 Vol.1, No.2, 63-92 (2009) Natural Science http://dx.doi.org/10.4236/ns.2009.12011 Copyright © 2009 SciRes. OPEN ACCESS REVIEW Recent advances in developing web-servers for predicting protein attributes* Kuo-Chen Chou1,2, Hong-Bin Shen1,2 1Gordon Life Science Institute, San Diego, California 92130, USA; kcchou@gordonlifescience.org 2Institute of Image Process & Pattern Recognition, Shanghai Jiaotong University, Shanghai, China Received 7 August 2009; revised 25 August 2009; accepted 28 August 2009. ABSTRACT Recent advance in large-scale genome se- quencing has generated a huge volume of pro- tein sequences. In order to timely utilize the in- formation hidden in these newly discovered sequences, it is highly desired to develop com- putational methods for efficiently identifying their various attributes because the information thus obtained will be very useful for both basic research and drug development. Particularly, it would be even more useful and welcome if a user-friendly web-server could be provided for each of these methods. In this minireview, a sy- stematic introduction is presented to highlight the development of these web-servers by our group during the last three years. Keywords: Cell-PLoc; Signal-CF; Signal-3L; MemType-2L; EzyPred; HIVcleave; GPCR-CA; ProtIdent; QuatIdent; FoldRate 1. INTRODUCTION Proteomics, or “protein-based genomics”, is the large- scale study of proteins. It was born due to the explosion of protein sequences generated in the post genomic era [1] as well as the necessity to understand the biological process at the cellular or system level. To effectively conduct studies in proteomics, it is highly desired to develop high throughput tools by which one can timely identify various attributes of pro- teins in a large-scale manner. For instance, given an uncharacterized protein se- quence, how can we identify which subcellular location site it resides at? Does the protein stay in a single sub- cellular location or can it simultaneously exist in or move between two and more subcellular locations? Which part of the protein is its signal sequence? Is it a membrane protein or non-membrane protein? If it is the former, to which membrane protein type does it belong? Is it an enzyme or non-enzyme? If the former, to which main functional class and sub-functional class does it belongs to? Is it a protease on non-protease? If it is the former, to which protease type does it belong? Which sites of the protein can be cleaved by proteases such as HIV protease and SARS enzyme? Is it a GPCR (G-pro- tein coupled receptor) or non-GPCR? If it is the former, to which type of GPCR does it belongs to? What kind of quaternary structure does it belong to? What kind of fold pattern does it assume? How can we estimate its folding rate? The list of questions is vast. Although the answers to these questions can be deter- mined by conducting various biochemical experiments, the approach of purely doing experiments is both time- consuming and costly. Consequently, the gap between the number of newly discovered protein sequences and the knowledge of their attributes is becoming increas- ingly wide. For instance, in 1986 the Swiss-Prot databank contained merely 3,939 protein sequence entries (Table 1), but the num- ber has since jumped to 428,650 according to version 57.0 of 24-Mar-2009 (www.ebi.ac.uk/swiss-prot), meaning that the number of protein sequence entries now is more than 108 times the number from about 23 years ago. The rapid increase in protein sequence entries is also shown by the Figure 1, where a statistical illustration to show the growth of the UniProtKB/ TrEMBL Protein Database (http://www.ebi.ac.uk/unipro t/TrEMBLstats/) is given. In order to use these newly found proteins for basic research and drug discovery in a timely manner, it is highly desired to bridge such a gap by developing effec- tive computational methods to predict their 3D (three- dimensional) structures [2,3] as well as various func- tion-related attributes based on their sequence informa- tion alone. * Part of the contents in this article was presented in Shanghai Univer- sit in June of 2009. 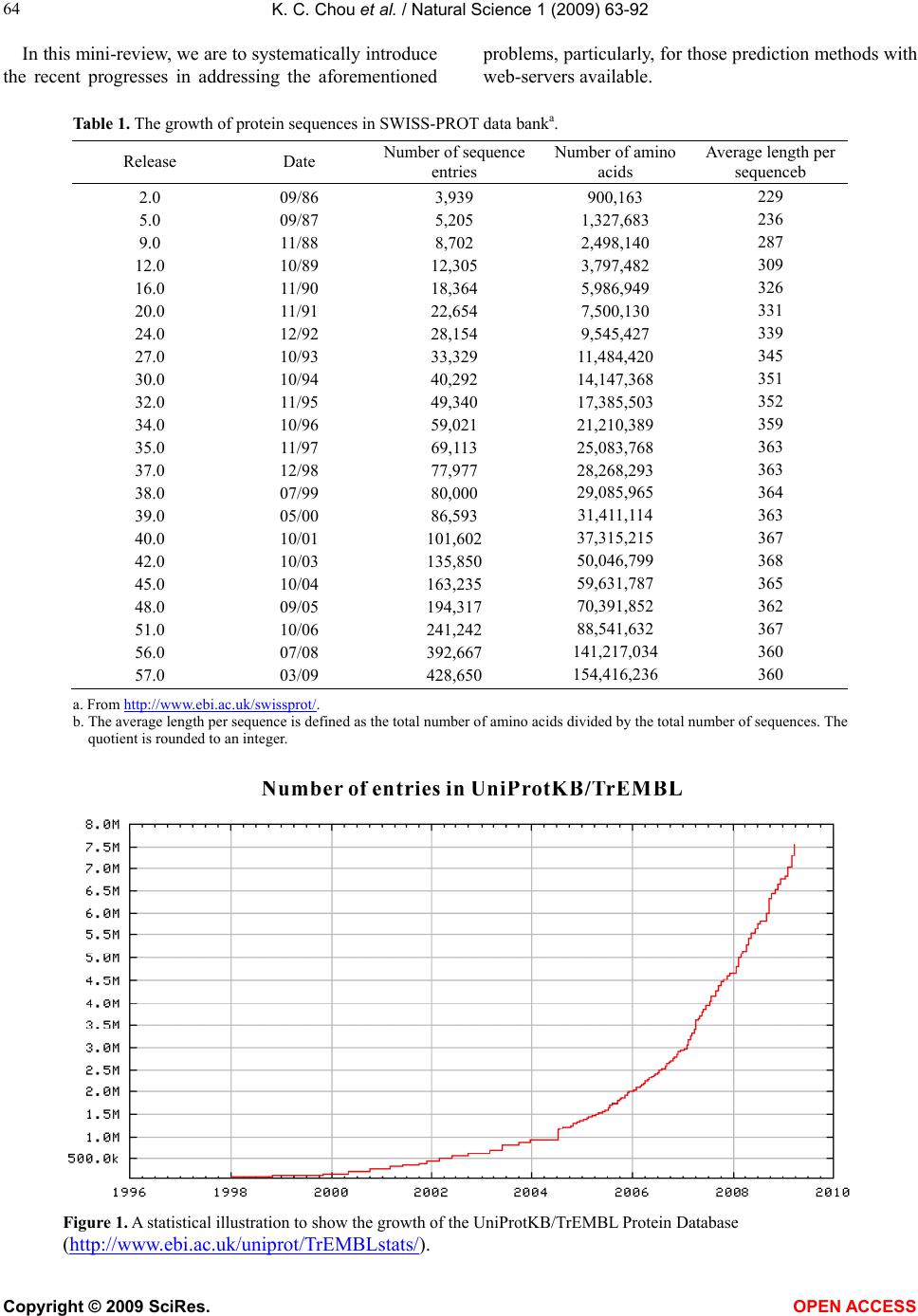 K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 64 In this mini-review, we are to systematically introduce the recent progresses in addressing the aforementioned problems, particularly, for those prediction methods with web-servers available. Table 1. The growth of protein sequences in SWISS-PROT data banka. Release Date Number of sequence entries Number of amino acids Average length per sequenceb 2.0 5.0 9.0 12.0 16.0 20.0 24.0 27.0 30.0 32.0 34.0 35.0 37.0 38.0 39.0 40.0 42.0 45.0 48.0 51.0 56.0 57.0 09/86 09/87 11/88 10/89 11/90 11/91 12/92 10/93 10/94 11/95 10/96 11/97 12/98 07/99 05/00 10/01 10/03 10/04 09/05 10/06 07/08 03/09 3,939 5,205 8,702 12,305 18,364 22,654 28,154 33,329 40,292 49,340 59,021 69,113 77,977 80,000 86,593 101,602 135,850 163,235 194,317 241,242 392,667 428,650 900,163 1,327,683 2,498,140 3,797,482 5,986,949 7,500,130 9,545,427 11,484,420 14,147,368 17,385,503 21,210,389 25,083,768 28,268,293 29,085,965 31,411,114 37,315,215 50,046,799 59,631,787 70,391,852 88,541,632 141,217,034 154,416,236 229 236 287 309 326 331 339 345 351 352 359 363 363 364 363 367 368 365 362 367 360 360 a. From http://www.ebi.ac.uk/swissprot/. b. The average length per sequence is defined as the total number of amino acids divided by the total number of sequences. The quotient is rounded to an integer. Figure 1. A statistical illustration to show the growth of the UniProtKB/TrEMBL Protein Database (http://www.ebi.ac.uk/uniprot/TrEMBLstats/). 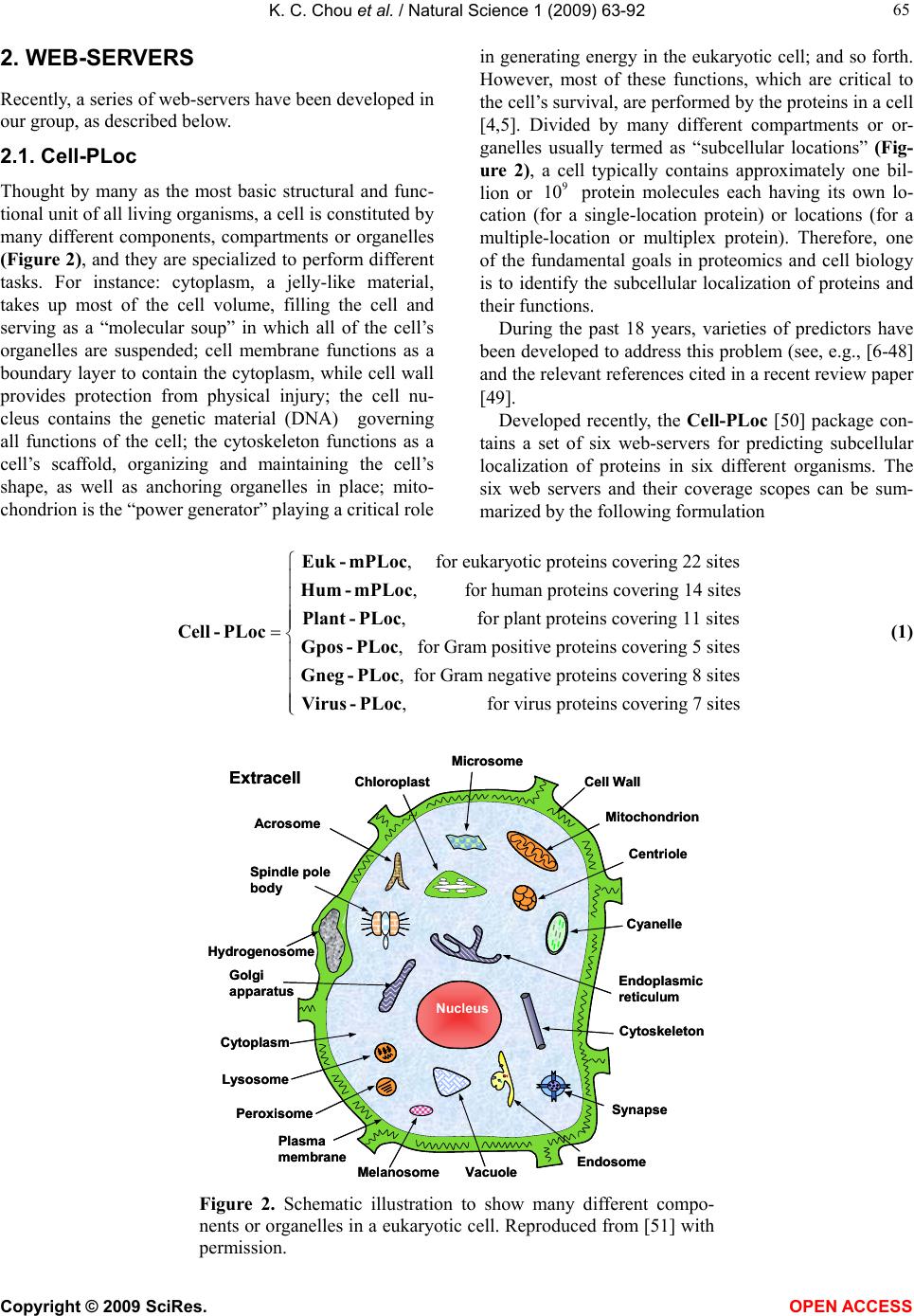 K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 65 2. WEB-SERVERS Recently, a series of web-servers have been developed in our group, as described below. 2.1. Cell-PLoc Thought by many as the most basic structural and func- tional unit of all living organisms, a cell is constituted by many different components, compartments or organelles (Figure 2), and they are specialized to perform different tasks. For instance: cytoplasm, a jelly-like material, takes up most of the cell volume, filling the cell and serving as a “molecular soup” in which all of the cell’s organelles are suspended; cell membrane functions as a boundary layer to contain the cytoplasm, while cell wall provides protection from physical injury; the cell nu- cleus contains the genetic material (DNA) governing all functions of the cell; the cytoskeleton functions as a cell’s scaffold, organizing and maintaining the cell’s shape, as well as anchoring organelles in place; mito- chondrion is the “power generator” playing a critical role in generating energy in the eukaryotic cell; and so forth. However, most of these functions, which are critical to the cell’s survival, are performed by the proteins in a cell [4,5]. Divided by many different compartments or or- ganelles usually termed as “subcellular locations” (Fig- ure 2), a cell typically contains approximately one bil- lion or 9 10 protein molecules each having its own lo- cation (for a single-location protein) or locations (for a multiple-location or multiplex protein). Therefore, one of the fundamental goals in proteomics and cell biology is to identify the subcellular localization of proteins and their functions. During the past 18 years, varieties of predictors have been developed to address this problem (see, e.g., [6-48] and the relevant references cited in a recent review paper [49]. Developed recently, the Cell-PLoc [50] package con- tains a set of six web-servers for predicting subcellular localization of proteins in six different organisms. The six web servers and their coverage scopes can be sum- marized by the following formulation , for eukaryotic proteins covering 22 sites , for human proteins covering 14 sites , for plant proteins covering 11 sites , f Euk- mPLoc Hum-mPLoc Plant - PLoc Cell - PLocGpos - PLocor Gram positive proteins covering 5 sites , for Gram negative proteins covering 8 sites , for virus proteins covering 7 sites Gneg - PLoc Virus - PLoc (1) Nucleus Plasma membrane Cytoplasm ChloroplastCell Wall Mitochondrion Endoplasmic reticulum Cytoskeleton Peroxisome Lysosome Golgi apparatus Centriole Ext rac ell Vacuole Cyanelle Hydrogenosome Spindle pole body Endosome Synapse Microsome Acrosome Melanosome NucleusNucleus Plasma membrane Cytoplasm ChloroplastCell Wall Mitochondrion Endoplasmic reticulum Cytoskeleton Peroxisome Lysosome Golgi apparatus Centriole Ext rac ell Vacuole Cyanelle Hydrogenosome Spindle pole body Endosome Synapse Microsome Acrosome Melanosome Figure 2. Schematic illustration to show many different compo- nents or organelles in a eukaryotic cell. Reproduced from [51] with permission.  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 66 where the character “m” in front of “PLoc” stands for “multiple”, meaning that the corresponding predictor can be used to deal with both single-location and multiple- location proteins. To use the web-server package, just do the following procedures. (1) Open the webpage http://chou.med.ha rvard.edu/b ioinf/Cell-PLoc/, and you will see the top page of the Cell-PLoc package [50] on your computer screen, as shown in Figure 3. (2) To pre- dict the subcellular localization of eukaryotic proteins, click the “Euk-mPLo c” button; to predict the subcellular localization of human proteins, click the “Hum-mP L o c ” button; to predict the subcellular localization of plant proteins, click the “Plant-PLoc” button; and so forth. (3) Now, you can follow the procedures (3) – (11) as de- scribed in [50] to get the desired results for the query proteins in the six different organisms. To maximize the convenience for the people working in the relevant areas, each of the six predictors in the Cell-PLoc package has been used to identify all the pro- tein entries in the corresponding organism (except those annotated with “fragment” or those with less than 50 amino acids) in the Swiss-Prot database that do not have subcellular location annotations or are annotated with uncertain terms such as “probable”, “potential”, “likely”, or “by similarity”. These large-scale predicted results can be directly downloaded by clicking the Download button after getting on the top page of each of the six web-servers. These results can serve two purposes: one is that they can be directly used by those who need the information immediately; the other is to set a preceding mark to examine the accuracy of these web-server pre- dictors by the future experimental results. For example, listed in Appendix A are 334 eukaryotic proteins. Their experimental annotated subcellular loca- tions were not available before Swiss-Prot 53.2 was re- leased on 26-June-2007. However, according to the large- scale predicted results by Euk-mPLoc that were submitted for publication on November-12-2006 as Supporting Infor- mation B in [51] and were also at the same time placed in the downloadable file called Tab_Euk-mPLoc at http://chou.med.harvard.edu/bioinf/euk-multi/ [50] or http://202.12 0.37.186/bio inf/euk-multi/ [51], the pre- dicted subcellular locations of the 334 eukaryotic pro- teins are given in column 4 of Appendix A, where for facilitating comparison the corresponding experimental results available about seven months later are also listed in column 5. From the table we can see the following: of the 334 eukaryotic proteins, 309 are with single location site and 25 with multiple location sites. Of the 309 single location proteins, only 22 were incorrectly predicted; of the 25 multiple location proteins, 2 (i.e., No.104 and No.322) were incorrectly predicted. It is interesting to see that the predicted result for No.104 was “Centriole; Nucleus” while the experimental observation “Cyto- plasm; Nucleus”, meaning only one of its two location sites was incorrectly predicted; and that the predicted result for No.322 was “Centriole; Cytoplasm; Nucleus” while the experimental observation “Nucleus; Cyto- plasm”, meaning both of its observed location sites were correctly predicted although the site of “Centriole” was over-predicted. Accordingly, the overall success rate for the 334 proteins is over 93% as proved later by experi- ments. Cell-PLoc: A package of web-servers for predicting subcellular localization of proteins in different organisms Euk-mPLoc Plant-PLoc Gneg-PLoc Hum- mPL o c Gpos-PLoc Virus-PLoc Figure 3. A semi-screenshot to show the Cell-PLoc web-page at (http://chou.med.harvard.edu/bioinf/Cell-PLoc/).  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 67 Although the predictors in the Cell-PLoc package [50] are very powerful, they have the following shortcomings. (1) In order for taking the advantage of Gene Ontology (GO) [52] approach [49], the input for a query protein must include its accession number. However, many pro- teins, such as synthetic and hypothetical proteins, as well as those newly-discovered proteins that have not been deposited into databanks yet, do not have accession numbers, and hence their subcellular locations cannot be predicted via the GO approach. (2) Since the current GO database is far from complete yet, many proteins cannot be meaningfully formulated in a GO space even if their accession numbers are available. (3) Although the PseAA (pseudo amino acid) composition [18,53] or PseAAC approach, a complement to the GO approach in Cell-PLoc, can take into account some partial sequence order effects, the original PseAAC [18] missed the func- tional domain (FunD) [23] and sequential evolution (SeqE) information [54,55]. To improve the aforemen- tioned shortcomings, the Cell-PLoc package is currently under developing to be a new version, the Cell-PLoc 2.0. At this stage, some of the predictors therein, such as Hum-mPLoc2.0[56], Plant-mPLoc [56], Gpos-mPLoc [57], and Gneg-mPLoc [58], have been completed, as will be briefed below. To show the difference of Hum-mPLoc 2.0 with the original Hum-mPLoc [44] in the Cell-PLoc package [55], let us see the following demonstration steps. Step 1. Open the webpage http://www.csbio .sjtu.edu.cn /bioinf/hum-multi-2/, and you will see its top page on your computer screen [50], as shown in Figure 4a. Step 2. Either type or copy and past the query protein sequence into the input box (depicted by the box at the center of Figure 4a). The input sequence should be in FASTA format (http://en.wikipedia.org/wiki/Fasta_format), as shown by clicking on the Example button right above the input box. For example, if you use the 1st query pro- tein sequence in the Example window, the input screen should look like the illustration in Figure 4b. Step 3. After clicking the Submit button, you will see “Cell membrane; Cytoplasm; Nucleus” shown on the screen (Figure 4c) after 15 seconds or so, indicating that the query protein is a multiplex protein that may simul- taneously exist in the three subcellular location sites, fully in agreement with experimental observations. Step 4. If using the 2nd query protein sequence in the Example window as an input, after clicking the Submit Figure 4. A semi-screenshot to show (a) the top page of the web-server Hum-mPLoc 2.0 at http://www.csbio.sjtu.edu.cn/bioinf/hum-multi-2/, (b) the input in FASTA format taken from the 1st query protein sequence in the Example window, (c) the output generated by clicking the Submit button in panel b, and (d) the output generated through the similar procedure but using the input taken from the 2nd query protein sequence in the Example window. 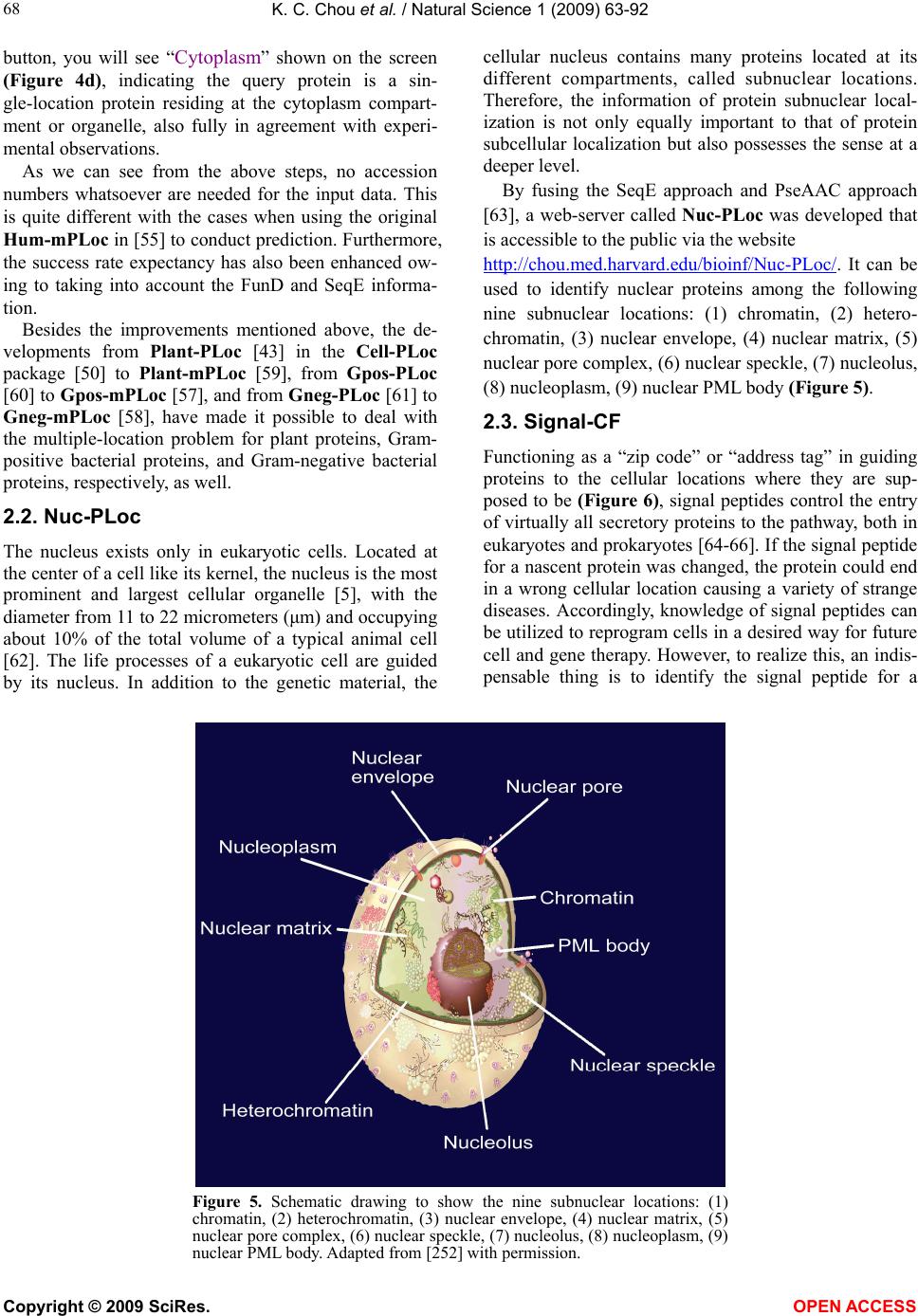 K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 68 button, you will see “Cytoplasm” shown on the screen (Figure 4d), indicating the query protein is a sin- gle-location protein residing at the cytoplasm compart- ment or organelle, also fully in agreement with experi- mental observations. As we can see from the above steps, no accession numbers whatsoever are needed for the input data. This is quite different with the cases when using the original Hum-mPLoc in [55] to conduct prediction. Furthermore, the success rate expectancy has also been enhanced ow- ing to taking into account the FunD and SeqE informa- tion. Besides the improvements mentioned above, the de- velopments from Plant-PLoc [43] in the Cell-PLoc package [50] to Plant-mPLoc [59], from Gpos-PLoc [60] to Gpos-mPLoc [57], and from Gneg-PLoc [61] to Gneg-mPLoc [58], have made it possible to deal with the multiple-location problem for plant proteins, Gram- positive bacterial proteins, and Gram-negative bacterial proteins, respectively, as well. 2.2. Nuc-PLoc The nucleus exists only in eukaryotic cells. Located at the center of a cell like its kernel, the nucleus is the most prominent and largest cellular organelle [5], with the diameter from 11 to 22 micrometers (μm) and occupying about 10% of the total volume of a typical animal cell [62]. The life processes of a eukaryotic cell are guided by its nucleus. In addition to the genetic material, the cellular nucleus contains many proteins located at its different compartments, called subnuclear locations. Therefore, the information of protein subnuclear local- ization is not only equally important to that of protein subcellular localization but also possesses the sense at a deeper level. By fusing the SeqE approach and PseAAC approach [63], a web-server called Nuc-PLoc was developed that is accessible to the public via the website http://chou. med.harvard.edu /bioinf/Nuc -PLoc/. It can be used to identify nuclear proteins among the following nine subnuclear locations: (1) chromatin, (2) hetero- chromatin, (3) nuclear envelope, (4) nuclear matrix, (5) nuclear pore complex, (6) nuclear speckle, (7) nucleolus, (8) nucleoplasm, (9) nuclear PML body (Figure 5). 2.3. Signal-CF Functioning as a “zip code” or “address tag” in guiding proteins to the cellular locations where they are sup- posed to be (Figure 6), signal peptides control the entry of virtually all secretory proteins to the pathway, both in eukaryotes and prokaryotes [64-66]. If the signal peptide for a nascent protein was changed, the protein could end in a wrong cellular location causing a variety of strange diseases. Accordingly, knowledge of signal peptides can be utilized to reprogram cells in a desired way for future cell and gene therapy. However, to realize this, an indis- pensable thing is to identify the signal peptide for a Figure 5. Schematic drawing to show the nine subnuclear locations: (1) chromatin, (2) heterochromatin, (3) nuclear envelope, (4) nuclear matrix, (5) nuclear pore complex, (6) nuclear speckle, (7) nucleolus, (8) nucleoplasm, (9) nuclear PML body. Adapted from [252] with permission.  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 69 Nucleus Plasma membrane Cytoplasm Mitochondria Endoplasmic reticulum Cytoskeleton Peroxisome Lysosome Golgi apparatus Centriole Extracell Microsome Signal protein NucleusNucleus Plasma membrane Cytoplasm Mitochondria Endoplasmic reticulum Cytoskeleton Peroxisome Lysosome Golgi apparatus Centriole Extracell Microsome Signal protein Figure 6. A schematic drawing to show: how the signal peptides of secretory proteins function as an “address tag” in directing the proteins to their proper cellular and extracellular locations. The signal peptide sequence is colored in puple, and the mature protein sequence in blue. Signal Peptidase -L s -4 -2 -1 +1 +2 +L m -3 Signal Peptidase -L s -4 -2 -1 +1 +2 +L m -3 Figure 7. A schematic drawing to show the signal sequence of a protein and how it is cleaved by the signal peptidase. An amino acid in the signal part is depicted as a red circle with a white number to indicate its sequential position, while that in the mature protein depicted as an open circle with a blue number. The signal sequence contains Ls residues and the mature protein Lm residues. The cleavage site is at the position (-1, +1), i.e., between the last residue of the signal sequence and the first residue of the mature protein. nascent protein. Many efforts have been made in this regards (see, e.g., [67-76] as well as the relevant refer- ences listed in a review article [77]). The signal peptide of a secretory protein is usually located at its N-terminal, and it will be cleaved off by a signal peptidase once the protein is translocated through  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 70 a membrane (Figure 7), where the cleavage site is commonly symbolized by (1, +1) , namely the posi- tion between the last residue of the signal peptide and the first residue of the mature protein. It can also be seen from Figure 7 that once the cleavage site is identified, the corresponding signal peptide is automatically known; and vice versa. The difficulty in predicting signal peptides is that for different secretory proteins, their signal peptides are quite different not only in sequence components and sequence orders but also in sequence lengths. Also, many previous methods were lacking of considering the coupling effects of the subsites around the cleavage sites, as analyzed in [78]. To address the above two problems, the web-server predictor called Signal-CF [79] was developed recently. Its features are reflected by its name, where “C” stands for “Coupling” and “F” for “Fusion”, meaning that Sig- nal-CF is formed by incorporating the subsite coupling effects along a protein sequence and by fusing the results derived from many width-different scaled windows through a voting system. Signal-CF is a 2-layer predictor: the 1st-layer predic- tion engine is to identify a query protein as secretory or non-secretory; if it is secretory, the process will be auto- matically continued with the 2nd-layer prediction engine to further identify the cleavage site of its signal peptide. The predictor is also featured by high success prediction rates with short computational time, and hence is par- ticularly useful for the analysis of large-scale datasets. Signal-CF is freely accessible at http://chou.med.h arvard.edu/b ioinf/Signal-CF/. 2.4. Signal-3L This is a 3-layer predictor developed for identifying the signal peptides of human, plant, animal, eukaryotic, Gram-positive, and Gram-negative proteins. The target of the 1st-layer is to identify a query protein as secretory or non-secretory. If the protein is identified as secretory, the process will be automatically continued by the 2nd- layer prediction engine to identify the potential cleavage sites (Figure 7) along its sequence. The 3rd-layer is to finally determine the unique cleavage site through a global sequence alignment operation. Signal-3L is ac- cessible to the public as a web-server at http://chou.med.harvard.edu/bioinf/Signal-3L/. Compared with Signal- CF, it might take a little longer computa- tional time but yield a little higher accuracy. Table 2. List of examples showing that signal peptides miss-predicted by SignalP-NN and/or SignalP-HMM are corrected by Sig- nal-3L. Proteina Experimentally verified signal peptidea SignalP 3.0-NN SignalP 3.0-HMM Signal-3L AAF91396.1 1-40 1-37 1-37 1-40 DKK1_HUMAN 1-31 1-22 1-28 1-31 MIME_HUMAN 1-20 1-19 1-19 1-20 NP_057466.1 1-21 1-19 1-19 1-21 NP_057663.1 1-35 1-30 1-46 1-35 NP_443122.2 1-21 1-22 1-22 1-21 NP_443164.1 1-26 1-33 1-33 1-26 Q6UXL0 1-28 1-29 1-29 1-28 STC1_HUMAN 1-17 1-21 1-18 1-17 TRLT_HUMAN 1-25 1-24 1-27 1-25 CD5L_HUMAN 1-19 1-18 1-19 1-19 EDAR_HUMAN 1-26 1-28 1-26 1-26 FZD3_HUMAN 1-22 1-17 1-22 1-22 IBP7_HUMAN 1-26 1-26 1-29 1-26 KLK3_HUMAN 1-17 1-17 1-23 1-17 NMA_HUMAN 1-20 1-20 1-26 1-20 NP_064510.1 1-22 1-22 1-23 1-22 NP_068742.1 1-24 1-24 1-25 1-24 NTRI_HUMAN 1-33 1-30 1-33 1-33 SY01_HUMAN 1-23 1-23 1-18 1-23 TIE1_HUMAN 1-21 1-21 1-22 1-21 TL19_HUMAN 1-26 1-23 1-26 1-26 TR14_HUMAN 1-38 1-36 1-38 1-38 TR19_HUMAN 1-29 1-29 1-25 1-29 XP_166856 1-17 1-17 1-20 1-17 XP_209141 1-22 1-23 1-22 1-22 a Data taken from [251]. The signal peptides experimentally verified and correctly predicted are in bold-face type colored in blue; those incorrectly predicted in red. (For interpretation of the references to color in this table caption, the reader is referred to the web version of this paper.)  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 71 Both Signal-CF and Signal-3L can be used to refine the results by other predictors in this area. For instance, listed in Table 2 are the signal peptides that were miss- predicted by SignalP-NN and/or SignalP-HMM in the SignalP package [75] but corrected by Signal-3L. Also, according to a recent report (see Table 1 of [80]) Signal-CF performed the best in predicting the long signal peptides, among the following eight web-server predictors: SignalP-NN [75], SignalP-HMM [75], Sig- nalP-NN or SignalP-HMM [75], Phobius [81], PrediSi [76], Signal-CF [79], Signal-3L [82], and Philius [83]. 2.5. MemType-2L Given a protein sequence, how can one identify whether it is a membrane protein or not? If it is, which membrane protein type it belongs to? It is important to address these problems because they are closely relevant to the biological function of the protein concerned and to its interaction process with other molecules in a biological system. Most functional units or organelles in a cell are “enveloped” by one or more membranes, which are the structural basis for many important biological functions. Although the basic structure of membranes is lipid bi- layer, many specific functions of the cell membrane are performed by the membrane proteins (see, e.g., [4,5]). For example, it is through membrane proteins that vari- ous chemical messages such as nerve impulses and hor- mone activity can be passed between cells (see, e.g., [84]); that cells can be attached to an extracellular matrix in grouping cells together to form tissues; that parts of the cytoskeleton can be attached to the cell membrane in order to provide shape; that the metabolism process and body’s defense mechanisms can be completed; as well as that molecules can be transported into and out of cells by such methods as proton pumps (see, e.g., [85-87]) and ion pumps (see, e.g., [88,89]), channel proteins [90-92] and carrier proteins (see, e.g., [93]). Membrane proteins possess different types, which are closely correlated with their functions. For instance, the transmembrane proteins can transport molecules across the membrane or function on both its sides, whereas proteins functioning on only one side of the lipid bilayer are often associated exclusively with either the lipid monolayer or a protein domain on that side. Therefore, information about membrane protein type can provide useful hints for determining the function of an unchar- acterized membrane protein. Furthermore, because of the fluid nature of their infrastructure, membrane proteins can move around the cell membrane so as to reach where their function is required. Therefore, it will certainly expedite the pace in determining the function and action process of uncharacterized membrane proteins if we can timely acquire the knowledge of their type. With the avalanche of protein sequences generated in the post genomic age and the fact that membrane proteins are encoded by 20-35% of genes [94], it is self-evident why it is so important to develop a sequence-based automated method for fast and effectively addressing the two prob- lems posed at the beginning of this Section. Stimulated by the encouraging results in predicting the structural classification of proteins based on their amino acid (AA) composition or AAC [95-103], the co- variant discriminant algorithm was introduced to identify the types of membrane proteins also based on their AA composition in 1999 [104]. However, the AA composi- tion does not contain any sequence order information. To avoid completely losing the sequence order information, the PseAA composition or PseAAC was introduced [18]. Since then, various prediction methods have been pro- posed in this area [53,105-118]. Recently, a user-friendly web-server predictor called “MemType-2L” was developed [54]. Compared with the other predictors which only cover 5-6 membrane types, MemType-2L can cover 8 membrane types (Fig- ure 8). MemType-2L is a 2-layer predictor: the 1st layer prediction engine is to identify a query protein as mem- brane or non-membrane; if it is membrane, the process will be automatically continued with the 2nd-layer pre- diction engine to further identify its type among the fol- lowing eight categories (Figure 8): (1) type I, (2) type II, (3) type III, (4) type IV, (5) multipass, (6) lipid-chain- anchored, (7) GPI-anchored, and (8) peripheral. MemType-2L is accessible to the public via the web- site at http://chou.med.harvard.ed u/bioinf/MemType/. 2.6. EzyPred Nearly all known enzymes are proteins that catalyze chemical reactions and are vitally important in the me- tabolic process. Given a protein sequence, how can we identify whether it is an enzyme or non-enzyme? If it is, which main functional class it belongs to? What about its sub functional class? These problems are closely correlated with the biological function of an uncharac- terized protein and its acting object and process [119]. Although their answers can be found by conducting various biochemical experiments, it is both time-con- suming and costly to do so solely by experimental ap- proaches. During the last six years, a number of predic- tors have been developed to address these problems [53,120-125]. Recently, a top-down automated method called “Ezy- Pred” was developed [126]. It not only covers all the six enzyme main-functional classes [127], but also many of their sub-functional classes (see Figure 9). EzyPred is a 3-layer predictor: the 1st layer prediction engine is for identifying a query protein as enzyme or non-enzyme;  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 72 C N (5) (6) (7) GPI (8) N N' C C N C C' N (1) (2) (3) (4) C N (5) (6)(6) (7) GPI (7) GPI (8) N N' C C N C C' N (1) (2) (3) (4) Figure 8. Schematic illustration showing the 8 types of membrane proteins: (1) type I transmembrane, (2) type II, (3) type III, (4) type IV, (5) multipass transmembrane, (6) lipid-chain-anchored membrane, (7) GPI-anchored mem- brane, and (8) peripheral membrane. As shown in the figure, types I, II, III, and IV are all of single-pass transmem- brane proteins; see [253] for a detailed description about their difference. Reproduced from [54] with permission. the 2nd layer for the main functional class; and the 3rd layer for the sub functional class. Within 90 seconds of submitting the sequence of a query protein into its input box, EzyPred will identify whether the query protein is enzyme or non-enzyme and, if it is an enzyme, to which main-functional class and sub-functional class it be- longs. EzyPred is accessible to the public as a web-server at http://chou.med.ha rvard.edu/b ioinf/EzyPred/. 2.7. ProtIdent Called by many as the biology’s version of Swiss army knives, proteases cut long sequences of amino acids into fragments and regulate most physiological processes. They are vitally important in life cycle and have become a main target for drug design (see, e.g., [2,128-134]). The actions of proteases are exquisitely selective (see, e.g. [135-139]), with each protease being responsible for splitting very specific sequences of amino acids under a preferred set of environmental conditions. According to their catalytic mechanisms, proteases are classified the following six types: (1) aspartic, (2) cysteine, (3) glu- tamic, (4) metallo, (5) serine, and (6) threonine [140]. Different types of proteases have different action mechanisms and biological processes. Therefore, it is important for both basic research and drug discovery to consider the following two problems. Given the sequence of a protein, can we identify whether it is a protease or non-protease? If it is, what protease type does it belong to? During the last three years, some efforts have been made in this regard [141,142]. However, none of these methods provided a web-server that can be easily used by the majority of experimental and pharmaceutical sci- entists to obtain the desired data. Very recently, a web-server called “ProtIdent” was developed [55] by fusing the FunD (functional domain) and SeqE (sequential evolution) information (Figure 10a). ProtIdent is a 2-layer predictor: the 1st layer is for identifying a query protein as protease or non-protease; if it is a protease, the process will automatically go to the second layer to further identify it among the six different mechanistic types (Figure 10b). Furthermore, a step-by-step protocol guide [143] was  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 73 provided for demonstrating how to use the ProtIdent web-server, by which one can get the desired 2-level re- sults for a query protein sequence in around 25 seconds. ProtIdent is freely accessible to the public via the site at http://www.csb io.sjtu.edu.cn/b ioinf/Protease. 2.8. GPCR-CA One of the largest families in the human genome is the one encoding the G-protein-coupled receptors (GPCRs), which are cell surface receptors. Owing to their charac- teristic transmembrane topology, GPCRs are also known as 7-transmembrane receptors, 7TM receptors, hepta- helical receptors, and serpentine receptors that “snake” Figure 9. A schematic drawing to use tree branches to classify enzyme and non-enzyme as well as the six main functional classes of enzymes and their subclasses.  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 74 Fusion RPS-Blast CDD …… Generate FunD descriptor for P Generate PsePSSM descriptor for P …… 1(1, μ)1(1, μ)1(2, μ)1(2, μ)1(10, μ)1(10, μ)2(1,0,μ)2(1,1,μ)2(1,1,μ)2(10,49,μ)2(10,49,μ) Final protease type Input protein sequence P OET-KNN Swiss- Prot OET-KNN PSI- Blast Base Input a protein sequence ProtIdent ensemble classifier ( = 2) Protease ProtIdent ensemble classifier ( = 6) Non-protease Stop Final protease type Training dataset I Training dataset II (a) (b) Figure 10. A flowchart to show (a) how to fuse the FunD approach and PsePSSM approach, and (b) how the two-layer Prot-Ident ensemble classifier works in identifying proteases and their functional types. See [55] for further explanation. Lipid bilayer 22 33 44 55 11 66 77 Intrace lluar E xt r ac ellular N C Figure 11. Schematic representation of a GPCR with a trademark of seven-transmembrane helices, depicted as cylinders and connected by alternating cytoplasmic and extracellular hydrophilic loops. The 7-helix bundle thus formed has a central pore on its extracellular surface. The red entity located in the central pore represents a ligand messenger.  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 75 across a cell membrane seven times (Figure 11). The major role of GPCRs is to transmit signals into the cell. GPCR-associated proteins may play at least the follow- ing four distinct roles in receptor signaling [144-147]: (1) directly mediate receptor signaling, as in the case of G proteins; (2) regulate receptor signaling through control- ling receptor localization and/or trafficking; (3) act as a scaffold, physically linking the receptor to various ef- fectors; (4) act as an allosteric modulator of receptor conformation, altering receptor pharmacology and/or other aspects of receptor function. Much effort has been invested for studying GPCRs by both academic institutions and pharmaceutical in- dustries. Today, approximately one third of the world small molecule drug markets are GPCR agonists and antagonists. The functions of many of GPCRs are still unknown, and it is both time-consuming and costly to determine their ligands and signaling pathways. Particularly, as membrane proteins, GPCRs are very difficult to crystal- lize and most of them will not dissolve in normal sol- vents. Accordingly, so far very few crystal GPCR struc- tures have been determined. Although the recently de- veloped state-of-the-art NMR technique is a very pow- erful in determining the 3D structures of membrane pro- teins [87,92-94,148], it is time-consuming and costly. In order to timely obtain the protein 3D structures for ra- tional drug design, the approach of structural bioinfor- matics has been often adopted (see, e.g., [84,149-153]). Unfortunately, such an approach fails to work in most GPCR-related cases because very few GPCRs have suf- ficiently high sequence similarity with existing struc- ture-known proteins, an indispensable condition for de- veloping a reasonable starting structure via structural bioinformatics [2,3]. Consequently, it is highly desired to develop automated methods that can fast and effectively identify the functional families of GPCRs according to their sequence information because the information thus obtained can help classifying drugs, a technique called “evolutionary pharmacology” quite useful for drug de- velopment. During the last 7 years or so, a number of methods were proposed in this regard [154-159]. Some of them were developed for identifying the main functional classes of GPCRs (see, e.g., [157]) and some for the sub- func- tional classes (see, e.g., [155]). None of these methods has provided a web-server for the public usage, and hence their practical application value is quite limited. (a1) (a2) (b1) (b2) Figure 12. The cellular automaton image generated according to Eqs.2-5 for (a1) the rhodopsin like family member with ac- cession number P41595; (a2) the rhodopsin like family member with accession number P18599; (b1) the secretin like family member with accession number O95838; and (b2) the secretin like family member with accession number Q02644. Panels (a1) and (a2) share a quite similar texture because the protein sequences from which the cellular automaton images were de- rived belong to a same GPCR family. And the same is true for panels (b1) and (b2).  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 76 (a) (b) (c) (d) (e) (f) Figure 13. The cellular automaton image generated according to Eqs.2-5 for a protein taken from (a) A-rhodopsin like family, (b) B-secretin like family, (c) C-metabotrophic/glutamate/pheromone family; (d) D-fungal pheromone family, (e) E-cAMP receptor family, and (f) F-Frizzled/Smoothemed family, respectively. The six panels have completely dif- ferent textures because they represent six different GPCR family members. Recently, a web-server predictor was developed [160] with the name as GPCR-CA, where “CA” stands for “Cellular Automaton” [161], meaning that the cellular automaton images have been utilized to reveal the pattern features hidden in piles of long and complicated protein sequences. Cellular automata are discrete dynamical sys- tems whose behavior is completely specified in terms of a local relation. A cellular automaton can be thought of as a stylized universe consisting of a regular grid of cells, each of which is in one of a finite number of possible states, updated synchronously in discrete time steps ac- cording to a local, identical interaction rule [162]. The procedures of generating the cellular automaton images for protein sequences can be briefed as follows. As a first step, each of the 20 native amino acids in a protein sequence is represented by a 5-digit strain ac- cording to the binary coding as defined in [163]. Thus, a protein consisting of N amino acids can be converted to a sequence with 5N digits (or grids); i.e,, 12 5 g()g()g()g(), (0) NN ttttt (2) where g()0 or 1 it (1, 2, , 5) iN as defined in [163]. Suppose the time for each updated step is con- secutively expressed by 0, 1, 2, , t, we have 12 5 12 5 12 5 g(0) g(0) g(0) g(0) g(1) g(1) g(1) g(1) g(2) g(2) g(2) g(2) NN NN NN 12 5 g()g()g() g() NN (3) where  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 77 -1 1 -1 1 -1 1 -1 1 0, if g()0, g()0, g()0 0, if g()0, g()0, g()1 1, if g()0, g()1, g()0 0, if g()0, g()1, g()1 g(1)1, iii iii iii iii i tt t tt t tt t tt t t -1 1 -1 1 -1 1 -1 1 (0, 1, , ) if g()1, g()0, g()0 0, if g()1, g()0, g()1 1, if g()1, g()1, g()0 0, if g()1, g()1, g()1 iii iii iii iii t tt t tt t tt t tt t (4) with the spatially periodic boundary conditions; i.e., 05 g()g ()N tt and 511 g()g() Ntt (5) Suppose: g() it, the thi grid at t, is filled with white color if g() 0 itand black if g() 1 it. Accord- ingly, each row of Eq.3 corresponds to a narrow ribbon mixed with white and black colors. Scanning these rib- bons successively on to a screen or sheet will generate a 2D (2-dimensional) black-and-white image. It has been observed that the image texture is basically steady after 100t. The image thus evolved is called the cellu- lar automaton image for the protein sequence concerned. The advantage of using the cellular automaton image to represent the protein is that it can help us visualize some special features hidden in its long and complex sequence [163]. For instance, the cellular automata images for proteins from a same GPCR family share a similar textu- re pattern (Figure 12), while those from different GPCR families have different texture patterns (Figure 13). Subsequently, the gray-level co-occurrence matrix factors extracted from the cellular automaton images were used to represent the samples of proteins through their pseudo amino acid composition [18,53], followed by utilizing the augmented covariant-discriminant clas- sifier [12,164] to operate the prediction of GPCR-CA. GPCR-CA is a 2-layer predictor: the 1st layer predic- tion engine is for identifying a query protein as GPCR on non-GPCR; if it is a GPCR protein, the process will be automatically continued with the 2nd-layer prediction engine to further identify its type among the following six functional classes: (1) rhodopsin-like, (2) secretin- like, (3) metabotrophic/glutamate/pheromone; (4) fungal pheromone, (5) cAMP receptor, and (6) Frizzled/Smoo- themed family. GPCR-CA is freely accessible at http://218.65.61.89:8080/bioinfo/GPCR-CA, by which one can get the desired 2-layer results for a query protein sequence within about 20 seconds. 2.9. HIVcleave During the past 17 years, the following two strategies have often been utilized to find drugs against AIDS (ac- quired immunodeficiency syndrome). One is to target the HIV (human immunodeficiency virus) reverse tran- scriptase (see, e.g., [165-171]); the other is to design HIV protease inhibitors [128,136,138,139,172-174]. Functioning as a dimer, the HIV protease is made up of two identical subunits, each having 99 residues, but with only one active site [136,174]. The essential func- tion of HIV protease is to cleave the precursor polypro- tens; loss of the cleavage-ability will stop the life cycle of infectious HIV, the culprit [175,176] of AIDS. To find the effective inhibitors against HIV protease, it is very helpful to understand the mechanism of how it cleaves the polyproteins and utilize the “distorted key” theory [136] to approach the problem, as illustrated be- low. HIV protease is a member of the aspartyl proteases that is highly substrate-selective and cleavage-specific. The HIV protease-susceptible sites in a given protein extend to an octapeptide region [177], with its amino acid residues sequentially symbolized by eight subsites 4 R, 3 R, 2 R, 1 R, 1' R, 2' R, 3' R, 4' R [178], as shown in Figure 14. The scissile bond is lo- cated between the subsites 1 R and 1' R. Occasionally, the susceptible sites in some proteins may contain one subsite less or one subsite more, corresponding to the case of a heptapeptide or nonapeptide, respectively. However, in investigating the cleavability of peptide sequences by HIV proteases, heptapeptides and nona- peptides need to be considered very rarely. This might be the result of a compromise between the following two factors. On one hand, according to the “rack mecha- nism” [179], the active site of HIV protease can be lik- ened to a “rack” during the peptide cleaving process. Thus, it appears that the more residues that are bound to the rack of enzyme, the more strained the peptide, and hence the more efficient the cleavage process. On the other hand, however, the active site of an HIV protease can hardly accommodate more than 8 residues. Conse- quently, for most cases, the protease-susceptible sites in proteins are strings of octapeptides as observed [135]. Thus, according to the “lock-and-key” mechanism in enzymology, an HIV protease-cleavable peptide must satisfy the substrate specificity, i.e., a good fit for bind- ing to the active site. However, such a peptide, after a modification of its scissile bond with some chemical procedure, will completely lose its cleavability but it can  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 78 HIV Protease R 2 R 4 R 3 R 1 R 1’ R 3’ R 2’ R 4’ N H H N N H H N C || O O || C C || O O || C H N H N N H N H O || C C || O C || O O || C S 4 S 2 S 1 ’ S 3 S 1 S 2 ’ S 3 ’ S 4 ’ HIV Protease R 2 R 4 R 3 R 1 R 1’ R 3’ R 2’ R 4’ N H H N N H H N C || O O || C C || O O || C H N H N N H N H O || C C || O C || O O || C S 4 S 2 S 1 ’ S 3 S 1 S 2 ’ S 3 ’ S 4 ’ Figure 14. Schematic representation of substrate bound to HIV protease based an analysis of protease-inhibitor crystal struc- tures. The active site of enzyme is composed of eight extended “subsites”, S4, S3, S2, S1, S1’, S2’, S3’, S4’, and their counterparts in a substrate extend to an octapeptide region, sequentially symbolized by R4, R3, R2, R1, R1’, R1’, R2’, R3’, R4’, respectively. The scissile bond is located between the subsites R1 and R1’. Reproduced with permission from Figure 3 of K.C. Chou [136]. (a) R 1’ R 2’ R 3’ R 4’ R 1 R 2 R 3 R 4 HIV Protease R 1’ R 2’ R 3’ R 4’ R 1 R 2 R 3 R 4 HIV Protease (b) R 1’ R 2’ R 3’ R 4’ R 1 R 2 R 3 R 4 HIV Protease R 1’ R 2’ R 3’ R 4’ R 1 R 2 R 3 R 4 HIV Protease Figure 15. Schematic illustration to show (a) a cleavable oc- tapeptide is chemically effectively bound to the active site of HIV protease, and (b) although still bound to the active site, the peptide has lost its cleavability after its scissile bond is modified from a hybrid peptide bond [254] to a single bond by some simple routine procedure. The eight residues of the pep- tide is sequentially symbolized R4, R3, R2, R1, R1’, R1’, R2’, R3’, R4’. The scissile bond is located between R1 and R1’. Adapted from [136] with permission. still bind to the active site of an enzyme. Actually, the molecule thus modified can be deemed as a “distorted key”, which can be inserted into a lock but can neither open the lock nor be pulled out from it. That is why a molecule modified from a cleavable peptide can sponta- neously become a competitive inhibitor against the en- zyme. An illustration about such a concept is given in Figure 15, where panel (a) shows an effective binding of a cleavable peptide to the active site of HIV protease, while panel (b) shows that the peptide has become a non-cleavable one after its scissile bond is modified al- though it can still tightly bind to the active site. Such a modified peptide, or “distorted key”, will automatically become an inhibitor candidate of HIV protease. Even for non-peptide inhibitors, it can also provide useful insights about the key binding groups, hydrophobic or hydro- philic environment, fitting conformation, et al. Accord- ingly, in search for the potential inhibitors, a matter of paramount importance is to discern what kind of pep- tides can be cleaved by HIV protease and what kind cannot be. Even if limited in the range of an octapeptide, it is by no means easy to address the question. This is because the number of possible octapeptides formed from 20 amino acids runs into 88log2010 20102.56 10 . It would be exhausting to experimentally test out such an astronomical number of octapeptides. However, if one could find an effective computational method for pre- dicting the cleavage sites in proteins by HIV protease,  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 79 the pace in search for the proper inhibitors of HIV pro- tease would be significantly expedited. Actually, during the last decade or so, various prediction methods have been developed in this regard [128,135,137-139,180- 186]. Recently, based on the discriminant function algo- rithm [136], a web server called HIVcleave [187] was established at the website http://chou.med.harvard.edu/bioinf/HIV/. For a given protein sequence, one can use HIVcleave to predict its cleavage sites by HIV-1 and HIV-2 proteases, respec- tively. 2.10. QuatIdent As the chief actors of various biological processes in a cell, proteins have the following four different structural levels: primary, secondary, tertiary, and quaternary [188]. The primary structure refers to the constituent amino acid sequence; the secondary, to the local spatial ar- rangement of a polypeptide’s backbone without regard to the conformations of its side chains; the tertiary, to the three-dimensional structure of an entire polypeptide; and the quaternary, to how many polypeptide chains (sub- units) involved in forming a protein and the spatial ar- rangement of its subunits. The concept of quaternary structure is derived from the fact that many proteins are composed of two or more subunits which associate with each other through non-covalent interactions and, in some cases, disulfide bonds. According to the number of subunits aggregated together in an oligomeric complex, protein quaternary structures can be classified into: monomer, dimer, trimer, tetramer, pentamer, and so forth [189]. A statistical distribution of different quaternary structural types is shown in Figure 16, from which we can see that the nature prefers those oligomers with even and/or small number of subunits, fully consistent with the findings by the previous investigators [190,191]. If the subunits in a complex are identical, then the complex is called homo-oligomer; otherwise hetero-oligomer. For example, the sodium channel is formed by a monomer [192] while the potassium channel by a homo-tetramer [88]; the phospholamban is formed by homo-pentamer [93,193] while the Gamma-aminobutyric acid type A (GABAA) receptor by a hetero-pentamer [84,194]; the M2 proton channel is formed by a homo-tetramer [87] while hemoglobin by a hetero-tetramer [195]. Facing the explosion of newly generated protein se- quences, we are challenged to develop an automated method for rapidly and reliably identify the quaternary structural attributes of uncharacterized proteins because they are closely relevant to the functions and mecha- nisms of proteins (see, e.g., [87,195]. Besides, the in- formation thus obtained is very useful in screening the candidates of proteins for their 3D structure determina- tion. It is known that many functionally important pro- teins exist in vivo as oligomers rather than single indi- vidual chains. For example, hemoglobin is a hetero- tetramer of two α chains and two β chains, and the four chains must be aggregated into one construct to perform its cooperative function during the oxygen- transporting process [195]. Also, the novel allosteric drug-inhibition mechanism for the M2 proton channel was recently revealed by the NMR observations [87,92]. It has been found through an in-depth analysis that such a subtle mechanism is closely correlated with a unique packing arrangement of four transmembrane helices from four identical protein chains [90,91,196]. For this kind of proteins, determination of their individual chains independently would be less interesting or should be avoided. Therefore, developing an effective method to predict the quaternary structural attributes of proteins based on their sequence information alone would pro- vide useful clues for both basic research and drug de- velopment. To address the challenge, the web-server predictor called “QuatIdent” [197] was developed recently by fusing the functional domain and sequential evolution information. QuatIdent is a 2-layer predictor. The 1st layer is for identifying a query protein as belonging to which one of the following ten main quaternary struc- tural attributes: (1) monomer, (2) dimer, (3) trimer, (4) tetramer, (5) pentamer, (6) hexamer, (7) heptamer, (8) octamer, (9) decamer, and (10) dodecamer. If the result thus obtained turns out to be anything but monomer, the process will be automatically continued to further iden- tify it belonging to a homo-oligomer or hetero-oligomer. QuatIdent is freely accessible to the public as a web server via the site at http://www.csbio.sjtu.edu.cn/bioinf/Quaternary/, by whi- ch one can get the desired 2-level results for a query protein sequence in around 25 seconds. And the longer the sequence is, the more time that is needed. 2.11. PQSA-Pred This is another web-server predictor [198] developed by hybridizing the functional domain composition approach and pseudo amino acid composition approach for pre- dicting protein quaternary structural attribute based on the sequence information alone. PQSA-Pred can be used to predict a query protein among the following three quaternary attributes according to its sequence in- formation: monomer, homo-oligomer, and heterooligo- mer. As a useful tool for crystallographic scientists in screening for their targets, PQSA-Pred is freely accessi- ble to the public via the website at http://218.65.61.89:8080/bioinfo/pqsa-pred . Besides QuatIdent [197] and PQSA-Pred [198], some other efforts were also made in this regard [189,199,200]. However, none of these methods provide a web-server that can be easily used by the public.  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 80 Monomer 14% Dimer 50% Trimer 6% Tetramer 21% Pentamer 2% Heptamer 5% 0.2% Octamer 1% Decamer 0.2% 0.3% 0.3%Dodecamer Others Hexamer Monomer 14% Dimer 50% Trimer 6% Tetramer 21% Pentamer 2% Heptamer 5% 0.2% Octamer 1% Decamer 0.2% 0.3% 0.3%Dodecamer Others Hexamer Figure 16. A pie chart to show the statistical distribution of different quaternary structural types in the nature derived from version 55.3 of Swiss-Prot database released 29-April-2008. Reproduced with permission from [197]. 2.12. PFP-Pred A protein can function properly only if it is folded into a very special and individual shape or conformation, i.e., has the correct secondary, tertiary and quaternary struc- ture [201]. Failure to fold into the intended 3D structure usually produces inactive proteins or misfolded proteins [202] that may cause cell death and tissue damage [203] and be implicated in prion diseases such as bovine spongiform encephalopathy (BSE, also known as “mad cow disease”) in cattle and Creutzfeldt-Jakob disease (CJD) in humans. All prion diseases are currently un- treatable and are always fatal [204]. Although the X-ray crystallography is a powerful tool in determining protein 3D structures, it usually takes months or even years to determine the structure of a sin- gle protein. Also, the determination might fail for those proteins (particularly membrane proteins) that are diffi- cult to crystallize. Although the nuclear magnetic reso- nance (NMR) technique is very powerful in determining membrane protein structures [87,93,94,148], it requires expensive equipments and take equally long or even longer time. The avalanche of protein sequences gener- ated in the Post Genomic Age has challenged us for de- veloping computational methods by which the structural information can be timely extracted from sequence da- tabases. Although the direct prediction of the 3D struc- ture of a protein from its sequence based on the least free energy principle [201,205] is scientifically quite sound and some encouraging results already obtained in eluci- dating the handedness problems and packing arrange- ments in proteins (see, e.g., [206-211]), it is far from successful yet for predicting its 3D structure owing to the notorious local minimum problem except for some very special cases or by utilizing some additional infor- mation from experiments (see, e.g., [212,213]). Actually, it is even not successful yet for simply predicting the overall fold of a query protein based on its sequence alone. For further information about protein folding, refer to a recent review [214] and the references cited therein. Again, although it is quite successful to predict the 3D structure of a protein according to the homology modeling approach [2,215] as reflected by a series of homology-modeled proteins for drug development [84,147,149-151,153,216-226], a hurdle exists when the query protein does not have any structure-known ho- mologous protein in the existing databases [3]. Facing this kind of situation, a different strategy, the so-called taxonomic approach [227] was developed to address the problem. According to such a strategy, pre- dicting the 3D structure of a protein may be first con- verted to a problem of classification; i.e., identifying which fold pattern it belongs to. Its underpinning is based on the assumption that the number of protein folds is limited [228-231]. The fold pattern of a protein is one level deeper than its structural classification [98,99,229], and hence is more challenging and complicated for prediction.  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 81 PFP-Pred [232] is one of these kinds of predictors. It was formed by a set of basic classifiers, with each trained in different parameter systems, such as predicted secondary structure, hydrophobicity, van der Waals vol- ume, polarity, polarizability, as well as different dimen- sions of pseudo amino acid composition, that were ex- tracted from a training dataset. The operation engine for the constituent individual classifiers was OET-KNN (Optimized Evidence-Theoretic K-Nearest Neighbors) rule [32,113,233]. Their outcomes were combined thru a weighted voting to give a final determination for classi- fying a query protein. The recognition was to find the true fold among the 27 possible patterns. The web-server of PFP-Pred is available to the public via the site http://chou.med.harvard.edu/bioinf/PFP-Pred/. 2.13. PFP-FunDSeqE This is an improved version of PFP-Pred by combining the functional domain information and the sequential evolution information through a fusion ensemble classi- fier [234], as reflected by parts of its name where “FunD” stands for “functional domain” while “SeqE” for “sequential evolution”. Compared with the other ex- isting methods for predicting the protein fold patterns, PFP-FunDSeqE can usually yield better results [234]. Its web-server is available at http://www.csbio.sjtu.edu.cn/bioinf/PFP-FunDSeqE/. 2.14. Pred-PFR Since each protein begins as a polypeptide translated from a sequence of mRNA as a linear chain of amino acids, it is interesting to study the folding rates of pro- teins from their primary sequences. Actually, protein chains can fold into the functional 3D structures with quite different rates, varying from several microseconds [235] to even an hour [236]. Since the 3D structure of a protein is determined by its primary sequence, we can assume the same is true for its folding rate. In view of this, we are challenged by an interesting question: Given a protein sequence, can we find its folding rate? Al- though the answer can be found by conducting various biochemical experiments, doing so is both time- con- suming and expensive. Also, although a number of pre- diction methods were proposed [237-242], they need the input from the 3D structure of the protein concerned, and hence the prediction is feasible only after its 3D struc- ture has been determined. However, according to data released on5-May-2009 by the RCSB Protein Data Bank (http://www.rcsb.org/pdb), the number of proteins with 3D structure known is only about 1.34% of the number of sequence-known proteins. Therefore, it is highly de- sired to develop an automated method that can rapidly and approximately predict the folding rates of proteins according to their sequence information alone. Some ef- forts have been made in this regard (see, e.g., [243,244]). Since the experimentally observed folding rate for a protein chain usually represents the “apparent folding rate constant” [245] as denoted by f , it is instructive to unravel its relationship with the detailed rate constants, as given below. The apparent folding rate constant f for a protein chain is defined via the following differential equation unfold funfold fold f unfold dP( )P() d dP()P() d t t t tKt t (6) where unfold P()t and fold P()t represent the concentra- tions of its unfolded state and folded state, respectively. Suppose the total protein concentration is 0 C, and ini- tially only the unfolded protein is present; i.e., unfold 0 P()tC and fold P()0t when 0t. Subse- quently, the protein system is subjected to a sudden change in temperature, solvent, or any other factor that causes the protein to fold. Obviously, the solution for Eq.6 is unfold 0f fold 0f P()exp P()1exp tC Kt tC Kt (7) It can be seen from the above equation that the larger the f , the faster the folding rate will be. Given the value of f , the half-life of an unfolded protein chain can be expressed by 1/2 f f ln1/ 20.693TK K (8) which can also be used to reflect the time that is needed for a protein chain to be half folded. However, the actual folding process is much more complicated than the one as described by Eq.6 even if the reverse rate for the folding system concerned can be ignored. As an illustra- tion, let us consider the following three-state folding mechanism 23 12 unfold interfold PPP k k (9) where inter P()t represents the concentration of an in- termediate state between the unfolded and folded states, 12 k is the rate constant for unfold P converting to inter P, and 23 k the rate constant for inter P converting to fold P. Thus we have the following kinetic equation  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 82 unfold 12 unfold inter 12 unfold23 inter fold 23 inter dP()P() d dP()P() P() d dP( )P() d tkt t tktkt t tkt t (1 0) To get the solution of Eq.10, let us use an intuitive dia- gram called “directed graph” or “digraph” (Figure 17a) [245,246] to represent Eq.9. To reflect the variation of the concentrations of the three protein states with time, the digraph is further transformed to the phase di- graph [245,246] as shown in Figure 17b, where s is an interim parameter associated with the Laplace transform as shown in Eq.11. unfold unfold 0 inter inter 0 fold fold 0 P() P()expd P() P()expd P()P()expd ttst ttst ttst (11) where unfold P , inter P and fold P are the phase concentra- tions of unfold P, inter P and fold P, respectively. Thus, ac- cording to the phase digraph of Figure 17b and using the graphic rule 4 [245,246], which is also called the graphic rule for non-steady-state kinetics” in litera- tures (see, e.g., [247]), we can directly write out the fol- lowing phase concentrations: 23023 00 unfold 12 2312 231212 23 P() sk sCsk CC s ksk sk ssk skskk (12.1) 12012 0 inter 12 23 231212 23 P() ksC kC s ksk ssk skskk (12.2) 12 23012 230 fold 12 23 231212 23 P() kkC kkC s sk sk ssk skskk (12.3) Through the above phase concentrations and using Laplace transform table (see, e.g., [248] or any standard mathematical tables), we can immediately obtain the desired concentrations for unfold P, inter P and fold P of Eq.10, as given by Eq.13. unfold 0 12 0 inter 23 12 0 fold12 230 23 12 12 23 12 23 12 P()e P()e e P()e e tC kC tkk C tkkC kk kt kt kt kt kt (13) Accordingly, it follows from Eq.13 that 23 12 fold1223012 23 23 12 unfold 23 1223 12 dP()ee1e P d k tkkC kk t t tkk kk kt k k (14) Comparing Eq.14 with Eq.6, we obtain the following equivalent relation 23 12 12 23 f 23 12 1e k kk Kkk kt (15) (a) (b) unfold Pinter P 12 k fold P 23 k unfold Pinter P 12 k fold P 23 k unfold Pinter P 12 k fold P 23 k 12 ks 23 kss unfold Pinter P 12 k fold P 23 k 12 ks 23 kss Figure 17. (a) The directed graph or digraph [245,246] for the three-state protein folding mechanism as schematically ex- pressed by Eq.9 and formulated by Eq.10. (b) The phase digraph obtained from of panel (a) according to graphic rule 4 for enzyme and protein folding kinetics [245,246], where s is an interim parameter (see the text for further explanation).  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 83 meaning that the apparent folding rate constant f is a function of not only the detailed rate constants, but also t. Accordingly, f is actually not a constant but will change with time. Only when 23 12 kk and 23 1k , can Eq.15 be reduced tof12 kand Eq.14 to folded 12 unfoldunfold dP( )P()P() df tktKt t (16) and f be treated as a constant. Even for a two-state protein folding system when the reverse effect needs to be considered, i.e., the system described by the following scheme and equation 12 21 unfoldfold PP k k (17) unfold 12unfold21 fold fold 12unfold21fold dP( )P()P() d dP()P()P() d tktkt t tktkt t (18) where 21 k represents the reverse rate constant convert- ing fold P back to unfold P. With the similar derivation by using the non-steady state graphic rule [245,246] as de- scribed above, we can get the following equivalent rela- tion [249] 12 1221 f1221 21 121221 exp exp kkk kkt kk kkt (19) indicating that, even for the two-state folding system of Eq.17, the apparent folding rate constant f can be treated as a constant only when 12 21 kk and 12 1k . It can be imagined that for a general multi-state fold- ing system, f will be much more complicated. Con- sequently, all the experimental apparent folding rate constants were actually measured under some special conditions. Recently, a web-server, called “Pred-PFR” (Predict- ing Protein Folding Rate), was developed for predicting the folding rate of a protein [249]. The predictor is fea- tured by fusing multiple individual predictors, each of which is established based on one special feature derived from the protein sequence. As a user-friendly web-server, Pred-PFR is freely accessible to the public at www.csbio.sjtu.edu.cn/bioinf/FoldingRate/. 2.15. FoldRate This is a different kind of protein folding rate predictor developed by fusing the folding-correlated features that can be either directly obtained or easily derived from the sequences of proteins [250]. FoldRate is freely accessible to the public at www.csbio.sjtu.edu.cn/bioinf/FoldRate/. Both Pred-PFR and Fold Rate can be used to predict the folding rate of a protein according to its sequence alone. The time by using the two web-server predictors to get the desired result for a query protein sequence is around 30 seconds. And the results obtained thus ob- tained are usually at least comparable with or even better than the existing methods that, however, need both the sequence and 3D structure information for prediction. 3. LIST OF WEB SERVERS For reader’s convenience, a brief description of each of the 15 web servers introduced in this article as well as its website address is given in Table 3. 4. CONCLUSION Web-server is a newly emerging thing in the Internet Age. Technically speaking, a web-server means a com- puter program that is responsible for accepting HTTP (Hypertext Transfer Protocol) requests from clients. By means of web-servers, many computational prediction methods, regardless how difficult their mathematics or how complicated their algorithms are, can be easily used by the vast majority of scientists without the need to understand the mathematical details. Written as a labo- ratory protocol with a “recipe” style, the web-servers introduced here are user friendly and can be very easily used. Therefore, they are particularly useful for bench scientists to generate various data or information in a timely manner that they may need for their research pro- jects. It is anticipated that all these web-servers are con- stantly evolving with continuously improving the train- ing datasets and prediction algorithms. To keep the users timely informed of the development, a short note will be published or an announcement will be placed in the relevant website.  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 84 Table 3. List of the 15 web servers introduced in this paper as well as their website addresses and targets. No. Name Website address Target 1 Cell-PLoc package http://chou.med.harvard.edu/bioinf/Cell-PLoc/ Protein subcellular localization [49] 2 Nuc-PLoc http://chou.med.harvard.edu/bioinf/Nuc-PLoc/ Protein subnuclear localization [63] 3 Signal-CF http://chou.med.harvard.edu/bioinf/Signal-CF/ Protein signal peptide [79] 4 Signal-3L http://chou.med.harvard.edu/bioinf/Signal-3L/ Protein signal peptide [82] 5 MemType-2L http://chou.med.harvard.edu/bioinf/MemType/ Membrane protein type [54] 6 EzyPred http://chou.med.harvard.edu/bioinf/EzyPred/ Enzyme functional class [126] 7 ProtIdent http://www.csbio.sjtu.edu.cn/bioinf/Protease/ Protease type [55] 8 GPCR-CA http://218.65.61.89:8080/bioinfo/GPCR-CA GPCR type [160] 9 HIVcleave http://chou.med.harvard.edu/bioinf/HIV/ HIV protease cleavage site [187] 10 QuatIdent www.csbio.sjtu.edu.cn/bioinf/Quaternary/ Protein quaternary structural attribute [197] 11 PQSA-Pred http://218.65.61.89:8080/bioinfo/pqsa-pred Protein quaternary structural attribute [198] 12 PFP-Pred http://www.csbio.sjtu.edu.cn/bioinf/PFP-Pred/ Protein fold pattern [232] 13 PFP-FunDSeqE www.csbio.sjtu.edu.cn/bioinf/PFP-FunDSeqE/ Protein fold pattern [234] 14 Pred-PFR www.csbio.sjtu.edu.cn/bioinf/FoldingRate/ Protein folding rate [249] 15 FoldRate www.csbio.sjtu.edu.cn/bioinf/FoldRate/ Protein folding rate [250] REFERENCES [1] Chou, K.C. (2002) A new branch of proteomics: predic- tion of protein cellular attributes. In Weinrer, P. W. and Lu, Q. (eds.), Gene Cloning & Expression Technologies, Chapter 4. Eaton Publishing, Westborough, MA, pp. 57-70. [2] Chou, K.C. (2004) Review: Structural bioinformatics and its impact to biomedical science. Current Medicinal Chemistry, 11, 2105-2134. [3] Chou, K.C. (2006) Structural bioinformatics and its im- pact to biomedical science and drug discovery. Frontiers in Medicinal Chemistry, 3, 455-502. [4] Alberts, B., Bray, D., Lewis, J., Raff, M., Roberts, K. and Watson, J.D. (1994) Molecular Biology of the Cell, chap.1. 3rd ed. Garland Publishing, New York & Lon- don. [5] Lodish, H., Baltimore, D., Berk, A., Zipursky, S.L., Ma- tsudaira, P. and Darnell, J. (1995) Molecular Cell Biol- ogy , Chap.3. 3rd ed. Scientific American Books, New York. [6] Nakai, K. and Kanehisa, M. (1991) Expert system for predicting protein localization sites in Gram-negative bacteria. Proteins: Structure, Function and Genetics, 11, 95-110. [7] Nakashima, H. and Nishikawa, K. (1994) Discrimination of intracellular and extracellular proteins using amino acid composition and residue-pair frequencies. J Mol Biol, 238, 54-61.  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 85 [8] Cedano, J., Aloy, P., P'erez-Pons, J.A. and Querol, E. (1997) Relation between amino acid composition and cellular location of proteins. J Mol Biol, 266, 594-600. [9] Nakai, K. and Horton, P. (1999) PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends in Biochemical Science, 24, 34-36. [10] Chou, K.C. and Elrod, D.W. (1998) Using discriminant function for prediction of subcellular location of pro- karyotic proteins. BBRC, 252, 63-68. [11] Reinhardt, A. and Hubbard, T. (1998) Using neural net- works for prediction of the subcellular location of pro- teins. Nucleic Acids Research, 26, 2230-2236. [12] Chou, K.C. and Elrod, D.W. (1999) Protein subcellular location prediction. Protein Engineering, 12, 107-118. [13] Yuan, Z. (1999) Prediction of protein subcellular loca- tions using Markov chain models. FEBS Letters, 451, 23-26. [14] Nakai, K. (2000) Protein sorting signals and prediction of subcellular localization. Advances in Protein Chemistry, 54, 277-344. [15] Murphy, R.F., Boland, M.V. and Velliste, M. (2000) To- wards a systematics for protein subcellular location: quantitative description of protein localization patterns and automated analysis of fluorescence microscope im- ages. Proc Int Conf Intell Syst Mol Biol, 8, 251-259. [16] Chou, K.C. (2000) Review: Prediction of protein struc- tural classes and subcellular locations. Current Protein and Peptide Science, 1, 171-208. [17] Emanuelsson, O., Nielsen, H., Brunak, S. and von Heijne, G. (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. Journal of Molecular Biology, 300, 1005-1016. [18] Chou, K.C. (2001) Prediction of protein cellular attrib- utes using pseudo amino acid composition. PROTEINS: Structure, Function, and Genetics (Erratum: ibid, 2001, Vol44, 60), 43, 246-255. [19] Feng, Z.P. (2001) Prediction of the subcellular location of prokaryotic proteins based on a new representation of the amino acid composition. Biopolymers, 58, 491-499. [20] Hua, S. and Sun, Z. (2001) Support vector machine ap- proach for protein subcellular localization prediction. Bioinformatics, 17, 721-728. [21] Feng, Z.P. and Zhang, C.T. (2001) Prediction of the sub- cellular location of prokaryotic proteins based on the hy- drophobicity index of amino acids. Int J Biol Macromol, 28, 255-261. [22] Feng, Z.P. (2002) An overview on predicting the subcel- lular location of a protein. In Silico Biol, 2, 291-303. [23] Chou, K.C. and Cai, Y.D. (2002) Using functional do- main composition and support vector machines for pre- diction of protein subcellular location. J Biol Chem, 277, 45765-45769. [24] Zhou, G.P. and Doctor, K. (2003) Subcellular location prediction of apoptosis proteins. PROTEINS: Structure, Function, and Genetics, 50, 44-48. [25] Pan, Y.X., Zhang, Z.Z., Guo, Z.M., Feng, G.Y., Huang, Z.D. and He, L. (2003) Application of pseudo amino acid composition for predicting protein subcellular location: stochastic signal processing approach. Journal of Protein Chemistry, 22, 395-402. [26] Park, K.J. and Kanehisa, M. (2003) Prediction of protein subcellular locations by support vector machines using compositions of amino acid and amino acid pairs. Bioin- formatics, 19, 1656-1663. [27] Gardy, J.L., Spencer, C., Wang, K., Ester, M., Tusnady, G.E., Simon, I., Hua, S., deFays, K., Lambert, C., Nakai, K. et al. (2003) PSORT-B: Improving protein subcellular localization prediction for Gram-negative bacteria. Nu- cleic Acids Research, 31, 3613-3617. [28] Huang, Y. and Li, Y. (2004) Prediction of protein subcel- lular locations using fuzzy k-NN method. Bioinf ormatic s, 20, 21-28. [29] Xiao, X., Shao, S., Ding, Y., Huang, Z., Huang, Y. and Chou, K.C. (2005) Using complexity measure factor to predict protein subcellular location. Amino Acids, 28, 57-61. [30] Gao, Y., Shao, S.H., Xiao, X., Ding, Y.S., Huang, Y.S., Huang, Z.D. and Chou, K.C. (2005) Using pseudo amino acid composition to predict protein subcellular location: approached with Lyapunov index, Bessel function, and Chebyshev filter. Amino Acids, 28, 373-376. [31] Lei, Z. and Dai, Y. (2005) An SVM-based system for predicting protein subnuclear localizations. BMC Bioin- formatics, 6, 291. [32] Shen, H.B. and Chou, K.C. (2005) Predicting protein subnuclear location with optimized evidence-theoretic K-nearest classifier and pseudo amino acid composition. Biochem Biophys Res Comm, 337, 752-756. [33] Garg, A., Bhasin, M. and Raghava, G.P. (2005) Support vector machine-based method for subcellular localization of human proteins using amino acid compositions, their order, and similarity search. J Biol Chem, 280, 14427-14432. [34] Matsuda, S., Vert, J.P., Saigo, H., Ueda, N., Toh, H. and Akutsu, T. (2005) A novel representation of protein se- quences for prediction of subcellular location using sup- port vector machines. Protein Sci, 14, 2804-2813. [35] Gao, Q.B., Wang, Z.Z., Yan, C. and Du, Y.H. (2005) Prediction of protein subcellular location using a com- bined feature of sequence. FEBS Lett, 579, 3444-3448. [36] Chou, K.C. and Shen, H.B. (2006) Predicting protein subcellular location by fusing multiple classifiers. Jour- nal of Cellular Biochemistry, 99, 517-527. [37] Guo, J., Lin, Y. and Liu, X. (2006) GNBSL: A new inte- grative system to predict the subcellular location for Gram-negative bacteria proteins. Proteomics, 6, 5099-5105. [38] Xiao, X., Shao, S.H., Ding, Y.S., Huang, Z.D. and Chou, K.C. (2006) Using cellular automata images and pseudo amino acid composition to predict protein subcellular lo- cation. Amino Acids, 30, 49-54. [39] Hoglund, A., Donnes, P., Blum, T., Adolph, H.W. and Kohlbacher, O. (2006) MultiLoc: prediction of protein subcellular localization using N-terminal targeting se- quences, sequence motifs and amino acid composition. Bioinformatics, 22, 1158-1165. [40] Lee, K., Kim, D.W., Na, D., Lee, K.H. and Lee, D. (2006) PLPD: reliable protein localization prediction from im- balanced and overlapped datasets. Nucleic Acids Res, 34, 4655-4666. [41] Zhang, Z.H., Wang, Z.H., Zhang, Z.R. and Wang, Y.X. (2006) A novel method for apoptosis protein subcellular localization prediction combining encoding based on  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 86 grouped weight and support vector machine. FEBS Lett, 580, 6169-6174. [42] Shi, J.Y., Zhang, S.W., Pan, Q., Cheng, Y.-M. and Xie, J. (2007) Prediction of protein subcellular localization by support vector machines using multi-scale energy and pseudo amino acid composition. Amino Acids, 33, 69-74. [43] Chou, K.C. and Shen, H.B. (2007) Large-scale plant protein subcellular location prediction. Journal of Cellu- lar Biochemistry, 100, 665-678. [44] Shen, H.B. and Chou, K.C. (2007) Hum-mPLoc: An ensemble classifier for large-scale human protein sub- cellular location prediction by incorporating samples with multiple sites. Biochem Biophys Res Commun, 355, 1006-1011. [45] Shen, H.B., Yang, J. and Chou, K.C. (2007) Euk-PLoc: an ensemble classifier for large-scale eukaryotic protein subcellular location prediction. Amino Acids, 33, 57-67. [46] Chen, Y.L. and Li, Q.Z. (2007) Prediction of apoptosis protein subcellular location using improved hybrid ap- proach and pseudo amino acid composition. Journal of Theoretical Biology, 248, 377–381. [47] Chen, Y.L. and Li, Q.Z. (2007) Prediction of the subcel- lular location of apoptosis proteins. Journal of Theoreti- cal Biology, 245, 775-783. [48] Mundra, P., Kumar, M., Kumar, K.K., Jayaraman, V.K. and Kulkarni, B.D. (2007) Using pseudo amino acid composition to predict protein subnuclear localization: Approached with PSSM. Pattern Recognition Letters, 28, 1610-1615. [49] Chou, K.C. and Shen, H.B. (2007) Review: Recent pro- gresses in protein subcellular location prediction. Ana- lytical Biochemistry, 370, 1-16. [50] Chou, K.C. and Shen, H.B. (2008) Cell-PLoc: A package of web-servers for predicting subcellular localization of proteins in various organisms. Nature Protocols, 3, 153-162. [51] Chou, K.C. and Shen, H.B. (2007) Euk-mPLoc: a fusion classifier for large-scale eukaryotic protein subcellular location prediction by incorporating multiple sites. Journal of Proteome Research, 6, 1728-1734. [52] Ashburner, M., Ball, C.A., Blake, J.A., Botstein, D., Butler, H., Cherry, J.M., Davis, A.P., Dolinski, K., Dwight, S.S., Eppig, J.T. et al. (2000) Gene ontology: tool for the unification of biology. Nature Genetics, 25, 25-29. [53] Chou, K.C. (2005) Using amphiphilic pseudo amino acid composition to predict enzyme subfamily classes. Bioin- formatics, 21, 10-19. [54] Chou, K.C. and Shen, H.B. (2007) MemType-2L: A Web server for predicting membrane proteins and their types by incorporating evolution information through Pse-PSSM. Biochem Biophys Res Comm, 360, 339-345. [55] Chou, K.C. and Shen, H.B. (2008) ProtIdent: A web server for identifying proteases and their types by fusing functional domain and sequential evolution information. Biochem Biophys Res Comm, 376, 321-325. [56] Shen, H.B. and Chou, K.C. (2009) A top-down approach to enhance the power of predicting human protein sub- cellular localization: Hum-mPLoc 2.0. Analytical Bio- chemistry, in press. [57] Shen, H.B. and Chou, K.c. (2009) Gpos-mPLoc: A top-down approach to improve the quality of predicting subcellular localization of Gram-positive bacterial pro- teins. Protein & Peptide Letters, submitted. [58] Shen, H.B. and Chou, K.C. (2009) Gneg-mPLoc: A top-down strategy to enhance the quality of predicting subcellular localization of Gram-negative bacterial pro- teins, to be submitted. [59] Chou, K.C. and Shen, H.B. (2009) Plant-mPLoc: A top-down strategy to augment the power for predicting plant protein subcellular localization, to be submitted. [60] Shen, H.B. and Chou, K.C. (2007) Gpos-PLoc: an en- semble classifier for predicting subcellular localization of Gram-positive bacterial proteins. Protein Engineering, Design, and Selection, 20, 39-46. [61] Chou, K.C. and Shen, H.B. (2006) Large-scale predic- tions of Gram-negative bacterial protein subcellular loca- tions. Journal of Proteome Research, 5, 3420-3428. [62] Alberts, B., Johnson, A., Lewis, J., Raff, M., Roberts, K. and Walter, P. (2002) Molecular biology of the cell, 4th edition. Garland Science, New York. [63] Shen, H.B. and Chou, K.C. (2007) Nuc-PLoc: A new web-server for predicting protein subnuclear localization by fusing PseAA composition and PsePSSM. Protein Engineering, Design & Selection, 20, 561-567. [64] Rapoport, T.A. (1992) Transport of proteins across the endoplasmic reticulum membrane. Science, 258, 931-936. [65] Zheng, N. and Gierasch, L.M. (1996) Signal sequences: the same yet different. Cell, 86, 849-852. [66] Chou, K.C. (2001) Prediction of signal peptides using scaled window. Peptides, 22, 1973-1979. [67] McGeoch, D.J. (1985) On the predictive recognition of signal peptide sequences. Virus Res, 3, 271-286. [68] von Heijne, G. (1986) A new method for predicting signal sequence cleavage sites. Nucleic Acids Research, 14, 4683-4690. [69] Folz, R.J. and Gordon, J.I. (1987) Computer-assisted predictions of signal peptidase processing sites. Biochem Biophys Res Comm, 146, 870-877. [70] Ladunga, I., Czako, F., Csabai, I. and Geszti, T. (1991) Improving signal peptide prediction accuracy by simu- lated neural network. Comput Appl Biosci, 7, 485-487. [71] Arrigo, P., Giuliano, F., Scalia, F., Rapallo, A. and Damiani, G. (1991) Identification of a new motif on nu- cleic acid sequence data using Kohonen's self-organizing map. Comput Appl Biosci, 7, 353-357. [72] Schneider, G. and Wrede, P. (1993) Signal analysis of protein targeting sequences. Protein Seq Data Anal, 5, 227-236. [73] Nielsen, H., Engelbrecht, J., Brunak, S. and von Heijne, G. (1997) Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Engineering, 10, 1-6. [74] Emanuelsson, O., Nielsen, H. and von Heijne, G. (1999) ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Pro- tein Science, 8, 978-984. [75] Bendtsen, J.D., Nielsen, H., von Heijne, G. and Brunak, S. (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol, 340, 783-795. [76] Hiller, K., Grote, A., Scheer, M., Munch, R. and Jahn, D. (2004) PrediSi: prediction of signal peptides and their  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 87 cleavage positions. Nucleic Acids Res, 32, W375-379. [77] Chou, K.C. (2002) Review: Prediction of protein signal sequences. Current Protein and Peptide Science, 3, 615-622. [78] Chou, K.C. (2001) Using subsite coupling to predict signal peptides. Protein Engineering, 14, 75-79. [79] Chou, K.C. and Shen, H.B. (2007) Signal-CF: a sub- site-coupled and window-fusing approach for predicting signal peptides. Biochem Biophys Res Comm, 357, 633-640. [80] Hiss, J.A. and Schneider, G. (2009) Architecture, function and prediction of long signal peptides. Brief Bioinform, 10, 569-578. [81] Kall, L., Krogh, A. and Sonnhammer, E.L. (2007) Ad- vantages of combined transmembrane topology and sig- nal peptide prediction--the Phobius web server. Nucleic Acids Res, 35, W429-432. [82] Shen, H.B. and Chou, K.C. (2007) Signal-3L: a 3-layer approach for predicting signal peptide. Biochem Bio- phys Res Comm, 363, 297-303. [83] Reynolds, S.M., Kall, L., Riffle, M.E., Bilmes, J.A. and Noble, W.S. (2008) Transmembrane topology and signal peptide prediction using dynamic bayesian networks. PLoS Comput Biol, 4, e1000213. [84] Chou, K.C. (2004) Modelling extracellular domains of GABA-A receptors: subtypes 1, 2, 3, and 5. Biochemical and Biophysical Research Communications, 316, 636-642. [85] Chou, K.C. (1993) Conformational change during photocycle of bacteriorhodopsin and its proton-pumping mechanism. Journal of Protein Chemistry, 12, 337-350. [86] Chou, K.C. (1994) Mini Review: A molecular piston mechanism of pumping protons by bacteriorhodopsin. Amino Acids, 7, 1-17. [87] Schnell, J.R. and Chou, J.J. (2008) Structure and mecha- nism of the M2 proton channel of influenza A virus. Na- ture, 451, 591-595. [88] Doyle, D.A., Morais, C.J., Pfuetzner, R.A., Kuo, A., Gulbis, J.M., Cohen, S.L., Chait, B.T. and MacKinnon, R. (1998) The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science, 280, 69-77. [89] Chou, K.C. (2004) Insights from modelling three-dimensional structures of the human potassium and sodium channels. Journal of Proteome Research, 3, 856-861. [90] Huang, R.B., Du, Q.S., Wang, C.H. and Chou, K.C. (2008) An in-depth analysis of the biological functional studies based on the NMR M2 channel structure of in- fluenza A virus. Biochem Biophys Res Comm, 377, 1243-1247. [91] Du, Q.S., Huang, R.B., Wang, C.H., Li, X.M. and Chou, K.C. (2009) Energetic analysis of the two controversial drug binding sites of the M2 proton channel in influenza A virus. Journal of Theoretical Biology, 259, 159-164. [92] Pielak, R.M., Jason R. Schnell, J.R. and Chou, J.J. (2009) Mechanism of drug inhibition and drug resistance of in- fluenza A M2 channel. Proceedings of National Acad- emy of Science, USA, 106, 7379-7384. [93] Oxenoid, K. and Chou, J.J. (2005) The structure of phospholamban pentamer reveals a channel-like archi- tecture in membranes. Proc Natl Acad Sci U S A, 102, 10870-10875. [94] Douglas, S.M., Chou, J.J. and Shih, W.M. (2007) DNA-nanotube-induced alignment of membrane proteins for NMR structure determination. Proc Natl Acad Sci U S A, 104, 6644-6648. [95] Nakashima, H., Nishikawa, K. and Ooi, T. (1986) The folding type of a protein is relevant to the amino acid composition. J Biochem, 99, 152-162. [96] Klein, P. and Delisi, C. (1986) Prediction of protein structural class from amino acid sequence. Biopolymers, 25, 1659-1672. [97] Klein, P. (1986) Prediction of protein structural class by discriminant analysis. Biochim Biophys Acta, 874, 205-215. [98] Chou, K.C. and Zhang, C.T. (1994) Predicting protein folding types by distance functions that make allowances for amino acid interactions. J Biol Chem, 269, 22014-22020. [99] Chou, K.C. (1995) A novel approach to predicting pro- tein structural classes in a (20-1)-D amino acid composi- tion space. Proteins: Structure, Function & Genetics, 21, 319-344. [100] Liu, W. and Chou, K.C. (1998) Prediction of protein structural classes by modified Mahalanobis discriminant algorithm. Journal of Protein Chemistry, 17, 209-217. [101] Chou, K.C., Liu, W., Maggiora, G.M. and Zhang, C.T. (1998) Prediction and classification of domain structural classes. PROTEINS: Structure, Function, and Genetics, 31, 97-103. [102] Chou, K.C. and Maggiora, G.M. (1998) Domain struc- tural class prediction. Protein Engineering, 11, 523-538. [103] Chou, K.C. (1999) A key driving force in determination of protein structural classes. Biochemical and Biophysi- cal Research Communications, 264, 216-224. [104] Chou, K.C. and Elrod, D.W. (1999) Prediction of mem- brane protein types and subcellular locations. PROTEINS: Structure, Function, and Genetics, 34, 137-153. [105] Cai, Y.D., Liu, X.J. and Chou, K.C. (2001) Artificial neural network model for predicting membrane protein types. Journal of Biomolecular Structure and Dynamics, 18, 607-610. [106] Guo, Z.M. (2002) Prediction of Membrane protein types by using pattern recognition method based on pseudo amino acid composition. Master Thesis, Bio-X Life Sci- ence Research Center, Shanghai Jiaotong University. [107] Cai, Y.D., Zhou, G.P. and Chou, K.C. (2003) Support vector machines for predicting membrane protein types by using functional domain composition. Biophysical Journal, 84, 3257-3263. [108] Cai, Y.D., Pong-Wong, R., Feng, K., Jen, J.C.H. and Chou, K.C. (2004) Application of SVM to predict mem- brane protein types. Journal of Theoretical Biology, 226, 373-376. [109] Wang, M., Yang, J., Liu, G.P., Xu, Z.J. and Chou, K.C. (2004) Weighted-support vector machines for predicting membrane protein types based on pseudo amino acid composition. Protein Engineering, Design, and Selection, 17, 509-516. [110] Chou, K.C. and Cai, Y.D. (2005) Prediction of membrane protein types by incorporating amphipathic effects. Journal of Chemical Information and Modeling, 45, 407-413.  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 88 [111] Liu, H., Wang, M. and Chou, K.C. (2005) Low-frequency Fourier spectrum for predicting mem- brane protein types. Biochem Biophys Res Commun, 336, 737-739. [112] Wang, M., Yang, J., Xu, Z.J. and Chou, K.C. (2005) SLLE for predicting membrane protein types. Journal of Theoretical Biology, 232, 7-15. [113] Shen, H.B. and Chou, K.C. (2005) Using optimized evi- dence-theoretic K-nearest neighbor classifier and pseudo amino acid composition to predict membrane protein types. Biochemical & Biophysical Research Communica- tions, 334, 288-292. [114] Shen, H.B., Yang, J. and Chou, K.C. (2006) Fuzzy KNN for predicting membrane protein types from pseudo amino acid composition. Journal of Theoretical Biology, 240, 9-13. [115] Wang, S.Q., Yang, J. and Chou, K.C. (2006) Using stacked generalization to predict membrane protein types based on pseudo amino acid composition. Journal of Theoretical Biology, 242, 941-946. [116] Shen, H.B. and Chou, K.C. (2007) Using ensemble clas- sifier to identify membrane protein types. Amino Acids, 32, 483-488. [117] Yang, X.G., Luo, R.Y. and Feng, Z.P. (2007) Using amino acid and peptide composition to predict membrane pro- tein types. Biochem Biophys Res Commun, 353, 164-169. [118] Pu, X., Guo, J., Leung, H. and Lin, Y. (2007) Prediction of membrane protein types from sequences and posi- tion-specific scoring matrices. J Theor Biol, 247, 259–265. [119] Afjehi-Sadat, L. and Lubec, G. (2007) Identification of enzymes and activity from two-dimensional gel electro- phoresis. Nature Protocols, 2, 2318-2324. [120] Chou, K.C. and Elrod, D.W. (2003) Prediction of enzyme family classes. Journal of Proteome Research, 2, 183-190. [121] Chou, K.C. and Cai, Y.D. (2004) Predicting enzyme fam- ily class in a hybridization space. Protein Science, 13, 2857-2863. [122] Cai, C.Z., Han, L.Y., Ji, Z.L. and Chen, Y.Z. (2004) En- zyme family classification by support vector machines. PROTEINS: Structure, Function, and Bioinformatics, 55, 66-76. [123] 1Cai, Y.D. and Chou, K.C. (2005) Predicting enzyme subclass by functional domain composition and pseudo amino acid composition. Journal of Proteome Research, 4, 967-971. [124] Huang, W.L., Chen, H.M., Hwang, S.F. and Ho, S.Y. (2006) Accurate prediction of enzyme subfamily class using an adaptive fuzzy k-nearest neighbor method. Bio- systems, 90, 405-413. [125] Zhou, X.B., Chen, C., Li, Z.C. and Zou, X.Y. (2007) Using Chou's amphiphilic pseudo-amino acid composi- tion and support vector machine for prediction of enzyme subfamily classes. Journal of Theoretical Biology, 248, 546–551. [126] Shen, H.B. and Chou, K.C. (2007) EzyPred: A top-down approach for predicting enzyme functional classes and subclasses. Biochem Biophys Res Comm, 364, 53-59. [127] Bairoch, A. (2000) The ENZYME Database in 2000. Nucleic Acids Research, 28, 304-305. [128] Poorman, R.A., Tomasselli, A.G., Heinrikson, R.L. and Kezdy, F.J. (1991) A cumulative specificity model for proteases from human immunodeficiency virus types 1 and 2, inferred from statistical analysis of an extended substrate data base. J Biol Chem, 266, 14554-14561. [129] Qin, H., Srinvasula, S.M., Wu, G., Fernandes-Alnemri, T., Alnemri, E.S., and Shi, Y. (1999) Structural basis of pro- caspase-9 recruitment by the apoptotic prote- ase-activating factor 1. Nature, 399, 549-557. [130] Chou, J.J., Li, H., Salvessen, G.S., Yuan, J. and Wagner, G. (1999) Solution structure of BID, an intracellular am- plifier of apoptotic signalling. Cell, 96, 615-624. [131] Watt, W., Koeplinger, K.A., Mildner, A.M., Heinrikson, R.L., Tomasselli, A.G. and Watenpaugh, K.D. (1999) The atomic resolution structure of human caspase-8, a key activator of apoptosis. Structure, 7, 1135-1143. [132] Chou, K.C., Wei, D.Q. and Zhong, W.Z. (2003) Binding mechanism of coronavirus main proteinase with ligands and its implication to drug design against SARS. (Erra- tum: ibid., 2003, Vol.310, 675). Biochem Biophys Res Comm, 308, 148-151. [133] Puente, X.S., Sanchez, L.M., Overall, C.M. and Lo- pez-Otin, C. (2003) Human and mouse proteases: a comparative genomic approach. Nat Rev Genet, 4, 544-558. [134] Chou, K.C., Wei, D.Q., Du, Q.S., Sirois, S., Shen, H.B. and Zhong, W.Z. (2009) Study of inhibitors against SARS coronavirus by computational approaches. In Lendeckel, U. and Hooper, N. (eds.), Viral proteases and antiviral protease inhibitor therapy. Proteases in Biology and Disease, Springer Publishing, 8. [135] Chou, K.C. (1993) A vectorized sequence-coupling model for predicting HIV protease cleavage sites in pro- teins. J Biol Chem, 268, 16938-16948. [136] Chou, K.C. (1996) Review: Prediction of HIV protease cleavage sites in proteins. Analytical Biochemistry, 233, 1-14. [137] You, L., Garwicz, D. and Rognvaldsson, T. (2005) Com- prehensive bioinformatic analysis of the specificity of human immunodeficiency virus type 1 protease. J Virol, 79, 12477-12486. [138] Rognvaldsson, T., You, L. and Garwicz, D. (2007) Bio- informatic approaches for modeling the substrate speci- ficity of HIV-1 protease: an overview. Expert Rev Mol Diagn, 7, 435-451. [139] Liang, G.Z. and Li, S.Z. (2007) A new sequence repre- sentation as applied in better specificity elucidation for human immunodeficiency virus type 1 protease. Bio- polymers, 88, 401-412. [140] Rawlings, N.D., Tolle, D.P. and Barrett, A.J. (2004) MEROPS: the peptidase database. Nucleic Acids Re- search, 32, D160-D164. [141] Chou, K.C. and Cai, Y.D. (2006) Prediction of protease types in a hybridization space. Biochem Biophys Res Comm, 339, 1015-1020. [142] Zhou, G.P. and Cai, Y.D. (2006) Predicting protease types by hybridizing gene ontology and pseudo amino acid composition. PROTEINS: Structure, Function, and Bio- informatics, 63, 681-684. [143] Shen, H.B. and Chou, K.C. (2009) Identification of pro- teases and their types. Analytical Biochemistry, 385, 153-160. [144] Heuss, C. and Gerber, U. (2000) G-protein-independent  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 89 signaling by G-protein-coupled receptors. Trends Neuro- sci, 23, 469-475. [145] Milligan, G. and White, J.H. (2001) Protein-protein in- teractions at G-protein-coupled receptors. Trends Phar- macol Sci, 22, 513-518. [146] Hall, R.A. and Lefkowitz, R.J. (2002) Regulation of G protein-coupled receptor signaling by scaffold proteins. Circ Res, 91, 672-680. [147] Chou, K.C. (2005) Coupling interaction between throm- boxane A2 receptor and alpha-13 subunit of guanine nu- cleotide-binding protein. Journal of Proteome Research, 4, 1681-1686. [148] Call, M.E., Schnell, J.R., Xu, C., Lutz, R.A., Chou, J.J. and Wucherpfennig, K.W. (2006) The structure of the zetazeta transmembrane dimer reveals features essential for its assembly with the T cell receptor. Cell, 127, 355-368. [149] Chou, K.C. (2004) Insights from modelling the 3D structure of the extracellular domain of alpha7 nicotinic acetylcholine receptor. Biochemical and Biophysical Re- search Communication, 319, 433-438. [150] Chou, K.C. (2004) Molecular therapeutic target for type-2 diabetes. Journal of Proteome Research, 3, 1284-1288. [151] Wei, D.Q., Du, Q.S., Sun, H. and Chou, K.C. (2006) Insights from modeling the 3D structure of H5N1 influ- enza virus neuraminidase and its binding interactions with ligands. Biochem Biophys Res Comm, 344, 1048-1055. [152] Wang, S.Q., Du, Q.S. and Chou, K.C. (2007) Study of drug resistance of chicken influenza A virus (H5N1) from homology-modeled 3D structures of neuramini- dases. Biochem Biophys Res Comm, 354, 634-640. [153] Wang, S.Q., Du, Q.S., Huang, R.B., Zhang, D.W. and Chou, K.C. (2009) Insights from investigating the inter- action of oseltamivir (Tamiflu) with neuraminidase of the 2009 H1N1 swine flu virus. Biochem Biophys Res Com- mun, 386, 432-436. [154] Elrod, D.W. and Chou, K.C. (2002) A study on the corre- lation of G-protein-coupled receptor types with amino acid composition. Protein Engineering, 15, 713-715. [155] Chou, K.C. and Elrod, D.W. (2002) Bioinformatical analysis of G-protein-coupled receptors. Journal of Pro- teome Research, 1, 429-433. [156] Bhasin, M. and Raghava, G.P. (2005) GPCRsclass: a web tool for the classification of amine type of G-protein-coupled receptors. Nucleic Acids Research, 33, W143-147. [157] Chou, K.C. (2005) Prediction of G-protein-coupled re- ceptor classes. Journal of Proteome Research, 4, 1413-1418. [158] Wen, Z., Li, M., Li, Y., Guo, Y. and Wang, K. (2007) Delaunay triangulation with partial least squares projec- tion to latent structures: a model for G-protein coupled receptors classification and fast structure recognition. Amino Acids, 32, 277-283. [159] Gao, Q.B. and Wang, Z.Z. (2006) Classification of G-protein coupled receptors at four levels. Protein Eng Des Sel, 19, 511-516. [160] Xiao, X., Wang, P. and Chou, K.C. (2009) GPCR-CA: A cellular automaton image approach for predicting G-protein-coupled receptor functional classes. Journal of Computational Chemistry, 30, 1414-1423. [161] Wolfram, S. (1984) Cellular automation as models of complexity. Nature, 311, 419-424. [162] Wolfram, S. (2002) A New Kind of Science. Wolfram Media Inc., Champaign, IL. [163] Xiao, X., Shao, S., Ding, Y., Huang, Z., Chen, X. and Chou, K.C. (2005) Using cellular automata to generate Image representation for biological sequences. Amino Acids, 28, 29-35. [164] Chou, K.C. (2000) Prediction of protein subcellular loca- tions by incorporating quasi-sequence-order effect. Bio- chemical & Biophysical Research Communications, 278, 477-483. [165] Althaus, I.W., Chou, J.J., Gonzales, A.J., Diebel, M.R., Chou, K.C., Kezdy, F.J., Romero, D.L., Aristoff, P.A., Tarpley, W.G. and Reusser, F. (1993) Steady-state kinetic studies with the non-nucleoside HIV-1 reverse transcrip- tase inhibitor U-87201E. J Biol Chem, 268, 6119-6124. [166] Althaus, I.W., Gonzales, A.J., Chou, J.J., Diebel, M.R., Chou, K.C., Kezdy, F.J., Romero, D.L., Aristoff, P.A., Tarpley, W.G. and Reusser, F. (1993) The quinoline U-78036 is a potent inhibitor of HIV-1 reverse transcrip- tase. J Biol Chem, 268, 14875-14880. [167] Althaus, I.W., Chou, J.J., Gonzales, A.J., Diebel, M.R., Chou, K.C., Kezdy, F.J., Romero, D.L., Aristoff, P.A., Tarpley, W.G. and Reusser, F. (1993) Kinetic studies with the nonnucleoside HIV-1 reverse transcriptase inhibitor U-88204E. Biochemistry, 32, 6548-6554. [168] Althaus, I.W., Chou, J.J., Gonzales, A.J., Diebel, M.R., Chou, K.C., Kezdy, F.J., Romero, D.L., Aristoff, P.A., Tarpley, W.G. and Reusser, F. (1994) Steady-state kinetic studies with the polysulfonate U-9843, an HIV reverse transcriptase inhibitor. Ex perien tia, 50, 23-28. [169] Althaus, I.W., Chou, J.J., Gonzales, A.J., Diebel, M.R., Chou, K.C., Kezdy, F.J., Romero, D.L., Thomas, R.C., Aristoff, P.A., Tarpley, W.G. et al. (1994) Kinetic studies with the non-nucleoside HIV-1 reverse transcriptase in- hibitor U-90152E. Biochemical Pharmacology, 47, 2017-2028. [170] Althaus, I.W., Chou, K.C., Franks, K.M., Diebel, M.R., Kezdy, F.J., Romero, D.L., Thomas, R.C., Aristoff, P.A., Tarpley, W.G. and Reusser, F. (1996) The benzyl- thio-pyrididine U-31,355 is a potent inhibitor of HIV-1 reverse transcriptase. Biochemical Pharmacology, 51, 743-750. [171] Chou, K.C., Kezdy, F.J. and Reusser, F. (1994) Review: Steady-state inhibition kinetics of processive nucleic acid polymerases and nucleases. Analytical Biochemistry, 221, 217-230. [172] McQuade, T.J., Tomasselli, A.G., Liu, L., Karacostas, V., Moss, B., Sawyer, T.K., Heinrikson, R.L. and Tarpley, W.G. (1990) A synthetic HIV-1 protease inhibitor with antiviral activity arrests HIV-like particle maturation. Science, 247, 454-456. [173] Meek, T.D., Lambert, D.M., Dreyer, G.B., Carr, T.J., Tomaszek, T.A., Jr., Moore, M.L., Strickler, J.E., De- bouck, C., Hyland, L.J., Matthews, T.J. et al. (1990) In- hibition of HIV-1 protease in infected T-lymphocytes by synthetic peptide analogues. Nature, 343, 90-92. [174] Wlodawer, A. and Erickson, J.W. (1993) Structure-based inhibitors of HIV-1 protease. Annu Rev Biochem, 62, 543-585.  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 90 [175] Barre-Sinoussi, F., Chermann, J.C., Rey, F., Nugeyre, M.T., Chamaret, S., Gruest, J., Dauguet, C., Axler-Blin, C., Vezinet-Brun, F., Rouzioux, C. et al. (1983) Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science, 220, 868-871. [176] Gallo, R.C., Salahuddin, S.Z., Popovic, M., Shearer, G.M., Kaplan, M., Haynes, B.F., Palker, T.J., Redfield, R., Oleske, J., Safai, B. et al. (1984) Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from pa- tients with AIDS and at risk for AIDS. Science, 224, 500-503. [177] Miller, M., Schneider, J., Sathyanarayana, B.K., Toth, M.V., Marshall, G.R., Clawson, L., Selk, L., Kent, S.B. and Wlodawer, A. (1989) Structure of complex of syn- thetic HIV-1 protease with a substrate-based inhibitor at 2.3 A resolution. Science, 246, 1149-1152. [178] Schechter, I. and Berger, A. (1967) On the size of the active site in protease. I. Papain. Biochem Biophys Res Comm, 27, 157-162. [179] Chou, K.C., Chen, N.Y. and Forsen, S. (1981) The bio- logical functions of low-frequency phonons: 2. Coopera- tive effects. Chemica Scripta, 18, 126-132. [180] Chou, K.C., Zhang, C.T. and Kezdy, F.J. (1993) A vector approach to predicting HIV protease cleavage sites in proteins. Proteins: Structure, Function, and Genetics, 16, 195-204. [181] Chou, J.J. (1993) Predicting cleavability of peptide se- quences by HIV protease via correlation-angle approach. Journal of Protein Chemistry, 12, 291-302. [182] Chou, K.C. and Zhang, C.T. (1993) Studies on the speci- ficity of HIV protease: an application of Markov chain theory. Journal of Protein Chemistry, 12, 709-724. [183] Chou, J.J. (1993) A formulation for correlating properties of peptides and its application to predicting human im- munodeficiency virus protease-cleavable sites in proteins. Biopolymers, 33, 1405-1414. [184] Zhang, C.T. and Chou, K.C. (1993) An alter- nate-subsite-coupled model for predicting HIV protease cleavage sites in proteins. Protein Engineering, 7, 65-73. [185] Thompson, T.B., Chou, K.C. and Zheng, C. (1995) Neu- ral network prediction of the HIV-1 protease cleavage sites. Journal of Theoretical Biology 177, 369-379. [186] Chou, K.C., Tomasselli, A.L., Reardon, I.M. and Hein- rikson, R.L. (1996) Predicting HIV protease cleavage sites in proteins by a discriminant function method. PROTEINS: Structure, Function, and Genetics, 24, 51-72. [187] Shen, H.B. and Chou, K.C. (2008) HIVcleave: a web-server for predicting HIV protease cleavage sites in proteins. Analytical Biochemistry, 375, 388-390. [188] Klotz, I.M., Darnell, D.W. and Langerman, N.R. (1975) Quaternary structure of proteins. In Neurath, H. and Hill, R. L. (eds.), The Proteins (3rd ed). Academic Press, New York, 1, 226-241. [189] Chou, K.C. and Cai, Y.D. (2003) Predicting protein qua- ternary structure by pseudo amino acid composition. PROTEINS: Structure, Function, and Genetics, 53, 282-289. [190] Goodsell, D.S. and Olson, A.J. (2000) Structural symme- try and protein function. Annu Rev Biophys Biomol Struct, 29, 105-153. [191] Levy, E.D., Boeri Erba, E., Robinson, C.V. and Teichmann, S.A. (2008) Assembly reflects evolution of protein complexes. Nature, 453, 1262-1265. [192] Chen, Z., Alcayaga, C., Suarez-Isla, B.A., O'Rourke, B., Tomaselli, G. and Marban, E. (2002) A "minimal" sodium channel construct consisting of ligated S5-P-S6 segments forms a toxin-activatable ionophore. J Biol Chem, 277, 24653-24658. [193] Oxenoid, K., Rice, A.J. and Chou, J.J. (2007) Comparing the structure and dynamics of phospholamban pentamer in its unphosphorylated and pseudo-phosphorylated states. Protein Sci, 16, 1977-1983. [194] Tretter, V., Ehya, N., Fuchs, K. and Sieghart, W. (1997) Stoichiometry and assembly of a recombinant GABAA receptor subtype. Journal of Neuroscience, 17, 2728-2737. [195] Perutz, M.F. (1964) The Hemoglobin Molecule. Scien- tific American, 211 , 65-76. [196] Wei, H., Wang, C.H., Du, Q.S., Meng, J. and Chou, K.C. (2009) Investigation into adamantane-based M2 inhibi- tors with FB-QSAR. Medicinal Chemistry, 5, 305-317. [197] Shen, H.B. and Chou, K.C. (2009) QuatIdent: A web server for identifying protein quaternary structural attrib- ute by fusing functional domain and sequential evolution information. Journal of Proteome Research, 8, 1577–1584. [198] Xiao, X., Wang, P. and Chou, K.C. (2009) Predicting protein quaternary structural attribute by hybridizing functional domain composition and pseudo amino acid composition. Journal of Applied Crystallography, 42, 169-173. [199] Garian, R. (2001) Prediction of quaternary structure from primary structure. Bioinformatics, 17, 551-556. [200] Zhang, S.W., Chen, W., Yang, F. and Pan, Q. (2008) Us- ing Chou's pseudo amino acid composition to predict protein quaternary structure: a sequence-segmented PseAAC approach. Amino Acids, 35, 591-598. [201] Anfinsen, C.B. and Scheraga, H.A. (1975) Experimental and theoretical aspects of protein folding. Adv Protein Chem, 29, 205-300. [202] Aguzzi, A. (2008) Unraveling prion strains with cell biology and organic chemistry. Proc Natl Acad Sci U S A, 105, 11-12. [203] Dobson, C.M. (2001) The structural basis of protein folding and its links with human disease. Philos Trans R Soc Lond B Biol Sci, 356, 133-145. [204] Prusiner, S.B. (1998) Prions. Proc Natl Acad Sci U S A, 95, 13363-13383. [205] Anfinsen, C.B. (1973) Principles that govern the folding of protein chains. Science, 181, 223-230. [206] Chou, K.C. and Scheraga, H.A. (1982) Origin of the right-handed twist of beta-sheets of poly-L-valine chains. Proceedings of National Academy of Sciences, USA, 79, 7047-7051. [207] Chou, K.C., Maggiora, G.M., Nemethy, G. and Scheraga, H.A. (1988) Energetics of the structure of the four-alpha-helix bundle in proteins. Proceedings of Na- tional Academy of Sciences, USA, 85, 4295-4299. [208] Chou, K.C., Nemethy, G. and Scheraga, H.A. (1990) Review: Energetics of interactions of regular structural elements in proteins. Accounts of Chemical Research, 23, 134-141.  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 91 [209] Chou, K.C., Nemethy, G. and Scheraga, H.A. (1984) Energetic approach to packing of a-helices: 2. General treatment of nonequivalent and nonregular helices. Journal of American Chemical Society, 106, 3161-3170. [210] Chou, K.C., Nemethy, G., Pottle, M. and Scheraga, H.A. (1989) Energy of stabilization of the right-handed beta-alpha-beta crossover in proteins. Journal of Mo- lecular Biology, 205, 241-249. [211] Chou, K.C. and Carlacci, L. (1991) Energetic approach to the folding of alpha/beta barrels. Proteins: Structure, Function, and Genetics, 9, 280-295. [212] Chou, K.C. (1992) Energy-optimized structure of anti- freeze protein and its binding mechanism. Journal of Molecular Biology, 223, 509-517. [213] Carlacci, L., Chou, K.C. and Maggiora, G.M. (1991) A heuristic approach to predicting the tertiary structure of bovine somatotropin. Biochemistry, 30, 4389-4398. [214] Scheraga, H.A., Khalili, M. and Liwo, A. (2007) Pro- tein-folding dynamics: overview of molecular simulation techniques. Annu Rev Phys Chem, 58, 57-83. [215] Holm, L. and Sander, C. (1999) Protein folds and fami- lies: sequence and structure alignments. Nucleic Acids Research, 27, 244-247. [216] Chou, K.C. (1995) The convergence-divergence duality in lectin domains of the selectin family and its implica- tions. FEBS Letters, 363, 123-126. [217] Chou, K.C., Jones, D. and Heinrikson, R.L. (1997) Pre- diction of the tertiary structure and substrate binding site of caspase-8. FEBS Letters, 419, 49-54. [218] Chou, J.J., Matsuo, H., Duan, H. and Wagner, G. (1998) Solution structure of the RAIDD CARD and model for CARD/CARD interaction in caspase-2 and caspase-9 re- cruitment. Cell, 94, 171-180. [219] Chou, K.C., Tomasselli, A.G. and Heinrikson, R.L. (2000) Prediction of the Tertiary Structure of a Cas- pase-9/Inhibitor Complex. FEBS Letters, 470, 249-256. [220] Chou, K.C. and Howe, W.J. (2002) Prediction of the tertiary structure of the beta-secretase zymogen. BBRC, 292, 702-708. [221] Du, Q.S., Wang, S., Wei, D.Q., Sirois, S. and Chou, K.C. (2005) Molecular modelling and chemical modification for finding peptide inhibitor against SARS CoV Mpro. Analytical Biochemistry, 337, 262-270. [222] Zhang, R., Wei, D.Q., Du, Q.S. and Chou, K.C. (2006) Molecular modeling studies of peptide drug candidates against SARS. Medicinal Chemistry, 2, 309-314. [223] Wang, J.F., Wei, D.Q., Li, L., Zheng, S.Y., Li, Y.X. and Chou, K.C. (2007) 3D structure modeling of cytochrome P450 2C19 and its implication for personalized drug de- sign. Biochem Biophys Res Commun (Corrigendum: ibid, 2007, Vol357, 330), 355, 513-519. [224] Wang, J.F., Wei, D.Q., Lin, Y., Wang, Y.H., Du, H.L., Li, Y.X. and Chou, K.C. (2007) Insights from modeling the 3D structure of NAD(P)H-dependent D-xylose reductase of Pichia stipitis and its binding interactions with NAD and NADP. Biochem Biophys Res Comm, 359, 323-329. [225] Wang, J.F., Wei, D.Q., Chen, C., Li, Y. and Chou, K.C. (2008) Molecular modeling of two CYP2C19 SNPs and its implications for personalized drug design. Protein & Peptide Letters, 15, 27-32. [226] Wang, J.F., Wei, D.Q., Du, H.L., Li, Y.X. and Chou, K.C. (2008) Molecular modeling studies on NADP-dependence of Candida tropicalis strain xylose reductase. The Open Bioinformatics Journal, 2, 72-79. [227] Ding, C.H. and Dubchak, I. (2001) Multi-class protein fold recognition using support vector machines and neu- ral networks. Bioinformatics, 17, 349-358. [228] Finkelstein, A.V. and Ptitsyn, O.B. (1987) Why do globular proteins fit the limited set of folding patterns? Prog Biophys Mol Biol, 50, 171-190. [229] Chou, K.C. and Zhang, C.T. (1995) Review: Prediction of protein structural classes. Critical Reviews in Bio- chemistry and Molecular Biology, 30, 275-349. [230] Dubchak, I., Muchnik, I., Mayor, C., Dralyuk, I. and Kim, S.H. (1999) Recognition of a protein fold in the context of the Structural Classification of Proteins (SCOP) clas- sification. PROTEINS: Structure, Function, and Genetics, 35, 401-407. [231] Murzin, A.G., Brenner, S.E., Hubbard, T. and Chothia, C. (1995) SCOP: a structural classification of protein data- base for the investigation of sequence and structures. Journal of Molecular Biology, 247, 536-540. [232] Shen, H.B. and Chou, K.C. (2006) Ensemble classifier for protein fold pattern recognition. Bioinformatics, 22, 1717-1722. [233] Chou, K.C. and Shen, H.B. (2006) Predicting eukaryotic protein subcellular location by fusing optimized evi- dence-theoretic K-nearest neighbor classifiers. Journal of Proteome Research, 5, 1888-1897. [234] Shen, H.B. and Chou, K.C. (2009) Predicting protein fold pattern with functional domain and sequential evo- lution information. Journal of Theoretical Biology, 256, 441-446. [235] Qiu, L.L., Pabit, S.A., Roitberg, A.E. and Hagen, S.J. (2002) Smaller and faster: The 20-residue Trp-cage pro- tein folds in 4 microseconds. Journal of American Chemical Society, 124, 12952-12953. [236] Goldberg, M.E., Semisotnov, G.V., Friguet, B., Kuwajima, K., Ptitsyn, O.B. and Sugai, S. (1990) An early immuno- reactive folding intermediate of the tryptophan synthease beta 2 subunit is a 'molten globule'. FEBS Lett, 263, 51-56. [237] Plaxco, K.W., Simons, K.T. and Baker, D. (1998) Contact order, transition state placement and the refolding rates of single domain proteins. J Mol Biol, 277, 985-994. [238] Ivankov, D.N., Garbuzynskiy, S.O., Alm, E., Plaxco, K.W., Baker, D. and Finkelstein, A.V. (2003) Contact or- der revisited: influence of protein size on the folding rate. Protein Science, 12, 2057-2062. [239] Zhou, H. and Zhou, Y. (2002) Folding rate prediction using total contact distance. Biophys Journal, 82, 458-463. [240] Gromiha, M.M. and Selvaraj, S. (2001) Comparison between long-range interactions and contact order in de- termining the folding rate of two-state proteins: applica- tion of long-range order to folding rate prediction. J Mol Biol, 310, 27-32. [241] Nolting, B., Schalike, W., Hampel, P., Grundig, F., Gantert, S., Sips, N., Bandlow, W. and Qi, P.X. (2003) Structural determinants of the rate of protein folding. J Theor Biol, 223, 299-307. [242] Ouyang, Z. and Liang, J. (2008) Predicting protein fold- ing rates from geometric contact and amino acid se-  K. C. Chou et al. / Natural Science 1 (2009) 63-92 Copyright © 2009 SciRes. OPEN ACCESS 92 quence. Protein Science, 17, 1256-1263. [243] Ivankov, D.N. and Finkelstein, A.V. (2004) Prediction of protein folding rates from the amino acid se- quence-predicted secondary structure. Proc Natl Acad Sci USA, 101, 8942-8944. [244] Gromiha, M.M., Thangakani, A.M. and Selvaraj, S. (2006) FOLD-RATE: prediction of protein folding rates from amino acid sequence. Nucleic Acids Res, 34, W70-74. [245] Chou, K.C. (1990) Review: Applications of graph theory to enzyme kinetics and protein folding kinetics. Steady and non-steady state systems. Biophysical Chemistry, 35, 1-24. [246] Chou, K.C. (1989) Graphical rules in steady and non-steady enzyme kinetics. J Biol Chem, 264, 12074-12079. [247] Lin, S.X. and Neet, K.E. (1990) Demonstration of a slow conformational change in liver glucokinase by fluores- cence spectroscopy. J Biol Chem, 265, 9670-9675. [248] Beyer, W.H. (1988) CRC Handbook of Mathematical Science, (6th Edition), Chapter 10, page 544. CRC Press, Inc., Boca Raton, Florida. [249] Shen, H.B., Song, J.N. and Chou, K.C. (2009) Prediction of protein folding rates from primary sequence by fusing multiple sequential features. Journal of Biomedical Sci- ence and Engineering (JBiSE), 2, 136-143 (open acces- sible at http://www.srpublishing.org/journal/jbise/). [250] Chou, K.C. and Shen, H.B. (2009) FoldRate: A web-server for predicting protein folding rates from pri- mary sequence. The Open Bioinformatics Journal, 3, 31-50 (open accessible at http://www.bentham.org/open/tobioij/). [251] Zhang, Z. and Henzel, W.J. (2004) Signal peptide predic- tion based on analysis of experimentally verified cleav- age sites. Protein Sci, 13, 2819-2824. [252] Spector, D.L. (2001) Nuclear domains. J Cell Sci, 114, 2891-2893. [253] Spiess, M. (1995) Heads or tails - what determines the orientation of proteins in the membrane. FEBS Lett, 369, 76-79. [254] Schulz, G.E. and Schirmer, R.H. (1985) Principles of Protein Structure, Chapter 2, Springer-Verlag, New York. 17-18.
|