Journal of Water Resource and Protection
Vol. 1 No. 3 (2009) , Article ID: 686 , 6 pages DOI:10.4236/jwarp.2009.13026
Effect of Packing Materials and Other Parameters on the Air Stripping Process for the Removal of Ammonia from the Wastewater of Natural Gas Fertilizer Factory
1Assistant Professor, Department of Civil and Environmental Engineering, Shah Jalal University of Science and Technology, Sylhet-3114, Bangladesh
2Professor, Department of Civil Engineering, Bangladesh University of Engineering and Technology, Dhaka, Bangladesh
E-mail: rakib_env@yahoo.com
Received May 23, 2009; revised June 7, 2009; accepted June 30, 2009
Keywords: Ammonia, air stripping, packing materials, stripping constant
ABSTRACT
Air-stripping method was used to remove ammonia from the wastewater collected from natural gas fertilizer factory. Different materials were used as packing materials for the air stripping system. The effect of pH over 10.5, air-water flow ratio, nature of packing materials, height of materials and initial influent concentration of ammonia on air stripping unit were investigated. An attempt has been made to find out the stripping constant. Stripping constant was found to be .001, 0014, .001 and .0009 for coal, plastic ring, stone chips and wood chips, respectively. Best result was found for plastic ring for its higher surface area. Wood chips did not give good result, because the chips amalgamate with each other and hence reduces the surface area.
1. Introduction
Industrial pollution is an area of growing environmental concern in Bangladesh. In Bangladesh, industry contributes about 26 per cent to GDP [1]. The Natural Gas Fertilizer Factory Ltd. (NGFFL), Fenchuganj, Sylhet is a pride and pioneer enterprise of the Bangladesh Chemical Industries Corporation (BCIC), was established in December, 1961 in the hilly picturesque surroundings of Fenchuganj to meet the urea demand of the country [2]. NGFFL produces urea. The process involves traditional chemical technology and deals with many chemicals. A part of the chemicals disposed as an industrial effluent in Kushiara River, which is responsible for the of river water quality. Among all the pollutants discharged from a fertilizer factory ammonia has the most severe effect on the receiving water body, soil and air. Ammonia is toxic to fish and aquatic organisms, even in very low concentrations. When levels reach 0.06 mg/L, fish can suffer gill damage. When levels reach 0.2 mg/L, sensitive fish like trout and salmon begin to die. As levels near 2.0 mg/L, even ammonia-tolerant fish like carp begin to die. Ammonia levels greater than approximately 0.1 mg/L usually indicate polluted waters [3]. Bangladesh Industrial Effluent Standards [4], for ammonia nitrogen is 100 mg/L. For the year 2005, the ammonia nitrogen was found above the limit [5]. As NGFFL produces excessive amount of ammonia as effluent and discharges it into the river Kushiara, so treatment of wastewater of NGFFL is very important. This research work has been undertaken to assess the effectiveness of air stripping unit (by using different packing materials) to remove ammonia from wastewater of NGFFL.
2. Air Stripping
Air stripping involves the mass transfer of VOCs that are dissolved in water from the water phase to the air phase. A one-dimensional mass transfer equation is used to describe the mass transfer flux of VOCs transferring from the water phase to the air phase. The equilibrium relationship is linear and is defined by Henry’s Law [6]. This mass transfer can be accomplished in a packed -column, a low profile, or a diffused-air air stripper [8]. The following methods are available for determining the size of a packed-column air stripper: Commercial software (Iowa State University), Manufacturer’s software (Carbon-air Environmental Systems), Analytical equations [7, 9, 10, 11] and McCabe-Thiele graphical method [11].
Ammonia stripping is a simple desorption process used to lower the ammonia content of a wastewater stream. Some wastewaters contain large amounts of ammonia and/or nitrogen-containing compounds that may readily form ammonia. It is often easier and less expensive to remove nitrogen from wastewater in the form of ammonia than to convert it to nitrate-nitrogen before removing it [12]. Ammonia Stripping seek to maximize the volatile ammonia component [13]. Ammonium ions in wastewater exist in equilibrium with gaseous ammonia as shown in the following equation:
NH4+ ←→ NH3 + H+(1)
In ammonia stripping, lime or caustic is added to the wastewater until the pH reaches 10.8 to 11.5 standard units, which converts ammonium hydroxide ions to ammonia gas [14].
3. Methodology
For the air-stripping experiments, one stripping tower of polyvinyl Chloride tube (1981.2 mm height X 50.8 mm internal diameter) was constructed (Figure 1). The tower was packed with plastic ring of 12.7 mm dia, wood chips of 19.05 mm, stone chips of 19.05 mm and coal chips of 19.05 mm (Figure 2) to promote the down flow of liquid in a thin, gentle stream. Five hundred to seven hundred ml of wastewater was used for each experiment. The effluent was collected from the bottom of the tower to measure the concentration of ammonia after collecting 100 and 300 ml of treated wastewater each time. Average was taken as the final concentration. Forced air was introduced directly into the column. The airflow rate was maintained 15 l/min for each experimental set. The pH levels were maintained 10.5 for each experiment to convert the ammonium ion to ammonia gas by adding Calcium Oxide. As the pump capacity was fixed, different air to liquid flow ratio was maintained by changing the water flow rates. A handoperated valve was used to regulate the wastewater flow rate. The effect of pH over 10.5, air-water flow ratio, nature of packing materials and height of materials and initial influent concentration of ammonia on air stripping unit were investigated.
An attempt has been made to find out the stripping constant. For each combination of airflow rate, water flow rate and effluent concentration, the fraction remaining after stripping was calculated by dividing the effluent concentration by the influent concentration. The

Figure 1. Air stripping experiment (Laboratory setup).

Figure 2. Different types of materials used as packing materials.
fractional results were plotted on log scale against the volumetric air-to-water ratio. An exponential curve was fitted to the data. When there is no airflow, the stripping factor is zero; the effluent concentration should theoretically be equal to the influent concentration. Therefore, the yintercept should be one. So the curve fit was forced through it. Empirically derived stripping constants were obtained from the exponential equations of the curve fitted lines The stripping constants can be used to predict treatment performance. The first-order decay model was used to determine stripping constant:
Ce/Ci = e-Kq (3)
Ce = effluent water concentration Ci = influent water concentration K = stripping constant q = volumetric air-to-water ratio Digital pH meter (HANNA, HI 98204) was used for the determination of pH. The ammonia was determined by DR-4000 spectrophotometer UVvisible wavelengths at λ=425 nm (Nessler method, using Nessler reagent, Mineral Stabilizer and Polyvinyl Alcohol Dispersing Agent, Hach 8038).
4. Result
4.1. Effect of Packing Height
Packing height has a great influence on stripping process. Laboratory experiments show that removal efficiency increased with the increase of packing height due to the increment of contact time between air and water. Without packing materials, with a constant air-water ratio 2000, the removal was found around 50%. pH of the wastewater was adjusted to 10.5. In case of plastic ring, only 63.59% removal was found for 1 feet height of packing materials and removal efficiency was being gradually increased and gave 91.60% removal for 5 feet height. Same results were found for other three materials. Coal, stone chips, and wood chips gave 60.89%, 60.73% and 60.43% removal for 1 feet height and 87.82%, 85.24% and 84.5% removal for 5 feet height respectively. The effect of packing height for various packing materials has been shown in figure 3.
4.2. Effect of pH on Air Stripping
Above pH 10.5, the effectiveness of the stripping has been studied. The effect of pH on the ammonia removal efficiency of the air-stripping method is shown in figure 4. The best result obtained from the study was 88.94%, 94.15%, 87.8% and 87.4% ammonia reduction by coal chips, plastic ring, stone chips and wood chips respectively at or over pH 11.5. Above pH 10.5, the ammonia removal efficiency was not greatly influenced by pH. For example, in case of coal as a packing material at pH 10.5 with an air flow rate 15 l/min, 87.8 % removal efficiency
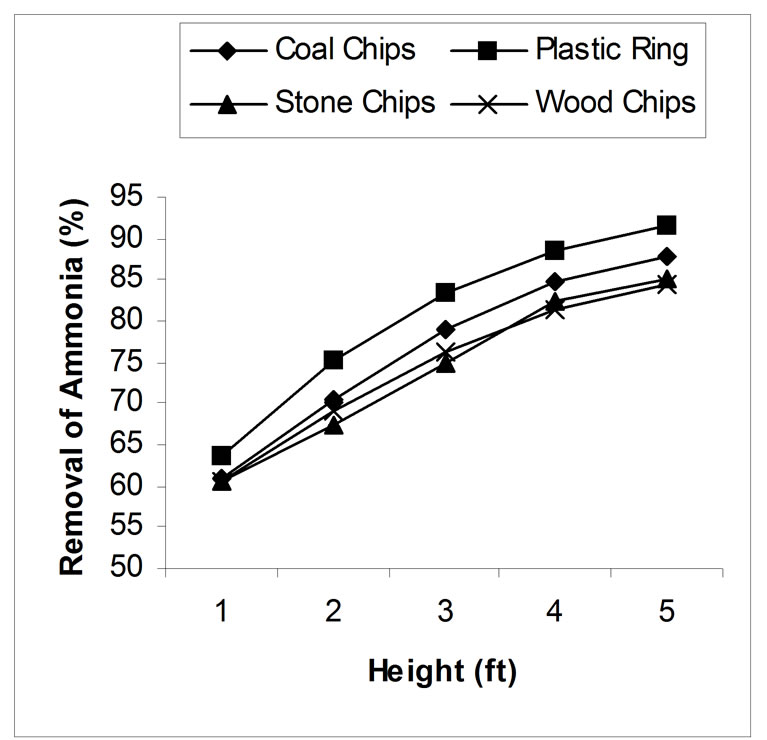
Figure 3. Effect of packing height on removal of ammonia.
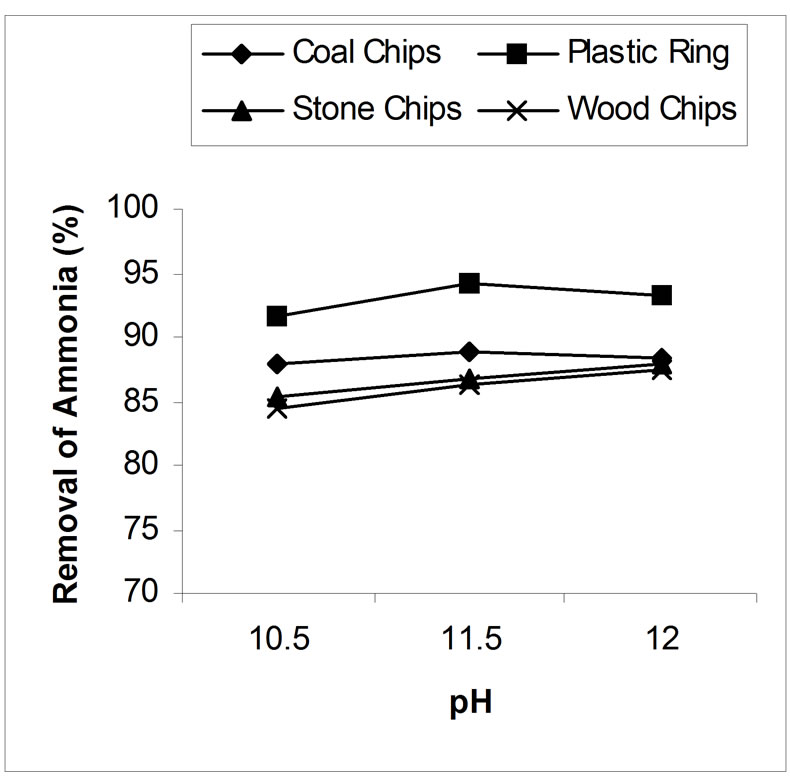
Figure 4. Effect of pH on removal of ammonia.
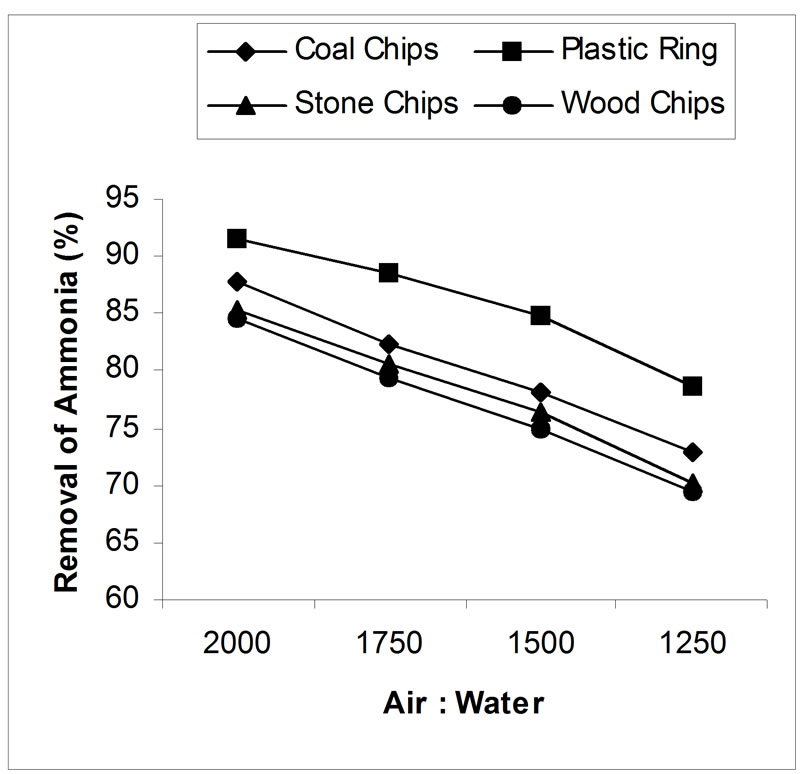
Figure 5. Effect of Air-Water ratio on removal of ammonia.
was achieved, while at pH 11.5 with the same air flow rate 88.9% removal was achieved. But it is evident from the experiment that more calcium oxide is required to raise the pH 11.5 than pH 10.5. Such large additions of lime would increase the formation of heavy calcium carbonate scale within the stripping tower and as a result the efficiency of the system would decrease and severe maintenance problem would occur [15]. How ever, since the effects of pH on removal efficiency were not found to be significant at levels above pH 10.5, there appears to be some reason for raising the level any higher.
4.3. Effect of Air-Water Ratio
The effect of air-water ratio on removal efficiency is presented in figure 5. All the results show very high levels of ammonia removal. An increase of air to water flow ratio from 1250 to 2000 (air flow is constant) facilitated the stripping process. In case of coal, plastic ring, stone and wood chips; removal efficiency was increased from 72.84% to 87.82%, 78.7% to 91.60%, 70% to 85.3% and 69.5% to 84.5% respectively by increasing the air to water flow ratio 1250 to 2000. In all the cases, packing depth was 5 feet and pH was adjusted to 10.5.
4.4. Effect of Initial Concentration
Effect of initial concentration on stripping unit was investigated. Influent concentration was varied from 80 mg/L to 1574 mg/L. In case of coal, plastic ring, stone and wood chips the removal efficiencies were in the range of 86.21% to 91.76%, 90.43% to 92.39%, 80% to 88.31%, and 83.72% to 88.20% respectively. Air to water flow ratio was maintained 2000 and packing depth in all the cases were 5 feet and pH was adjusted to 10.5. The detail results have been shown in table 1.
4.5. Effect of Packing Materials
Quality of packing materials has an influence over efficiency of stripping column. Among the four types of packing materials used as packing materials, plastic ring

Table 1. Effect of influent concentration on air stripping unit.
gave the best performance due to its high surface area. Among the other three materials, coal gave comparatively better performance. It may be due to its adsorbing property. Wood as packing materials was not found suitable because they amalgamated with each other within a very short time and reduced the surface area. Stone is a heavy material. So it will increase the cost of the building of column. Coal chips may be a good option. It gives good performance. But the major drawback is its self-weight. Plastic ring will be the best choice to use as packing materials. It is lighter, gave the maximum surface area and can be produced locally. Air strippers often become fouled from iron, calcium, manganese, or biological growth. Packed-column air strippers must either have the packing removed for cleaning or be washed with an acid solution [16].
4.6. Effluent Concentration Prediction
The first-order decay model was used to determine stripping constant according to the Equation (1). Stripping constant was found .001, .0014, .001 and .0009 for coal, plastic ring, stone chips and wood chips respectively. The equation with the empirically derived stripping constants was used to predict effluent water concentration. To verify the prediction model, some known concentration of ammonia contaminated Wastewater was passed through the air-stripping unit by keeping the air-water ratio 2000. A curve of experimental concentration vs. predicted concentration has been plotted in figure 6 to evaluate the accuracy of the model. From the model prediction curves, Equation (4), (5), (6) and (7) have been generated to find out the value of effluent concentration of ammonia when the packing materials are coal, plastic ring, stone chips and wood chips respectively. Those equations will be valid only when the pH of the raw water is adjusted to 10.5 and the height of the packing materials is 5 feet.
Ce = Ci e-.001q(4)
Ce = Ci e-.0014q(5)
Ce = Ci e-.001q(6)
Ce = Ci e-.0009q(7)
Where
Ce = effluent water concentration
Ci = influent water concentration
q = volumetric air-to-water ratio
4.7. Ammonia in the Air
In air stripping unit, wastewater is treated and becomes free from ammonia. But it posses a great threat to the ambient air. Huge amount of ammonia (828 to 1401 ppm) has been found at the outlet of the stripping unit by using mass balance equation. Recovery of ammonia as either ammonium sulfate or nitrate by scrubbing with sulfuric or nitric acid can be a solution [17].
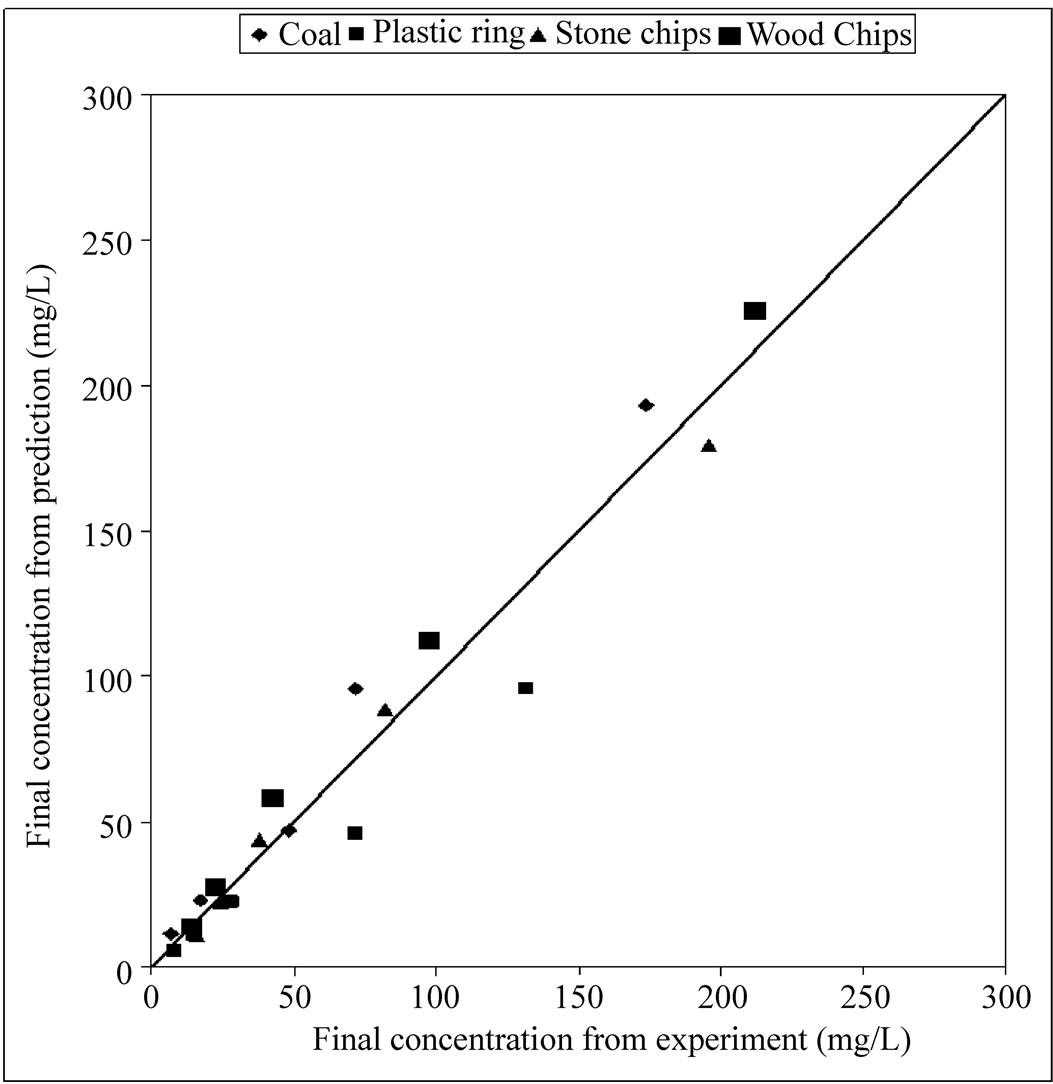
Figure 6. verification of predicted model.
5. Conclusion
Air-stripping method achieved very high rates of ammonia removal and the results indicated that it could be a great solution for wastewater treatment of NGFFL. Its use requires a large addition of lime (approximately 2 to 3 gm per liter of wastewater; amount is not fixed as the alkalinity of the wastewater was found to vary), a high airflow rate (NGFFL uses huge amount of air to generate fertilizer. They can transfer air through a bypass line to the air-stripping unit.) and the provision of a tower containing some packing materials. Different materials were used as packing materials. Best result was found using plastic ring for its higher surface area. It can be produced locally by using 1/2¢¢ PVC pipe and it is relatively cheap in Bangladesh.
REFERENCES
- ESCAP, “Private enterprise development for export promotion in south Asia,” United Nations Economic And Social Council, Fourteenth meeting 2–4, December 2002.
- M. N. A. Khan, P. Datta, A. S. M. Dakua, and S. I., “An assessment of Water Quality Deterioration of the River Kushiara due to Effluent Discharge from Natural Gas Fertilizer Factory,” B. Sc. Engg. Thesis, CEE Dept, Shah Jalal University of Science & Tech., Sylhet., 2002.
- USEPA, “Agency Quality Criteria for Water,” EPA # 440/5-86-001, US Environmental Protection Agency, Washington, DC. 1986.
- DOE, “The Environment Conservation Rules,” Department of Environment, Ministry of Environment and Forest, Government of the People’s Republic of Bangladesh, 1997.
- U. Dhar and M. Rahman, “Surface water quality modeling: A case study on Kushiara River,” B.Sc. Engg. Thesis, CEE Dept, Shah Jalal University of Science & Tech., Sylhet, 2006.
- M. Kavanaugh, and R. R. Trussell, “Design of aeration towers to strip volatile contaminants from drinking water,” Journal AWWA, pp. 684–692, December 1980.
- H. Shulka, and R. E. Hicks, “Process design manual for stripping of organics,” EPA- 600/2- 84-139, 1984.
- E. Mead and J. Leibbert, “A comparison of packed -column and low-profile sieve tray air strippers,” Proceedings of the 1998 Conference on Hazardous Waste Research, 1998.
- W. Ball, M. D. Jones, and M. C. Kavanaugh, “Mass transfer of volatile organic compounds in packed tower aeration,” J. Water Pollution Control Federation, Vol. 56, No. 2, pp. 127–136, 1984.
- J. M. Montgomery, Consulting Engineers Inc., “Water treatment principles & design,” John Wiley & Sons, New York, pp. 237–261, 1985.
- R. Treybal, “Mass-transfer operations,” McGraw-Hill, New York, 3rd ed., pp. 275–313, 1980.
- L. Culp, Wesner, M. George and L. Culp, “Handbook of advanced wastewater treatment,” 2nd ed., Van Nostrand Reinhold Co., New York, 1978.
- P. H. Liao, A. Chen and K. V. Lo, “Removal of nitrogen from swine manure wastewaters by ammonia stripping,” Bioresource Technology 54, Elsevier Science Limited, Great Britain, pp. 17–20, 1995.
- USEPA, “Wastewater technology fact sheet ammonia stripping,” EPA 832-F-00-019 US Environmental Protection Agency, Washington, D. C., 2000.
- USEPA, “Nitrogen removal by ammonia stripping,” EPA/670/2-73-040, US Environmental Protection Agency, Washington, D. C., 1973.
- Jaeger Products, Inc., 1611 Peachleaf, Houston, TX 77039, jpadmin@jaeger.com/1006foul.htm.
- S. C. Bhatia, “Environmental pollution and control in chemical process industries,” Khanna Publishers, Delhi, pp. 644–651, 2001.

