19.  T. S. Skelton et al. / Open Journal of Immunology 1 (2011) 15-26 Copyright © 2011 SciRes. http://www.scirp. org/journal/OJI/Openly accessible at 26 [58] Lisik, W., Tejpal, N., Gong, Y., et al. (2009) Down regu- lation of genes involved in T cell polarity and motility during the induction of heart allograft tolerance by al- lochimeric MHC I. PlosOne, 4, e8020. doi:10.1371/journal.pone.0008020 [59] Skelton, T.S., Tejpal, N., Gong, Y., et al. (2010) Down- regulation of RhoA and changes in T cell cytoskeleton correlate with the abrogation of allograft rejection. Transplant Immunology, 23, 185-193. doi:10.1016/j.trim.2010.06.009 [60] Wheeler, A.P. and Ridley, A.J. (2004) Why three Rho proteins? RhoA, RhoB, RhoC and cell motility. Experi- mental Cell Research, 301, 43-49. doi:10.1016/j.yexcr.2004.08.012 [61] Semiletova, N., Shen, X.D., Baibakov, B., et al. (2010) Intensity of transplant chronic rejection correlates with level of graft-infiltrating regulatory cells. The Journal of Heart and Lung Transplantation, 29, 335-341. doi:10.1016/j.healun.2009.08.003
|