 Open Jo urnal of Obstetr ics and Gynecology, 2011, 1, 71-83 OJOG doi:10.4236 /ojog.2011.13014 Published Online September 2011 (http://www.SciRP.org/journal/ojog/). Published Online September 2011 in SciRes. http://www.scirp.org/journal/OJOG Prognostic factors for survival after neoadjuvant chemotherapy for advanced ovarian cancer: meta-analysis Malcolm John Farquharson1*, Nadeem Ahmed Siddiqui2 1Department of Obstetrics and Gynecology, Crosshouse Hospital, Ayrshire, UK. 2Department of Gynecological Oncology, Glasgow Royal Infirmary, Glasgow, UK. E-mai l : * HHUUmalcolmfarquharson@nhs.netUUHH Received 25 July 2011; revised 20 August 2011; accepted 29 August 2011. ABSTRACT Background: In advanced disease current practice is staging and primary debulking laparotomy followed by platinum-based chemotherapy. The effort to achie- ve ‘optimal debulking’ is associated with a compli- cation risk of 8% - 63% and a mortality rate of 1% - 6%. Neoadjuvant chemotherapy has been proposed as an alternative option. Objectives: This meta-analy- sis aimed to determine prognostic factors influencing survival in patients with advanced ovarian cancer following neoadjuvant chemotherapy. Search St rategy : Clinical trials citing the terms ‘advanced ovarian can- cer’, ‘ovarian cancer’, ‘neoadjuvant chemotherapy’ and ‘surgery’ were identified by searching Pubmed and ScienceDirect between January 1st 2000 and September 30th 2010. Data Collection and analysis: The trials included used platinum-based chemothe- rapy a nd i nvolved stage III/IV disease that underwent neoadjuvant chemotherapy followed by surgery. Prog- nostic variables were identified for analysis including number/type of chemotherapy, % stage IV disease, % max i ma l cy toreductive surg ery a nd w hether a lympha- denectomy was performed. The % bowel surgery and ultra-radical surgery was also analysed. Main Results: Twenty six trials were identified as suitable for anal- ysis and included 3 non-randomised Phase II studies, 2 retrospective case-control studies, 17 from retros- pective analysis and 1 RCT. A significant association betwe en taxane use vs plat inum only (p = 0.019), year of publication (p = 0.032), % maximal interval cytor- eduction (p = 0.046) and median overall survival was identified. No significant survival benefit was dem- onstrated with number of chemotherapy cycles (p = 0.065), lymphadenectomy (p = 0.813) and % bowel surgery performed (p = 0.606). Conclusions: The addition of taxane and % maximal cytoreduction achieved is associated with improved overall survival. There is, however no evidence that lymphadenecto- my, number of chemotherapy cycles or bowel surgery influences survival. Keywords: Neoa dj uvant Che mo the ra py; Surviva l; Advanced Ovarian Cancer; Prognostic Factors 1. INTRODUCTIO N Ovarian cancer is the 2nd commonest gynaecological malignancy with epithelial tumours accounting for 90%. In the UK, there are approximately 6500 new cases of ovarian cancer per year. They are usually discovered at an advanced stage (FIGO stage III/IV) resulting in the overall poor prognosis [1]. The 5 year survival rate for early stage cancer is about 90% whereas the 5 year sur- vival rate in advanced disease is 10% - 30% [2]. In advanced ovarian disease the standard current prac- tice is a staging and primary debulking laparotomy followed by platinum based chemotherapy [3]. Optimal cytoreduction during initial surgery has become widely accepted as the most important prognostic indicator. Over the last 20 years the view on what constitutes ‘maximal debulking’ has changed from leaving no tu- mour deposit >2 cm to leaving no macroscopic disease being acceptable. Increasingly more radical surgery is being performed in an effort to free the patient of macro- scopic disease [4]. A m et a -analysis of 2637 women from retrospective non-randomised c ontrol trials de monstrate d a survival benefit for those patients with advanced di- sease who had no macroscopic disease or tumour deposit >2 cm [5]. There has been no evidence that a survival benefit exists with residual tumour >2 cm and often optimal debulking is simply not possible due to the extent of the disease [6]. In this scenario initial surgery may be ques- tionable if residual tumour <2 cm is not achievable. The effort to achieve ‘maximal/optimal debulking’ is associated with a complication risk of 8% - 63% [7]. Mortality following debulking surgery has been reported from 1% - 6% with a recent systematic review reporting  M. J. Farquharson et al . / Open Journal of Obstetrics and Gynecology 1 (2011) 71-83 Copyright © 2011 Sci Res. OJOG the mean mortality at 2.8% [8]. A laparotomy is a major operation that requires hospital admission and time for recovery that may delay the start of chemotherapy. How- ever two studies have s hown no evidence that a delay in starting chemotherapy influences the survival rate [9,10]. Neoadjuvant chemotherapy has been proposed as an alternative option in advanced ovarian cancer. A recent RCT showed that neoadjuvant chemotherapy was not infer ior t o primar y debulk ing surger y in ter ms of the s ur- vival rate [4]. It can be argued that surgery following chemotherapy may be technically easier than if surgery was undertaken initially. In addition a prospective study has shown that there is an improved patient quality of life and functional status following neoadjuvant chemo- therapy [11]. The survival benefit and prognosis of upfront surgery in advanced ovarian cancer is well known and estab- lished . Howe ver the use of neoadj uvant c hemothe rapy is less well researched. The aim of this meta-analysis is to determine the prognostic factors influencing overall sur- vival rate in patients with advanced ovarian cancer following neoa djuva nt chemothe rapy. 2. METHODS A meta-analysis was performed based on the recom- mendations of the Preferred Reporting Items for System- atic Reviews and Meta-analyses (PRISMA) statement [12]. 2.1. Search Strat eg y A literature search has been carried out using the National Library of Medicine and National Institutes of Health (Pubmed) and ScienceDirect for all clinical trials on the use of neoadjuvant chemotherapy in advanced ovarian cancer. The following headings and keywords were used in the literature search; ‘advanced ovarian cancer’, ‘ovarian cancer’, ‘neoadjuvant chemotherapy’ and ‘surgery’. The search included all English language articles and was limited to the period of January 1st 2000 to September 30th 2010. The references of each article were reviewed for any relevant articles which were subsequently included in the analysis. The terms have been expanded to include all subcategories in an attempt to obtain all published trials that fit the selection criteria. 2.2. Selection Criteria The trials included have used platinum based chemothe- rapy and involve advanced epithelial ovarian cancer stage III/IV that was staged according to the FIGO classifica tion. The cohorts includ ed must ha ve undergone neoadjuvant chemotherapy followed by cytoreductive surgery. The excluded trials were those where chemotherapy regimens other than platinum based were used, early stage ovarian cancer and no inclusion of the proportion of patients with stage III or IV disease. Articles were excluded that did not include the median survival time (months). Also excluded were articles that did not men- tion criteria for residual tumour and define what they considered optimal debulking surgery. Further cohorts were not included if the number of chemotherapy cycles administered and the different chemotherapy regimens were not recorded. 2.3. Studies Identified A total of 333 potentiall y relevant studie s were iden tified based on the above search criteria. After screening the titles and abstracts, 291 studies were excluded for the following reasons: (1) unrelated to subject (n = 115), (2) review article s (n = 96), (3) non ovarian patients (n = 60), (4) case reports (n = 15), (5) preclinical trials (n = 5). Further assessment of these studies for more detailed information identified 16 ineligible studies due to intra- peritoneal or intra-arterial chemotherapy (n = 8), no me- dian overall survival (n = 5), no residual tumour criteria (n = 2) and the use of adjuvant chemotherapy (n = 1). Finally 26 studies were identified as suitable for inclu- sion in the meta-anal ysis (Figure 1). 2.4. Extraction of Data The baseline characteristics of patients were recorded incl uding the medi an and range of age, the hi stolo gy and grade of disease and the number of patients within each cohort. The percentage of patients with stage III/IV disease were recorded as well as a breakdown of Stage III A, B a nd C if inc lude d. T he residual tu mour criteria o f each study was identified and maximal cytoreduction was con sidered to have occurred if residual disease mea- sured less than 1cm in largest diameter. Additional information included the study design and the year of study. The staging procedure used to diagnose and determine severity were collected and also the pre- operative disease severity including the main site of disease. Other information included percentage of patients achieving maximal debulking within the cohort, rate of bowel surgery performed, whether or not lymphadenec- tomy was performed and the site of the residual disease following debulking surgery. The percentage of patients in each cohort who did not go on to have cytoreductive surgery following neoadjuvant chemotherapy was re- corded and the outcomes of these patients. Whether ultra radical surgery was done or not among the cohorts was collected in addition to what procedures were performed. 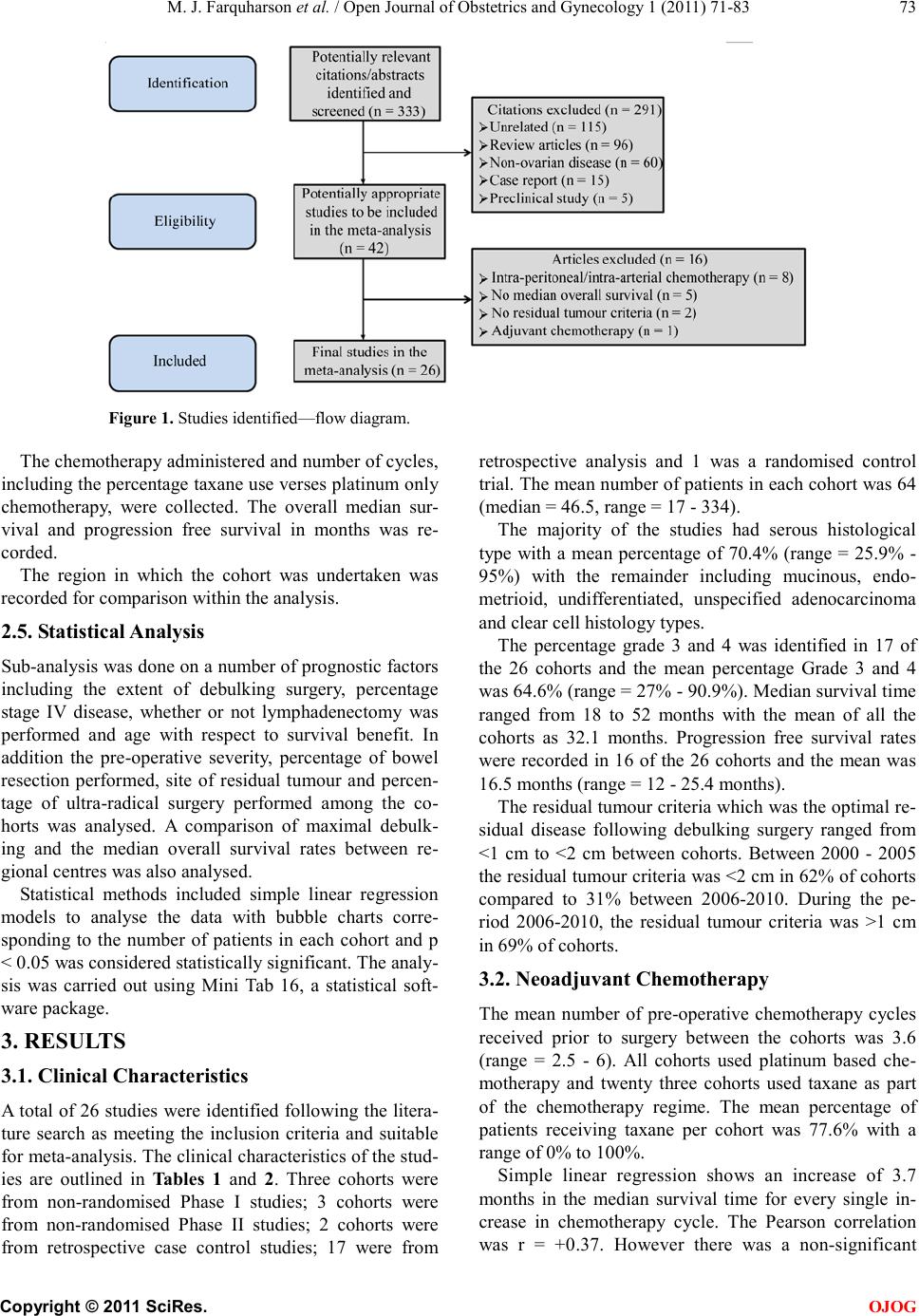 M. J. Farquharson et al . / Open Journal of Obstetrics and Gynecology 1 (2011) 71-83 Copyright © 2011 Sci Res. OJOG Figure 1. Studies identified—flow diagram. The chemotherapy administered and number of cycles, including the percentage taxane use verses platinum onl y chemotherapy, were collected. The overall median sur- vival and progression free survival in months was re- corded. The region in which the cohort was undertaken was recorded for comparison within the analysis. 2.5. S tatistica l Analysis Sub-a nal ysis was done on a number of prognostic factors including the extent of debulking surgery, percentage stage IV disease, whether or not lymphadenectomy was performed and age with respect to survival benefit. In addition the pre-operative severity, percentage of bowel resection performed, site of residual tumour and percen- tage of ultra-radical surgery performed among the co- horts was analysed. A comparison of maximal debulk- ing and the median overall survival rates between re- gional centres was also analysed. Statistical methods included simple linear regression models to analyse the data with bubble charts corre- sponding to the number of patients in each cohort and p < 0.05 was considered statistically significant. The analy- sis was carried out using Mini Tab 16, a statistical soft- ware package. 3. RESULTS 3.1. Clinical Characteristics A total of 26 studies were identi fied follo wing the liter a- ture search as meeting the inclusion criteria and suitable fo r me ta -a nalysis. The clinical characteristics of the stud- ies are outlined in Tables 1 a nd 2. Three cohorts were from non-randomised Phase I studies; 3 cohorts were from non-randomised Phase II studies; 2 cohorts were from retrospective case control studies; 17 were from retrospective analysis and 1 was a randomised control trial. The mean number of patients in each cohort was 64 (median = 46.5, range = 17 - 334). The majority of the studies had serous histological type with a mean percentage of 70.4% (range = 25.9% - 95%) with the remainder including mucinous, endo- metrioid, undifferentiated, unspecified adenocarcinoma and clear cell histology types. The percentage grade 3 and 4 was identified in 17 of the 26 cohorts and the mean percentage Grade 3 and 4 was 64.6% (range = 27% - 9 0 . 9% ). Me d ia n s urvi va l t ime ranged from 18 to 52 months with the mean of all the cohorts as 32.1 months. Progression free survival rates were recorded in 16 of the 26 cohorts and the mean was 16.5 months (range = 12 - 25.4 months). The residual tumour criteria which was the optimal re- sidual disease following debulking surgery ranged from <1 cm to <2 cm between cohorts. Between 2000 - 2005 the residual tumour criteria was <2 cm in 62% of cohorts compared to 31% between 2006-2010. During the pe- riod 2006-2010, the residual tumour criteria was >1 cm in 69% of cohorts. 3.2. Neoadjuvant Chemotherapy The mean number of pre-operative chemotherapy cycles received prior to surgery between the cohorts was 3.6 (range = 2.5 - 6). All cohorts used platinum based che- motherapy and twenty three cohorts used taxane as part of the chemotherapy regime. The mean percentage of patients receiving taxane per cohort was 77.6% with a range of 0% to 100%. Simple linear regression shows an increase of 3.7 months in the median survival time for every single in- crease in chemotherapy cycle. The Pearson correlation was r = +0.37. However there was a non-significant 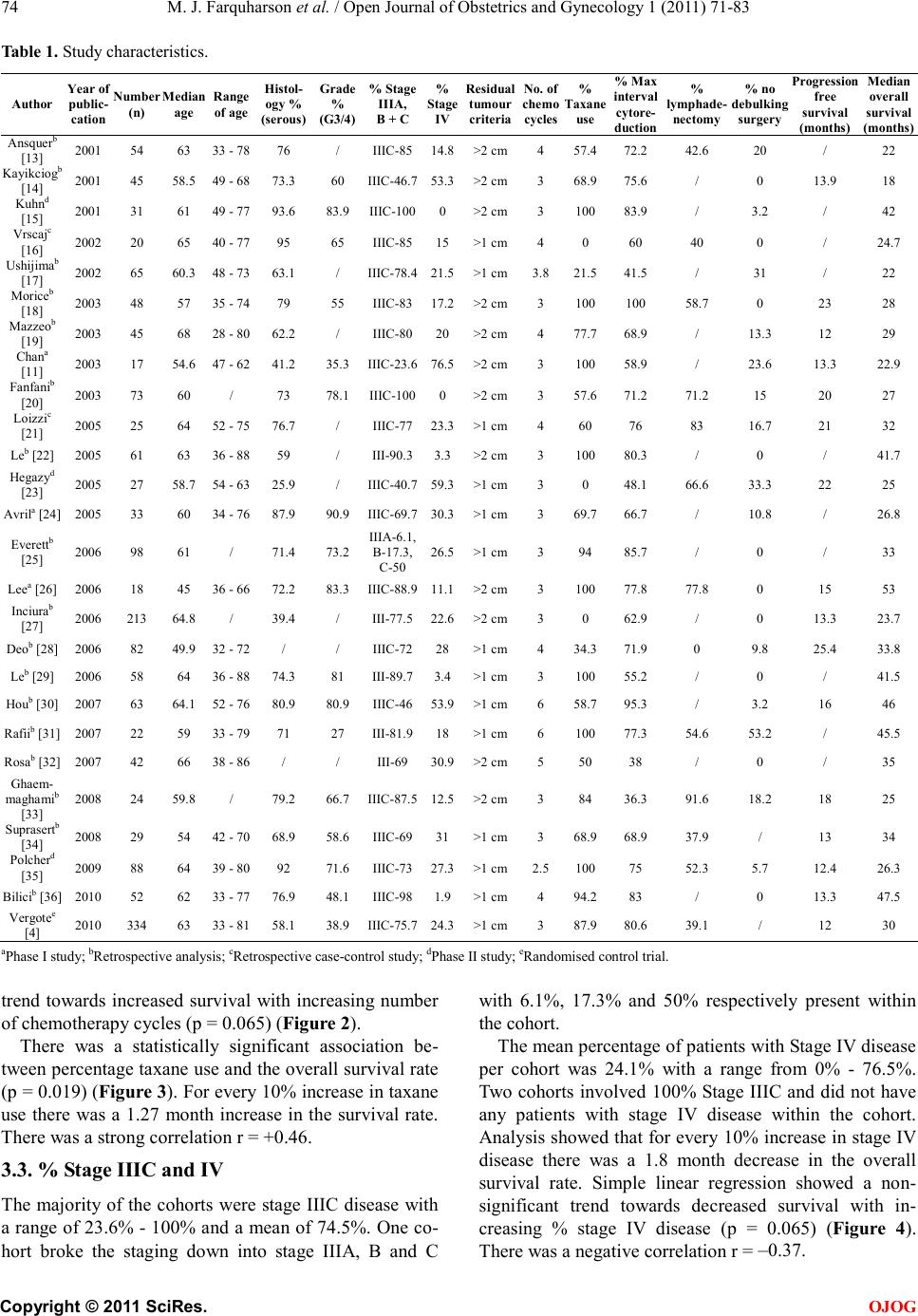 M. J. Farquharson et al . / Open Journal of Obstetrics and Gynecology 1 (2011) 71-83 Copyright © 2011 Sci Res. OJOG Table 1. Study charact er istics. Author Year of public- cation (n) Median age Range of age Histol- ogy % (serous) Grade % (G3/4) % Stage IIIA, B + C % Stage IV Residual tumour criteria No. of chemo cycles % Tax use % Max interval cytore- duction % lymphade- nectomy % no debulking surgery Progres free survival (months) Median overall survival (months) [13] 2001 54 63 33 - 78 76 / IIIC-85 14.8 >2 cm 4 57.4 72.2 42.6 20 / 22 2001 45 58.5 49 - 68 73.3 60 IIIC-46.7 53.3 >2 cm 3 68.9 75.6 / 0 13.9 18 2001 31 61 49 - 77 93.6 83.9 IIIC-100 0 >2 cm 3 100 83.9 / 3.2 / 42 2002 20 65 40 - 77 95 65 IIIC-85 15 >1 cm 4 0 60 40 0 / 24.7 Ushijima 2002 65 60.3 48 - 73 63.1 / IIIC-78.4 21.5 >1 cm 3.8 21.5 41.5 / 31 / 22 Morice 2003 48 57 35 - 74 79 55 IIIC-83 17.2 >2 cm 3 100 100 58.7 0 23 28 Mazzeo [19] 2003 45 68 28 - 80 62.2 / IIIC-80 20 >2 cm 4 77.7 68.9 / 13.3 12 29 [11] 2003 17 54.6 47 - 62 41.2 35.3 IIIC-23.6 76.5 >2 cm 3 100 58.9 / 23.6 13.3 22.9 [20] 2003 73 60 / 73 78.1 IIIC-100 0 >2 cm 3 57.6 71.2 71.2 15 20 27 [21] 2005 25 64 52 - 75 76.7 / IIIC-77 23.3 >1 cm 4 60 76 83 16.7 21 32 Leb [22] 2005 61 63 36 - 88 59 / III-90.3 3.3 >2 cm 3 100 80.3 / 0 / 41.7 2005 27 58.7 54 - 63 25.9 / IIIC-40.7 59.3 >1 cm 3 0 48.1 66.6 33.3 22 25 Avrila [24] 2005 33 60 34 - 76 87.9 90.9 IIIC-69.7 30.3 >1 cm 3 69.7 66.7 / 10.8 / 26.8 Everettb [25] 2006 98 61 / 71.4 73.2 III A -6.1, B-17.3, 26.5 >1 cm 3 94 85.7 / 0 / 33 Leea [26] 2006 18 45 36 - 66 72.2 83.3 IIIC-88.9 11.1 >2 cm 3 100 77.8 77.8 0 15 53 [27] 2006 213 64.8 / 39.4 / III-77.5 22.6 >2 cm 3 0 62.9 / 0 13.3 23.7 Deob [28] 2006 82 49.9 32 - 72 / / IIIC-72 28 >1 cm 4 34.3 71.9 0 9.8 25.4 33.8 Leb [29] 2006 58 64 36 - 88 74.3 81 III-89.7 3.4 >1 cm 3 100 55.2 / 0 / 41.5 Houb [30] 2007 63 64.1 52 - 76 80.9 80.9 IIIC-46 53.9 >1 cm 6 58.7 95.3 / 3.2 16 46 Rafiib [31] 2007 22 59 33 - 79 71 27 III-81.9 18 >1 cm 6 100 77.3 54.6 53.2 / 45.5 Rosab [32] 2007 42 66 38 - 86 / / III-69 30.9 >2 cm 5 50 38 / 0 / 35 Ghaem- maghamib [33] 2008 24 59.8 / 79.2 66.7 IIIC-87.5 12.5 >2 cm 3 84 36.3 91.6 18.2 18 25 [34] 2008 29 54 42 - 70 68.9 58.6 IIIC-69 31 >1 cm 3 68.9 68.9 37.9 / 13 34 [35] 2009 88 64 39 - 80 92 71.6 IIIC-73 27.3 >1 cm 2.5 100 75 52.3 5.7 12.4 26.3 Bilicib [36] 2010 52 62 33 - 77 76.9 48.1 IIIC-98 1.9 >1 cm 4 94.2 83 / 0 13.3 47.5 2010 334 63 33 - 81 58.1 38.9 IIIC-75.7 24.3 >1 cm 3 87.9 80.6 39.1 / 12 30 aPhase I study; bRet rospective analysis; cRetrospective case-control study; dPha se II study; eRandomised control trial. trend towards increased survival with increasing number of chemotherapy cycles (p = 0.065) (Figure 2). There was a statistically significant association be- tween percentage taxane use and the overall survival rate (p = 0.019) (Figu re 3). For every 10% increase in taxane use there was a 1.27 month increase in the survival rate. There was a strong correlation r = +0.46. 3.3. % Stage IIIC and IV The majority of the cohorts were stage IIIC disease with a range of 23.6% - 100% and a mean of 74.5%. One co- hort broke the staging down into stage IIIA, B and C with 6.1%, 17.3% and 50% respectively present within the cohort . The mean percentage of patients with Stage IV disease per cohort was 24.1% with a range from 0% - 76.5%. Two cohorts involved 100% Stage IIIC and did not have any patients with stage IV disease within the cohort. Analysis showed that for every 10% increase in stage IV disease there was a 1.8 month decrease in the overall survival rate. Simple linear regression showed a non- significant trend towards decreased survival with in- creasing % stage IV disease (p = 0.065) (Figure 4). There was a negative correlation r = –0.37. 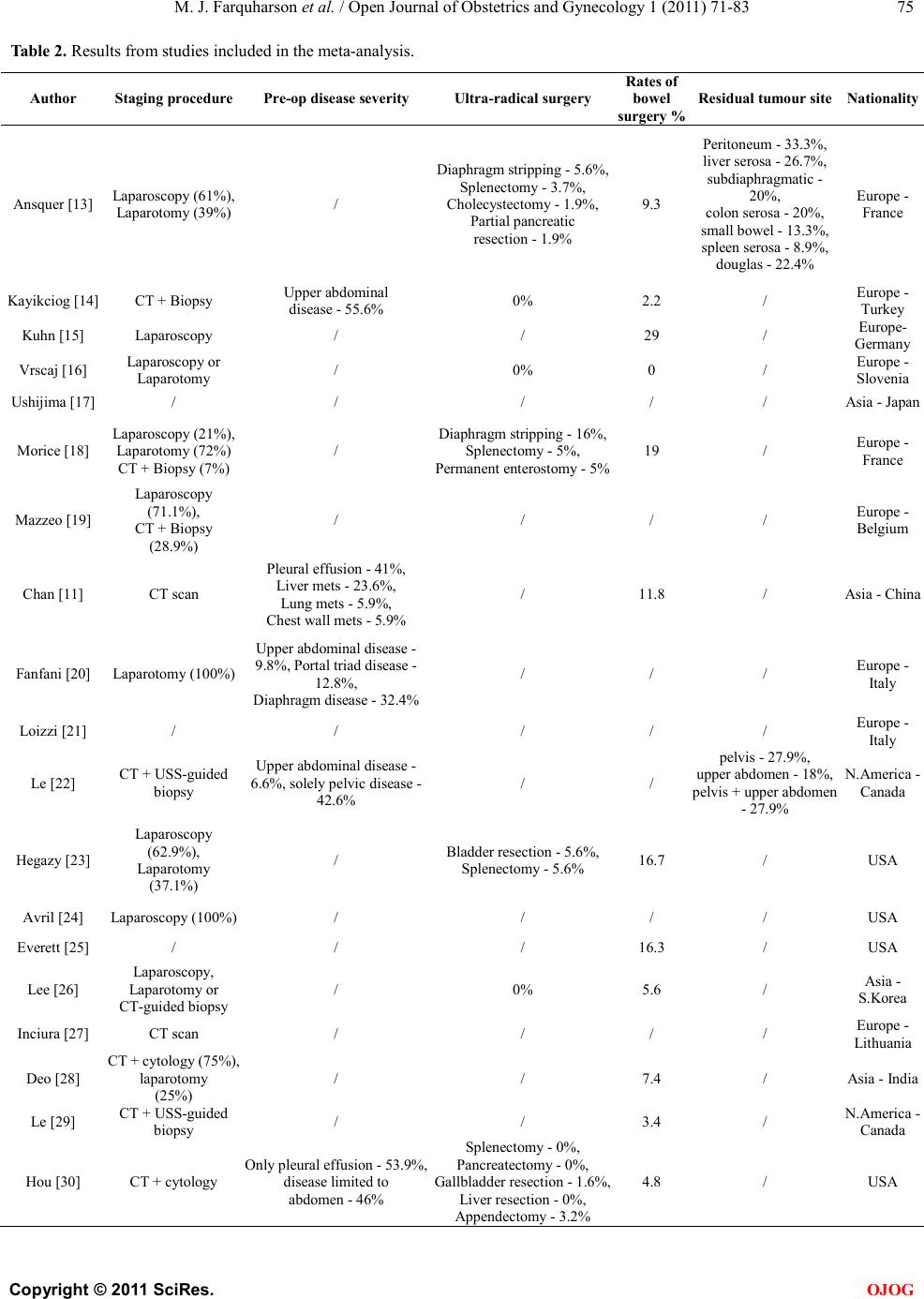 M. J. Farquharson et al . / Open Journal of Obstetrics and Gynecology 1 (2011) 71-83 Copyright © 2011 Sci Res. OJOG Table 2. Results from studies included in the meta-anal ysis. Author Staging proce dure Pre-op disea se sev erity Ultra-radical sur gery bowel Residual tumour si te Nationality Ansquer [13] Laparoscopy (61%), Laparotomy (39%) / Diaphragm stripping - 5.6%, Splenectomy - 3.7%, Cholecystectomy - 1.9%, Partial pancre atic resection - 1.9% 9.3 Peritoneum - 33.3% , liver seros a - 26.7%, subdiaphragmatic - 20%, colon serosa - 20%, small bowel - 13.3%, spleen serosa - 8.9%, douglas - 22.4% Europe - France Kayikc iog [14] CT + Biopsy disease - 55. 6% 0% 2.2 / Turkey Kuhn [ 15 ] Laparoscopy / / 29 / Germany Vrscaj [16] Laparoscopy or La p arotomy / 0% 0 / Ushijima [17] / / / / / Asia - Japan Morice [18] Laparoscopy (21%), Laparotomy (72%) CT + Biopsy (7%) / Diaphragm stripping - 16%, Splenectomy - 5%, Permanent enterostomy - 5% 19 / Europe - France Ma zzeo [19] (71.1%), CT + Biopsy (28.9%) / / / / Europe - Belgium Chan [11] CT sc a n Pleural effusion - 41%, Liver mets - 23.6%, Lung mets - 5.9%, Ch est wall m ets - 5.9% / 11.8 / Asia - China Fanfani [20] Laparotomy (100%) Upper abdominal disease - 9.8%, Portal t riad di sease - 12.8%, Diaphragm disease - 32.4% / / / Europe - Italy Loizzi [21] / / / / / Le [22] CT + US S-guided biopsy Upper abdominal disease - 6.6%, solely pelvic disease - 42.6% / / upper abdomen - 18%, pelvi s + upper abd om en - 27.9% N.America - Canada Hegazy [2 3] Laparoscopy (62.9%), Laparotomy (37.1%) / Bladder resection - 5.6%, Splenectomy - 5.6% 16.7 / USA Avril [24] Laparoscopy (100%) / / / / USA Everett [25] / / / 16.3 / USA Lee [26] Laparotomy or / 0% 5.6 / Asia - S. Kor e a Inciura [27] CT scan / / / / Europe - Lithuania Deo [28] laparotomy (25%) / / 7.4 / Asia - India Le [29] / / 3 .4 / Hou [30] CT + c ytology Only pleural eff us ion - 53.9%, disease limited to abdomen - 46% Pancreatectomy - 0%, Gallbladder resection - 1.6%, Liver resection - 0%, 4.8 / USA 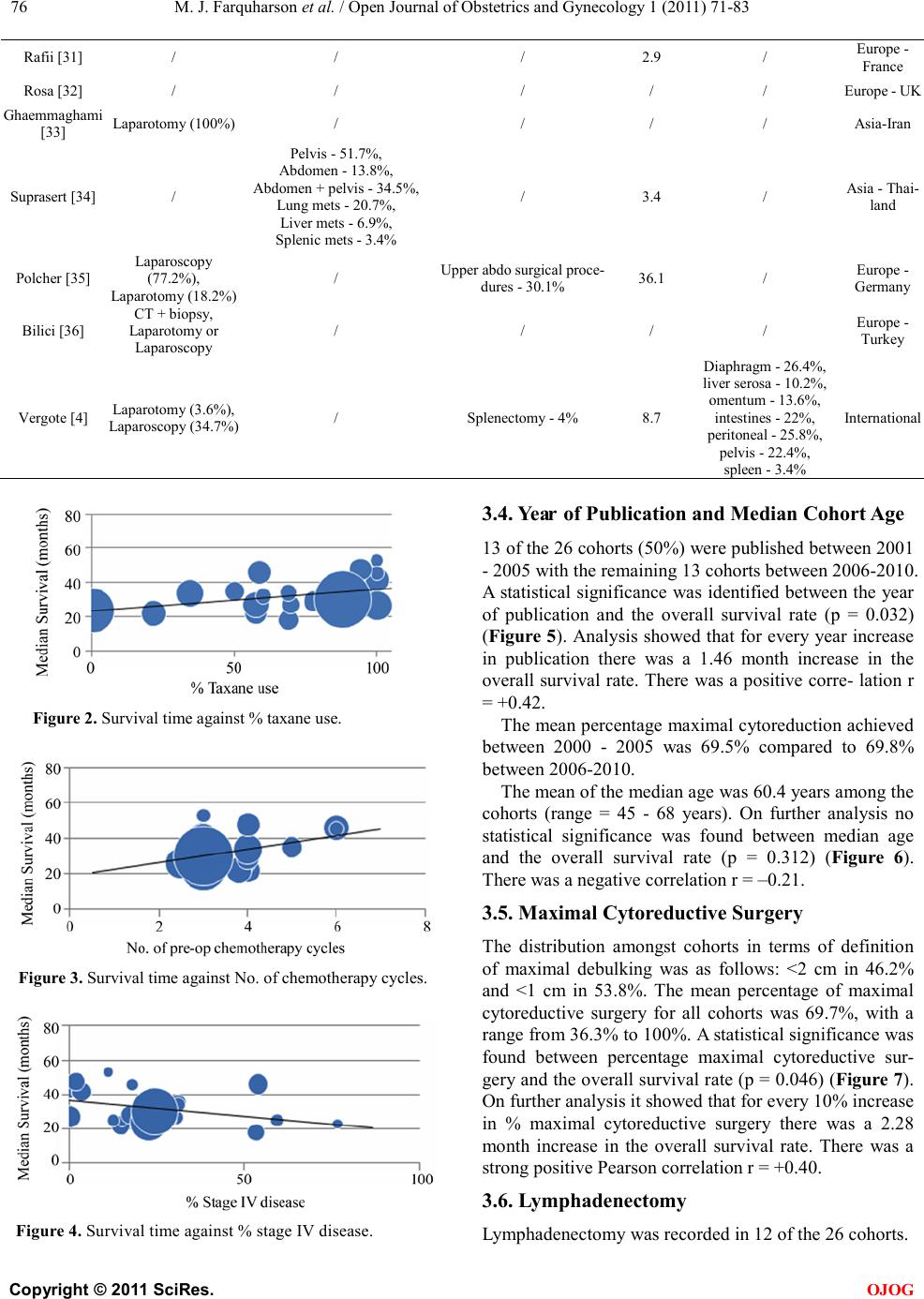 M. J. Farquharson et al . / Open Journal of Obstetrics and Gynecology 1 (2011) 71-83 Copyright © 2011 Sci Res. OJOG Rafii [31] / / / 2.9 / Rosa [32] / / / / / Europe - UK [33] Lapa rotomy (100%) / / / / Asi a -Iran Suprasert [34] / Pelvis - 51.7%, Abdomen - 13.8%, Abdom en + pel vis - 34.5%, Lung mets - 20.7%, Liver mets - 6.9%, Splenic mets - 3.4% / 3 .4 / Asia - Thai- land Polcher [35] (77.2%), / Upper abdo surgical proce- dures - 30.1% 36.1 / Europe - Germany Bilic i [ 36 ] CT + biops y, Laparotomy or Laparoscopy / / / / Europe - Turkey Vergo te [4] Laparotomy (3.6%), Laparoscopy (34.7%) / Splenectomy - 4% 8.7 Diaphragm - 26.4%, liver seros a - 10.2%, omentum - 13.6%, intestines - 22%, peritoneal - 25.8%, pelvis - 22.4%, spleen - 3.4% International Figure 2. Survival time against % taxane use. Figure 3. Survival time against No. of chemotherapy cycles. Figure 4. Surviva l time against % stage IV disease. 3.4. Year of Publication and Median Cohort Age 13 of the 26 cohorts (50%) were published between 2001 - 2005 with the remaining 13 cohorts between 2006-2010. A statistical significance was identified between the year of publication and the overall survival rate (p = 0.032) (Figu re 5). Analysis showed that for every year increase in publication there was a 1.46 month increase in the overall survival rate. There was a positive corre- lation r = +0.42. The mean percentage maximal cytoreduction ac hieved between 2000 - 2005 was 69.5% compared to 69.8% between 2006-2010. The mean of the median age was 60.4 years among the cohorts (range = 45 - 68 years). On further analysis no statistical significance was found between median age and the overall survival rate (p = 0.312) (Figure 6). There was a negative correlation r = –0.21. 3.5. Maximal Cytoreductive Surgery The distribution amongst cohorts in terms of definition of maximal debulking was as follows: <2 cm in 46.2% and <1 cm in 53.8%. The mean percentage of maximal cytoreductive surgery for all cohorts was 69.7%, with a range fro m 36.3% to 10 0%. A statistical significance was found between percentage maximal cytoreductive sur- ger y and the o verall s urvival rate (p = 0.046) (Figure 7). On further analysis it showed that for every 10% increase in % maximal cytoreductive surgery there was a 2.28 month increase in the overall survival rate. There was a strong positive Pearson correlation r = +0.40. 3.6. Lymphadenectomy Lymphade necto my was recorded in 12 of the 26 cohorts.  M. J. Farquharson et al . / Open Journal of Obstetrics and Gynecology 1 (2011) 71-83 Copyright © 2011 Sci Res. OJOG Figure 5. Survival time against year of publication. Figure 6. Survival time agai nst median age of patien ts. Figure 7. Survival time against % maximal cytoreduction achieved. In the remaining 14 cohorts it was unclear whether lym- phadenectomy was performed or not as part of the de- bulking s urgery. The mean percentage of lymphadenectomy performed among the 12 cohorts was 55%, with a range from 0% to 91.6%. No statistical significance was found between the percentage lymphadenectomy performed and the overall survival rate (p = 0.813) (Figure 8). There was a weak positive correlation r = +0.07. 3.7. Comparison between Centres The cohort studies were from a broad range of countries from Europe, Asia and North America. Twelve of the cohorts were from Europe, 7 were from Asia, 6 were from North America and the recent randomised control trial from Vergote et al. [4] was an international paper. The mean o f the o ver all s urvi val r ate was 3 1.9 month s in Figure 8. Survival time against % of Lymphadenectomy performed among the cohorts. Europe, 29.8 months in Asia and 35.6 months in North America (Figure 9). The percentage of maximal cytoreduction achieved between the centres was 72.4% in Europe, 61.6% in Asia and 71.8% in North America (F igure 10). Lymphadenectomy was performed in 7 of the 12 Euro- pean cohorts (58%), in 3 of the 7 Asian cohorts (43%) and in 1 of the 6 North American cohorts (17%). 3.8. Bowel Resection and Ultra Radical Surgery The percentage of bowel resections performed among the cohorts ranged from 0% - 36.1%. Sixteen of the 26 co- horts recorded whether bowel resection was performed or not. Two studies differentiated bowel resection as large or small bowel and one study identified the site of resection as a rectosigmoid resection. The mean bowel resection rate was 9.8% between cohorts. Further analysis showed t hat for every 10% increase in percentage bowel surgery there was a decrease of 1.4 months in the overall survival rate. There was no statis- tically significant relationship found between the median overall survival rate and percentage bowel surgery per- formed (p = 0.606). There was a weak correlation r = –0.14. The mean percentage maxima l cyto reduct ion ac hieve d among the cohorts that performed bowel surgery was 74.2% compared to the remaining cohorts that did not mention or perform bowel surgery of 62.5%. There was a higher percentage of Stage IV disease of 28.7% in the cohorts that performed bowel surgery compared to 16.7% in those cohorts that did not perform the surgery. Nine of the 26 cohorts mentioned if ultra radical de- bulking surgery was performed. Six of the nine studies actually performed ultra radical surgery. A variety of pro- cedures were performed amongst the cohorts including splenectomy, cholecystectomy, diaphragm stripping, per- manent enterostomy, bladder resection, liver resection, appendicectomy and pancreatectomy. One study just mentioned upper abdominal surgical procedures and did not specify the individual procedures. The rate of sple- nectomies performed ran ged from 0% - 5.6%, with a mean 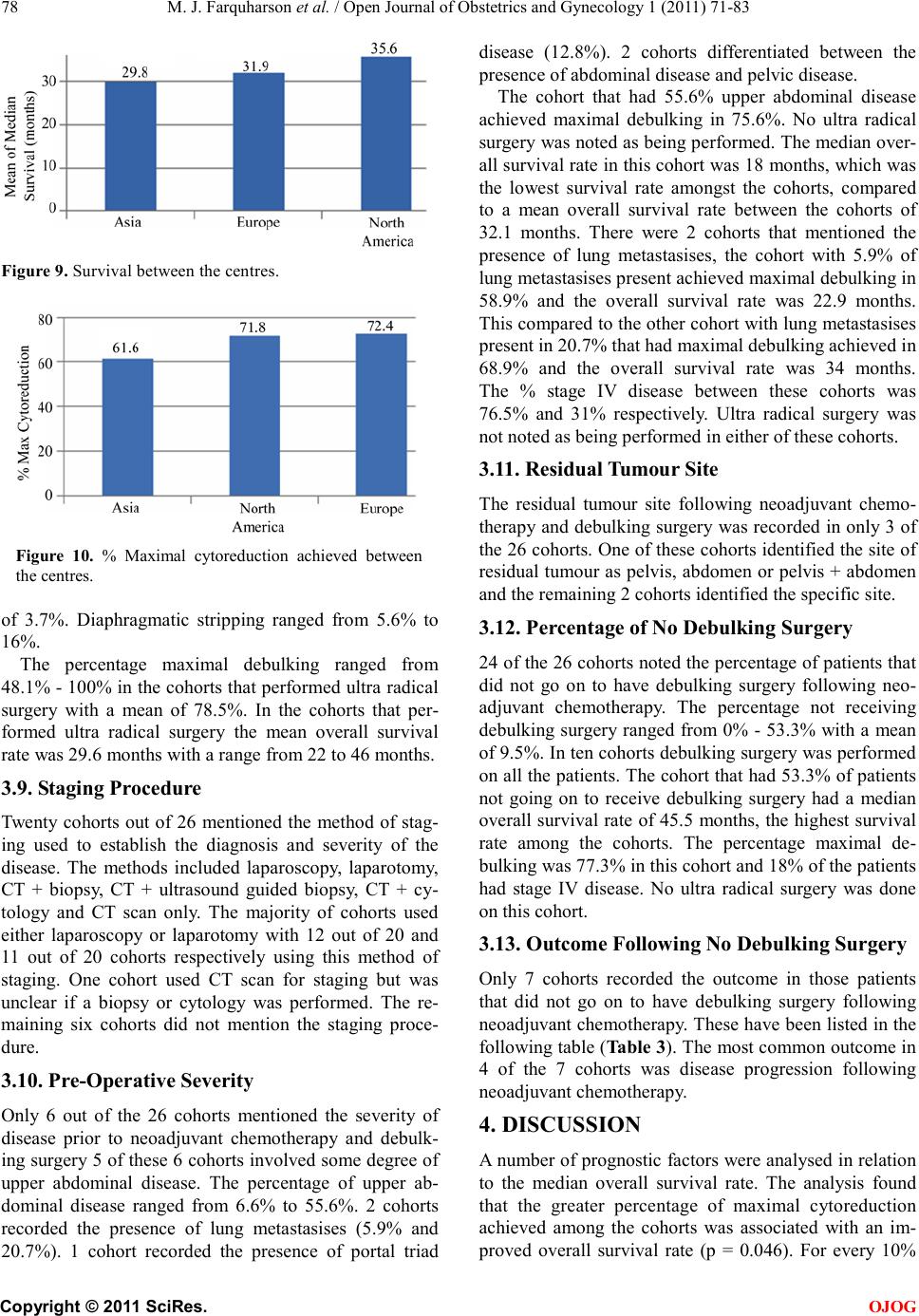 M. J. Farquharson et al . / Open Journal of Obstetrics and Gynecology 1 (2011) 71-83 Copyright © 2011 Sci Res. OJOG Figure 9. Survival between the centres. Figure 10. % Maximal cytoreduction achieved between the cen tres. of 3.7%. Diaphragmatic stripping ranged from 5.6% to 16%. The percentage maximal debulking ranged from 48.1% - 100% in the cohorts that performed ultra radical surgery with a mean of 78.5%. In the cohorts that per- formed ultra radical surgery the mean overall survival rate was 29.6 months with a range from 22 to 46 months. 3.9. St aging Procedure Twenty cohorts out of 26 mentioned the method of stag- ing used to establish the diagnosis and severity of the disease. The methods included laparoscopy, laparotomy, CT + biopsy, CT + ultrasound guided biopsy, CT + cy- tology and CT scan only. The majority of cohorts used either laparoscopy or laparotomy with 12 out of 20 and 11 out of 20 cohorts respectively using this method of staging. One cohort used CT scan for staging but was unclear if a biopsy or cytology was performed. The re- maining six cohorts did not mention the staging proce- dure. 3.10. Pre -Operative Severity Only 6 out of the 26 cohorts mentioned the severity of disease prior to neoadjuvant chemotherapy and debulk- ing surgery 5 of these 6 cohorts involved some degree of upper abdominal disease. The percentage of upper ab- dominal disease ranged from 6.6% to 55.6%. 2 cohorts recorded the presence of lung metastasises (5.9% and 20.7%). 1 cohort recorded the presence of portal triad disease (12.8%). 2 cohorts differentiated between the presence of abdominal disease and pelvic disease. The cohort that had 55.6% upper abdominal disease achieved maximal debulking in 75.6%. No ultra radical surger y was note d as b ei ng pe rfo r med. T he median o ve r- all survival ra te in thi s cohort was 18 mon ths, whi ch wa s the lowest survival rate amongst the cohorts, compared to a mean overall survival rate between the cohorts of 32.1 months. There were 2 cohorts that mentioned the presence of lung metastasises, the cohort with 5.9% of lung met a stasi se s p re se nt a c hi e ve d ma xi mal de b ul ki n g i n 58.9% and the overall survival rate was 22.9 months. This co mpared to the other cohort with lung metasta sises present in 20.7 % that had maximal debulking achieved in 68.9% and the overall survival rate was 34 months. The % stage IV disease between these cohorts was 76.5% and 31% respectively. Ultra radical surgery was not noted as being performed in either of these cohorts. 3.11. Residual Tumour Site The residual tumour site following neoadjuvant chemo- therapy and debulking surgery was recorded in only 3 of the 26 cohor ts. One of these cohorts ide ntified the site of residual tumour as pelvis, abdomen or pelvis + abdomen and the remaining 2 cohor ts id e ntified the specific s ite . 3.12. Percentage of No Debulking Surgery 24 of the 26 cohorts noted the percentage of patients that did not go on to have debulking surgery following neo- adjuvant chemotherapy. The percentage not receiving debulking surgery ranged from 0% - 53.3% with a mean of 9. 5%. In ten c oho rts d eb ulking surge ry was performed on all the patie nts. The cohor t that had 53.3 % of patients not going on to receive debulking surgery had a median over all survi val rate of 45 .5 mont hs, the highest survi val rate among the cohorts. The percentage maximal de- bulking was 77.3% in this cohort and 18% of the patients had stage IV disease. No ultra radical surgery was done on this cohort. 3.13. Outcome Following No Debulking Surgery Only 7 cohorts recorded the outcome in those patients that did not go on to have debulking surgery following neoadjuvant che mot herap y. T hese ha ve bee n listed in the following table ( Table 3). The most common outcome in 4 of the 7 cohorts was disease progression following neoa djuva nt chemothe rapy. 4. DISCUSS IO N A n umber of prognostic factors were analysed in relation to the median overall survival rate. The analysis found that the greater percentage of maximal cytoreductio n achieved among the cohorts was associated with an im- proved overall survival rate (p = 0.046). For every 10%  M. J. Farquharson et al . / Open Journal of Obstetrics and Gynecology 1 (2011) 71-83 Copyright © 2011 Sci Res. OJOG Table 3. Ou t co me s following no debulking surgery. Author Outcome following no debulking sur gery Ansquer [13] 4 pati en ts di ed before 3 cycles o f chemo Kuhn [ 15 ] 1 patient had a PE Ushijima [17] 2 patients did not receive c hemo due to poor prognosis, 18 received chemo, 3 refused surgery - all died Ma zzeo [19 ] 1 patient had IHD, 1 refused surgery + died, 3 had d isease progr ession Loizzi [21] 5 patients had dis ease progressi on + died within 6 month s Hou [30] 1 patient due to co -morbidities - remained disease free, 1 had dis ease progression and died within 9 months Ghaemmaghami [33] 4 patients had disease progression increase in percentage optimal debulking there was a 2.28 month increase in the survival rate. This is consis- tent with a number of studies and it is now commonly accepted that optimal debulking surgery is the single best prognostic indicator for overall survival. The addition of taxane to the chemotherapy regime was also found to improve the overall survival (p = 0.019). For every 10% increase in taxane use there was a 1.27 month increase in the overall survival rate. However there was no association between the number of chemo- therapy cycles and survival rates which differed from a pre vious me ta -anal ysi s b y B ri s to w et a l. that found a sig- nificant association [37]. The percentage stage IV disease did show the expected decrease in the overall survival rate with an increase in the percentage stage IV disease but this was not a signi- ficant trend. This may have been because the remaining patients had e xte nsive sta ge III C disea se whic h is a ssoci - ated with a poor survival rate and therefore survival rates between stage I IIC and IV may not be dissimilar. For every year increase in publication there was a sta- tistically significant increase in the overall survival rate (1.46 months). The mean percentage maximal cytoreduc- tion was similar between 2000-2005 and 2006-2010 (69.5% and 69.8%) but the definition of optimal debulk- ing as <1 cm became much more acceptable. With 38% of cohorts defining optimal debulking as <1 cm between 2000-2005 and 69% between 2006-2010. This change in definition of optimal debulking combined with similar cytoreduction rates is a possible reason for the improved survival ra t es over time. The mean of the median age was 60.4 years among the cohorts. There was a negative correlation (r = –0.21) be- tween age a nd the overall survival rate but not a statisti- cally significant association (p = 0.312). A more useful meas ureme nt woul d ha ve bee n the perfo rmanc e stat us o f patients as this is more likely to be associated with a poor survival rate than increased age. In comparing centres, there was a better overall sur- vival rate in North America (35.6 months) than both Europe (31.9 months) and Asia (29.8 months). The per- centage optimal cytoreduction achieved was similar in Europe (72.4%) and North America (71.8%) but much lower in Asia (61.6%). This would be a possible explana- tion for the improved survival rates in North America and Europe compared to Asia but it is difficult to com- ment further as there were relatively few cohorts and not an equal distribution betwee n re gio ns. A wide va r ie t y of sta gi ng p roc edures were used among the cohorts. Laparoscopy and laparotomy were the most common procedures performed with 14 out of the 20 cohorts using either procedure. Pre-operative severity and residual tumour site were poorly recorded among the cohorts with only 6 of the 26 cohorts and 3 of the 26 cohorts respectively recording the relevant data. I t is therefore d ifficult to analyse due to the limited data. There was a wide range of upper abdominal disease pre-operatively (6.6% - 55.6%) and the cohort with 55.6% of abdominal disease was associated with the lowest overall survival rate of 18 months. Resid ual tu mour s ite follo wi ng d ebul kin g su rgery can- not be reliably analysed as so few cohorts recorded this information. It would have been interesting to compare the pre-operative severity with both the optimal debulk- ing achieved and the overall survival rate. Also to be able to correlate the cohorts that had a poor optimal debulking percentage with the site of residual tumour. We know that upper abdominal disease is associated with a re- duced likelihood of achieving complete cytoreduction. The percentage of patients who did not go on to have debulking surgery ranged from 0% - 53.3%. The reason why debulking surgery was not performed was not rou- tinely mentioned among the cohorts. The cohort with 53.3% of patients not goi ng o n t o ha ve d e b ulki ng s urgery had a high ove rall surv ival rat e o f 45.5 months. Ho wever this may have been because 77.3% in the cohort achie- ved maximal debulking and only 18% had stage IV dis- ease. The outcome of patients who had no debulking surgery was poorly recorded with only 7 cohorts noting the outcome. The percentage of bowel resection performed among the cohorts did not correspond to an increase in survival  M. J. Farquharson et al . / Open Journal of Obstetrics and Gynecology 1 (2011) 71-83 Copyright © 2011 Sci Res. OJOG rates. There was in fact a decrease in the survival rate of 1.4 months for every 10% increase in bowel resection but this was not statistically significant (p = 0.606). However in the cohorts that performed bowel resections there was a greater percentage of optimal debulking achieved (74.2%) compared to the cohorts that did not perform bowel surgery (62.5%). There was also a much higher percentage of Stage IV disease amo ng the c ohor ts that performed bowel surgery (28.7%) compared to those that did not (16.7%). The greater percentage of Stage IV disease in some cohorts meant that there were a greater number of patients with widespread and extensive dis- ease. Bowel resection may have been necessary in some cohorts more than others in an effort to achieve optimal debulking. This may be a possible explanation for why there was a slight decrease in the survival rate in those cohorts that performed bowel surgery as a greater num- ber of patients had extensive disease before debulking surgery was attempted. It is also unclear whether a spe- cialist or a general surgeon performed the bowel surgery within the cohorts. Most experts believe that the less residual disease pre- sent a fter surgery the better the overall survival rate. The question still remains how radical and aggressive should surgeons be in an effort to achieve ‘optimal’ debulking. If optimal cytoreduction is achieved this does not how- ever mean the patient will have a similar survival rate than a patient who initially had disease less than 1cm without cytoreduction. This is supported by Hoskins et al. who showed that patients with large volume disease with optimal debulking did not have a similar survival rate to those patients who presented with small volume disease [38]. It has been suggested that large volume disease may have a more aggressive pathology than small volume disease and this requires further study. In this meta-analysis, ultra radical surgery was per- formed in six cohorts in an effort to achieve optimal de- bulking. Only 9 cohorts recorded if ultra radical surgery was performed or not. A wide range of procedures were performed including splenectomies, liver resections and pancreatectomies. The percentage achieving maximal debulking among the cohorts that performed ultra radical surgery ranged from 48.1% to 100% with a mean of 78.5%. The mean over all s urvival r ate a mong the coho rts tha t had ultra radical surgery was 29.6 months which is below the overall mean of all cohorts of 32.1 months. The range of overall survival rates among these cohorts was 22 - 46 months. The reason for the one study that had a survival rate of 46 months was unclear as no patient had a sple- nectomy, pancreatectomy or liver resectio n in this coho rt however optimal debulking was achieved in 95.3% of patients. The overall survival rate of 29.6 months could be less than the mean of all the cohorts as the effort to achieve optimal debulking may be associated with ex- tensi ve mor b idity. T he post-operative complications were not recorded from the cohorts and this would have been useful to compare morbidity associate d with ultra radica l surgery and lymphadenectomy. The data regarding ultra radical surgery is however difficult to interpret and ana- lyse as it was performed in so few studies and further research needs to be done to establish any benefit. This meta-analysis has not looked at the use of CA- 125 monitoring in terms of resectability and prognosis, however a number of studies have suggested it may be a useful indicator. It is widel y accepted that C A-125 levels decrease during first line che motherap y and is associated with a better pr ognosis [39]. A st udy by Bilici et a l. [36] showed that following neoadjuvant chemotherapy, a sta- tistically significant decrease in CA-125 levels was de- termined and this is consistent with similar studies fol- lowing ad juvant che motherapy. CA-125 levels have failed to be precise and reliable in determining the resectability of advanced ovarian cancer. Ho weve r st ud ies ha ve s hown t hat a CA -125 that does not normalise following neoadjuvant chemotherapy is asso- ciated with a worse outcome. An alternative more reli- able method may be the use of laparoscopy routinely to establish the severity of disease prior to undertaking de- bulking s urgery. CT scan criteria for determining the resectability of the tumour has been reported to be a reliable indicator. Qay- yum et el found that pre-operative CT and MRI were equally accurate in detecting inoperable disease with a positive predictive value of 94% and a negative predic- tive value of 96% [40]. There is however no uniformly accepted CT scan criteria for predicting resec ta bility. The ascites volume has been shown by Kuhn et al. [15] to have an influence on the survival rate and tumour re- sectability. The ascites volume is often associated with extensive and diffuse peritoneal disease and the mea- surement of ascites volume alongside other investiga- tions may well be useful in the prediction of tumour re- sectability. This meta-analysis did not show a statistical signifi- cant association between lymphadenectomy and the overall survival rate (p = 0.813) however less than half the cohorts recorded whether lymphadenectomy was per- formed or not. This was consi stent wit h a RCT by Pa nici et al. that noted an improved progression free survival but no t ove rall sur vi val i n wome n fol lo win g a systematic lymphadenectomy [41]. A much reported hypothesis is that lymph node invol- vement in ovarian cancer is chemoresistant and is often termed a ‘sanctuary’ site with regards to its response to chemotherapy. A possible explanation for this resistance  M. J. Farquharson et al . / Open Journal of Obstetrics and Gynecology 1 (2011) 71-83 Copyright © 2011 Sci Res. OJOG from Eisenkop et al. is that retroperitoneal nodal metas- tases have a reduced blood supply compared to intra- abdominal disease and this results in lower levels of cy- totoxic age nts re ach ing t he no d es [4 2] . It is unclear if the poor prognosis of patients with positive lymph nodes is due to the tumour’s aggressiveness or that nodal in- volvement is resistant to che motherapy [43]. Lymphadenectomy is commonly performed outside the UK however wi thi n t he U K very fe w ce ntr e s p er form the procedure routinely. The recent pre-publication from the National Institute for Health and Clinical Excellence (NICE) (Guideline February 2011) does not mention the role of systematic lymphadenectomy in advanced ova- rian cancer which suggests it may be a while before the procedure becomes common practice within the UK [44]. The recent study by Vergote et al. [4] in September 2011 was the first randomised control trial comparing primary debulking surgery followed by chemotherapy versus neoadjuvant chemotherapy followed by debulking surgery. The study concluded that neoadjuvant chemo- therapy followed by interval debulking was not inferior to the standard primary debulking surgery in the treat- ment of advanced ovarian cancer. However both groups had little or no upp er abdominal surgery performed. This may have been a coincidence with relatively few patients requiring the surgery or due to the aggressiveness of the debulking surgery. Either way this will have influenced the overall survival rate compared to other studies where there are a higher percentage of upper abdominal surgery often associated with a poorer prognosis. The study also had a wide variability between the rate of optimal and complete cytoreduction. With the range for complete cytoreduction at primary debulking be- tween 3.9% and 62.9%. This again would be expected to influence the overall survival rate. The number of cohorts included in this meta-analysis made a multiple linear regression analysis difficult so there was the potentia l for interactio ns between variables. There will have been a degree of selection bias but there was a strict inclusion criteria. However the criteria for neoadjuvant chemotherapy administration varied across the cohorts with some studies using CT imaging and other cohorts using laparoscopy or laparotomy to assess the disease severity and need for chemotherapy. The studies included in the meta-analysis were not all of the highest quality. There was only one randomised control study done on the subject and therefore a system- atic review including only randomised control trials was not possible. Additional prognostic factors that may have influ- enced survival rates were not examined and these in- cluded preoperative CA-125 levels, ascitic volume and performance status. In further studies it would be inter- esting to record post-operative co mplication s withi n eac h cohort to compare whether the aggressiveness of debulk- ing surgery is associated with additional morbid ity. This meta-analysis has shown a number of important prognostic factors that influence survival rates in patients that have had neoadjuvant chemotherapy followed by debulking surgery. The recent RCT by Vergote et al. has shown that neoadjuvant chemotherapy is not inferior to the traditional primary cytoreduction. Therefore it is likely that neoadjuvant chemotherapy will have an in- creasing role in the future management of advanced ovarian cancer. The results from the CHORUS trial are awaited to see if they support these findings. Further research will need to address the issue of identifyi ng pa- tients that are suitable for neoadjuvant chemotherapy or primary cytoreduction. This may be a combination of CT findings, pre-operative CA-125 levels, ascitic volume or performance status that can predict the resectability of the disease. 5. CONTRIBUTION TO AUTHORSHIP MJF is the first and main author of the manuscript. MJF conceived and designed the manuscript and analysed the data. NAS contributed to interpretation of the data and assisted in the preparation of the article. 6. DETAILS OF ETHICS APPROVAL/FUNDIN G This is a review of the literature, so no ethical approval was required. No funding source was involved. 7. ACKNOWLEDGEMENTS This paper was submitted as part of an e-dissertation in May 2010 for the Edinburgh Surgical Sciences Qualification (ESSQ). The ESSQ is a 3 year distance learning programme leading to a Masters in Surgical Sciences (HHUUwww.essq.rcsed.ac.ukUUHH). REFERENCES [1] P o mel , C., Barton, D.P.J., McNeish, I. and Shepherd, J. (2008) A statement for extensive primary cytoreductive surgery in advanced ovarian cancer. An International Journal of Obstetrics and Gynaecology, 115, 808-810. HHUUdoi:10.1111/j.1471-0528.2008.01692.xUU [2] Hariprasad, R., Kumar, L. and Kapoor, A. (2007) Neoadjuvant chemotherapy for advanced ovarian cancer- editorial. Indian Journal of Medical & Paediatric Oncology, 28, 4-5. [3] Scottish Intercollegiate Guidelines Network (SIGN) (2003) Epithelial ovarian cancer, Clinical Guideli ne 75. [4] Vergot e, I., Trope, C.G. and Armant, F. (2010) Neoad- juvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. The New England Journal of Medi- cine, 363, 943-953. HHUUdoi:10.1056/NEJMoa0908806UU [5] Allen, D.G., Heintz, A.P. and Touw, F.W. (1995) A  M. J. Farquharson et al . / Open Journal of Obstetrics and Gynecology 1 (2011) 71-83 Copyright © 2011 Sci Res. OJOG meta-anal ysis of residual diseas e and survival in st age III and IV carcino ma of th e ovar y. European Journal of Gyna- ecologic Oncology, 16, 349-356. [6] Morrison, J., Swanton, A., Collins, S. and Kehoe, S. (2007) Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Data- base of Systemat ic Reviews, 4, CD005343. HHUUdoi:10.1002/14 651858.CD005343.pub2UU [7] V enesmaa, P. and Ylikorkala, O. (1992) Morbidity and Mortality associated with primary and repeat operations for ovarian cancer. Obstetrics & Gynecology, 79, 168- 172. [8] Gerest ein, C.G., Damhuis, R.A., Burger, C.W. and Kooi, G.S. (2009) Postoperative mortality after primary cytor- eductive surgery for advanced stage epithelial ovarian cancer: A systematic review. Gynecologic Oncology, 114, 523-527. HHUUdoi:10.1016/j.ygyno.2009.03.011UU [9] Aletti, G.D., Long, H.J., Podratz, K.C. and Cliby, W.A. (2007) Is time to chemotherapy a determinant of prog- nosis in advancedstage ovarian cancer? Gynecolog ic Oncology, 104, 212-216. HHUUdoi:10.1016/j.ygyno.2006.07.045UU [10] Gadducci, A., Sartori, E., Landoni, F., et al. (2005) Rel- ationship between time interval from primary surgery to the start of taxaneplus platinum-based chemotherapy and clinical outcome of patients with advanced epithelial ovarian cancer: Result s of a multicenter retrospective Ita- lian study. Journal of Clinical Onc ology, 23, 751-758. HHUUdoi:10.1200/JCO.2005.03.065UU [11] Chan, Y.M., Ng, T.Y., Ngan , H.Y. and Wong, L.C. (2003) Quality of life in women treated with neoadjuvant chem- oth erapy for advanced o varian cancer: A prospecti ve lon- gitudinal study. Gynecologic Oncology, 88, 9-16. HHUUdoi:10.1006/gyno.2002.6849UU [12] Moher, D., Liber ati , A., Tetzlaff, J. and Altman, D.G. (2009) PRISMA group. preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. British Medicine Journal, 339, b253 5. HHUUdoi:10.1136/bmj.b2535UU [13] Ansquer, Y., Leblanc, E., Clough, K., Morice, P., Daupl at, J., Math ev et, P., et al. (2001) Neoadjuvant chemotherap y for unresectable ovarian carcinoma. Cance r, 91, 2329-2334. HHUUdoi:10.1002/10 97-0142(20010615)91:12<2329: :AID-CN CR1265>3.0.CO;2-UUU [14] Kayikciog, F., Kose, M.F., Boran, N., Caliskan, E. and Tulunay, G. (2001) Neoadjuvant chemotherapy or primary surgery in advanced epithelial ovarian carcinoma. Inter- national Jour nal of Gynecological Cancer, 11, 466-470. HHUUdoi:10.1046/j.1525-1438.2001.01064.xUU [15] Kuhn, W., R ut ke, S., Späthe, K., Schmalfeld t, B., Flo rack, G. and Von, H.B. (2001 ) Neoadjuvant chemotherapy followed by tumor debulking prolongs survival for pa- tients with poor prognosis in International Federation of Gynecolo gy and Obst etrics stage IIIC ovarian carcin oma. Cancer, 92, 2585-2591. HHUUdoi:10.1002/10 97-0142(20011115)92:10<2585: :AID-CN CR1611>3.0.CO;2-#UU [16] Vrščaj, M.U. and Rakar, S. (2002) Neoadjuvant chemo - therapy for advanced epithelial ovarian carcinoma: A retrospective case-control study. European Journal of Gynaecolocal Oncology, 23, 405-410. [17] Ushijima, K., Ota, S., Komai, K., Matsuo, G., Motoshima, S., Honda, S., et al. (2002) Clinical assessment of neo a- djuvant chemotherapy and interval cytoreductive surgery for unresectable advanced ovarian cancer. International Surgery, 87, 185-190. [18] Morice, P., Dubernard, G., Rey, A., Atallah, D., Pautier, P., Po mel , C., et al. (2003) Results of interval debulking surgery compared with primary debulking surgery in advanced stage ovarian cancer. Journal of the American Colloge of Surgeons, 197, 955-963. HHUUdoi:10.1016/j.jamcollsurg.2003.06.004UU [19] Mazzeo, F., Berlière, M., Kerger, J., Squifflet, J., Duck, L., D’Hondt, V., et al. (2005) Neoadjuvant chemoth- erapy in patients with primarily unresectable, advanced- stage ovarian cancer. Gynecolocal Oncology, 23, 7445- 7453. [20] Fanfani, F., Ferrandina, G., Corrado, G., Fagotti, A., Zahut, H.V., Mancuso, S., et al. (2002) Impact o f interval debulking surgery on clinical outcome in primary unresectable FIGO stage IIIC ovarian cancer patients. Oncology, 65, 316-322. HHUUdoi:10.1159/000074644UU [21] Loi z zi, V., Cormi o, G., Rossi, C.A., Di, G.A.R., Cucco vill o, A. and Selvaggi, L. (2005) Neoadjuvant chemotherapy in advanced ovarian cancer: A cas e-control study. International Journal of Gyne cological Cancer, 15, 217-223. HHUUdoi:10.1111/j.1525-1438.2005.15206.xUU [22] Le, T., Faught, W., Hopkins, L. and Fung, M.F.K. (2005) Primary chemotherapy and adjuvant tumor debulking in the management of advanced-stage epithelial ovarian cancer. International Journal of Gyneco log ical Can ce r, 15, 770-775. HHUUdoi:10.1111/j.1525-1438.2005.00134.xUU [23] Hegazy, M.A.F., Hegazi, R.A.F., Elshafei, M.A., Setit, A.E., Elshamy, M.R., Eltatoongy, M., et al. (2005) Neoa- djuvant chemotherapy versus primary surgery in advan- ced ovarian carcinoma. World Journal of Surgery Oncology, 3, 57-64. doi:10.1186/1477-7819-3-57UU [24] Avril, N., Sassen, S., Schmalfeldt, B., Naehrig, J., Rutke , S., Weber, W.A., et al. (2005) Prediction of response to neoadjuvant chemotherapy by sequential F-18-fluor- odeoxyglucose positron emission tomography in patients with advancedstage ovarian cancer. Jouanl of Clinical Oncology, 23, 7445-7453. HHUUdoi:10.1200/JCO.2005.06.965UU [25] Everett, E.N., French, A.E., Stone, R.L., Pastore, L.M., Jazaeri, A.A., Anderson, W.A., et al. (2006) Initial chemotherapy followed by surgical cytoreduction for the treatment of stage III/IV epithelial ovarian cancer. Am- erican Jouranl of Obstetrics and Gynecol ogy, 195, 568- 576. HHUUdoi:10.1016/j.ajog.2006.03.075UU [26] Lee, S., Kim, B., Lee, J., Park, C., Lee, J. and B ae, D. (2006) Preliminary results of neoadjuvant chemotherapy with paclitaxel and cisplatin in patients with advanced epith eli al o varian cancer who are i nad equat e for o pt imu m primary surgery. Journal of Obstetrics and Gynecology, 32, 99-106. [27] Inciura, A., Simavicius, A., Juozaityte, E., Kurtinaitis, J., Nadi sau sk iene, R., S ved as, E., et al. (2006) Comparison of adjuvant and neoadjuvant chemotherapy in the man- agement of advanced ovarian cancer: A retrospective study of 574 pa tients. Biomed Central, 6, 153. HHUUdoi:10.1186/14 71-2407-6-153UU [28] Deo, S.V.S., Goyal, H., Shukla, N.K., Rain a, V., Kuma r, L.,  M. J. Farquharson et al . / Open Journal of Obstetrics and Gynecology 1 (2011) 71-83 Copyright © 2011 Sci Res. OJOG Srinivas, G., et al. (2006) Neoadjuvant chemotherapy fol- lowed by surgical cytoreduction in advanced epithelial ova- rian cancer. Indian Journal of Cancer, 43, 117-121. HHUUdoi:10.4103/00 19-509X.27933UU [29] Le, T., Alshaikh, G., Hopkins, L., Faught, W. and Fung, M.F.K. (2006) Prognostic significance of postoperative morbidities in patients with advanced epithelial ovarian cancer treated with neoadjuvant chemotherapy and delayed primary surgical debulking. Annuals of Surggical Oncology, 13, 1711-1716. HHUUdoi:10.1245/s10434-006-9125-6UU [30] Hou, J.Y., Kelly, M.G., Yu, H., McAlpine, J.N., Azodi, M., Rut herford, T.J., et al. (2007) Neoadjuvant chemo- therapy lessens surgical morbidity in advanced ovarian cancer and leads to improved survival in stage IV disease. Gynecological Oncology, 105, 211-217. HHUUdoi:10.1016/j.ygyno.2006.11.025UU [31] Rafi i, A., Deval, B., Geay, J.F., Chopin, N. and Paoletti, X., P ar aiso, D., et al. (2007) Treatment of FI GO stage IV ovarian carcinoma: Results of primary surgery or interval surgery after neoadjuvant chemotherapy: A retrospective study. International Journal of Gynecological Cancer, 17, 777-783. HHUUdoi:10.1111/j.1525-1438.2007.00905.xUU [32] Rosa, D.D., Ton, N.C., Clamp, A., Mullamitha, S., Lau, S., Clayton, R., et al. (2007) The neoadjuvant approach in the treat ment of patien ts with ad vanced epit helial ovarian carcinoma. Clinical Oncology, 19, 125-128. HHUUdoi:10.1016/j.clon.2006.11.015UU [3 3] Ghaemmaghami, F., Kari mi-Zar chi , M., Modares-Gilan i, M., Mousavi, A. and Behtash, N. (2008) Clinical outcome of Iranian patients with advanced ovarian cancer with neoa- djuvant chemotherapy versus primary debulking surgery. Asian Pacific Journal of Cancer Prevention , 9, 719-724. [34] Supraser t, P., Tiyanon, J. and Kietpeerakool, C. (2008) Outcome of interval debulking in advanced ovarian cancer patients. Asian Pacific Journal of Cancer Prev- ention, 9, 519-524. [35] Pölch er, M., Mahner , S., Ortman n, O., Hilfrich, J., Di edri ch, K., B r eitbach, G., et al. (2009) Neoadjuvant chemotherapy with docetaxel in advanced ovarian cancer—a prospective multicenter phase II trial (PRIM OVAR). Oncologu Reports, 22, 605-613. [36] Bilici, A., Salep ci, T., Dane, F., Gu mu s, M., Ustaalioglu, B.B.O., Seker, M., et al. (2010) Neoadjuvant chemo- therapy followed by interval cytoreductive surgery in patien ts with unresectable, advanced stage epitheli al ova- rian cancer: A single centre experience. Archives of Gy- necology and Obs tetics, 282, 417-425. HHUUdoi:10.1007/s00404-009-1330-7UU [37] Bristow, R.E. and Chi, D.S. (2006) Platinum-based neoa- djuvant chemotherapy and interval surgical cytor-eduction for advanced ovarian cancer: A meta-analysis. Gyneco- logical Oncology, 103, 1070-1076. HHUUdoi:10.1016/j.ygyno.2006.06.025UU [38] Hoskins, W.J., Bundy, B.N., Thigpen, J.T. and Omur a, G.A. (1992) The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: A gynaecologic on- cology group study. Gynecological Oncology, 47, 159- 166. HHUUdoi:10. 1016/0090-8258(92 )90100-WUU [39] Pecorelli, S., Odicino, F. and Favalli, G. (2002) Interval debulking surgery in advanced epithelial ovarian cancer. Best Pra ctice and Research Clin ic al Obstetrics and Gyn- aecology, 16, 573-583. HHUUdoi:10.1053/beog.2002.0302UU [40] Qa yyum, A., Coakley, F.V., Westphalen, A.C., Hricak, H., Okuno, W.T. and Powell, B. (2005) Role of CT and MR imaging in predicting optimal cytoreduction of newly diagnosed primary epithelial ovarian cancer. Gyneco- logical Oncology, 96, 301-306. HHUdoi:10.1016/j.ygyno.2004.06.054UU [41] Panici, P.B., Maggioni, A., Hacker, N., Landoni, F., Acker-Mann, S., Campagnutta, E., et al. (2005) S ys - tematic aortic and pelvic lymphadenectomy versus res- ection of bulky nodes only in optimally debulked ad- vanced o varian can cer: A ran domized clinical trial. Jour- nal of the National Cancer Inst itue, 97, 560-566. HHUUdoi:10.1093/jnci/dji102UU [42] Eisenkop, S.M. and Spirtos, N.M. (2001) The clinical significance of occult macroscopically positive retrop- eritoneal nodes in patients with epithelial ovarian cancer. Gynecol ogical Oncology, 82, 143-149. HHUUdoi:10.1006/gyno.2001.6232UU [43] Kim, H.S., Ju, W., Jee, B.C., Kim, Y.B., Park, N.H. and Song, Y.S. (2010) Systematic lymphadenectomy for survival in epithelial ovarian cancer: A meta-analysis. International Journal of Gynecological Cancer, 20, 520- 528. HHUUdoi:10.1111/IGC.0b013e3181d6de1dUU [44] National Institute for Health and Clinical Excellence (NICE) (2011) Ovarian cancer: Full guideline for pre- publication check.
|