Journal of Biomedical Science and Engineering
Vol.6 No.8B(2013), Article ID:35781,9 pages DOI:10.4236/jbise.2013.68A2007
Differentially expressed genes identified by microarray analysis following oxymatrine treatment of hepatic stellate cell
![]()
1Department of Hepatology, Shanghai East Hospital Affiliated to Tongji University, Shanghai, China
2Department of Gerontology, Shanghai Putuo Central Hospital Affiliated to Shanghai Traditional Chinese Medicine University, Shanghai, China
Email: #wendywangjinjun@163.com, #xiongwujun@126.com
Copyright © 2013 Junwei Hu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 3 June 2013; revised 24 July 2013; accepted 7 August 2013
Keywords: Oxymatrine; Hepatic Stellate Cell; Microarray; Bioinformatics Analyses
ABSTRACT
AIM: To investigate the effects of oxymatrine on the gene expression profile of hepatic stellate cell (HSC) and provide novel insights into the mechanism of oxymatrine against hepatic fibrosis. Methods: HSC was isolated from normal SD by in situ perfusion of collagenase and pronase and density Nycodenz gradient centrifugation. MTT colorimetry was used to study the effect of oxymatrine on the proliferation of HSC. Total RNA and mRNA of quiescent HSC, culture-activated HSC and oxymatrine treated HSC were extracted. Effect of oxymatrine on HSC gene expression profile was detected by oligonucleotide microarray analysis with Affymetrix gene chip rat U230A. Differentially expressed genes were annotated with Gene Ontology (GO) and analyzed with Kyoto encyclopedia of genes and genomes (KEGG) pathway using the Database for Annotation, Visualization and Integrated Discovery. Results: Oxymatrine could inhibit the proliferation of HSC in a dose-dependent manner. A total of 4641 differentially expressed genes were identified by cDNA chip between activated and quiescent HSC, among which 2702 genes were upregulated, and 1939 genes were down-regulated in activated HSC. cDNA microarray uncovered downregulation of 56 genes in response to oxymatrine, the representative genes including alpha 2 type I procollagen, alpha-1 type I collagen, tissue inhibitor of metalloproteinase 1, interleukin 1 beta, early growth response 1, chemokine ligand 2, chemokine ligand 1, CTGF, TGFβ1. The most enriched GO terms included response to wounding, inflammatory response, cell migration, cell motility, wound healing, TGFβ receptor signaling pathway. KEGG pathway analysis revealed that oxymatrine affected the ECM-receptor interaction, focal adhesion, cytokine-cytokine recaptor interaction, TGFβ signaling pathway, MAPK signaling pathway. There were 37 genes upregulated significantly following oxymatrine treatment. The most enriched GO terms included oxidation reduction, negative regulation of lipoprotein oxidation, regulation of lipoprotein oxidation, steroid metabolic process, regulation of lipase activity. Six genes were confirmed with QPCR, consistent with microarray. Conclusion: The mechanism of oxymatrine in inhibiting liver fibrogenesis is associated with multi-genes and multi-pathways regulation.
1. INTRODUCTION
Liver fibrosis is a reparative reaction of the liver to various chronic disease states. It is also a common pathological change that occurs in all chronic hepatic diseases. The characteristics of liver fibrosis include misregulation of the synthesis and degradation of collagen in the extracellular matrix (ECM). Hepatic stellate cell (HSC) is a major source of ECM and activation of HSC is one of the key steps in the development of liver fibrosis. HSC is becoming a major target cell for antifibrotic therapy. To inhibit HSC, activation is one of the most important strategies for preventing liver fibrosis.
Traditional Chinese herbal medicines may contain therapeutic agents for treating hepatic fibrosis [1]. Oxymatrine is a kind of alkaloid extraction derived from a Chinese herb Sopbora flavescens Ait. It has been used for treating viral hepatitis B, hepatitis C and hepatic fibrosis in recent years in China. The mechanisms of oxymatrine include inhibiting HSC proliferation, inhibiting replication of viral hepatitis B and C, regulating immune function and so on [2-11]. But its in-depth molecular mechanism is still not clear and its molecular target is unknown. Gene chip technology is characterized by high communication, low consumption and miniaturization, thus providing a technological platform to identify and characterize changes in gene expression associated with the anti-hepatic fibrosis mechanism of traditional Chinese medicine [12].
In this study, microarray analysis was used to screen for differentially expressed genes in activated HSC following oxymatrine treatment. We expect that our results will help illustrate the molecular mechanisms involved in the HSC activation and highlight potential targets for oxymatrine against liver fibrosis.
2. MATERTIALS AND METHDOS
2.1. Cell Isolation, Identify and Culture
HSC was isolated from normal SD rat by collagenasepronase perfusion and subsequent density centrifugation on Nycodenz gradients as described previously [13]. Purity was tested by retinoid autofluorescence and exceeded 95% in all isolations. HSC was cultured in Dulbecco’s modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS), glutamine, HEPES buffer, and antibiotics.
2.2. Effect of Oxymatrine on HSC Proliferation
The third to fourth passage of HSC cells were spread onto 96-well flat-bottom plates at a density of 5 × 104/mL, 100 μL per well, and cultured in DMEM including 5% fetal calf serum. Cells were treated with oxymatrine (0, 50, 100, 200, 400 μg/mL) for 24 h, 48 h, 72 h respectively in a 5% CO2 humidified incubator at 37˚C. Each group was repeated for 6 wells. 200 μL of 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (5 mg/mL) was added into each well at the end of 24 h, 48 h, 72 h respectively. The supernatant was removed and 100 μL of DMSO was added to each well to dissolve the formazan product. Absorbance at 570 nm was measured using a microplate reader.
2.3. Microarray Experiments and Bioinformatics Analysis
Trizol was used to extract total RNA from HSC according to the manufacture’s instructions. Total RNA was purified using an RNeasy® kit (Qiagen, Germany) and the quality of RNA was determined. We used rat genome 430 2.0 array gene chips (Affymetrix, Santa Clara, CA). The arrays were hybridized, washed and scanned according to the standard Affymetrix protocol. The gene chip tests were performed by professional staffs of Shanghai Biochip Company. Chips were scanned with a Genechip Scanner 3000 7G (Affymetrix). Data analysis was performed using GCOS1.2 software. After background correction, we performed normalization for each array and gene. Data were filtered based on both signal intensity and detection call. Differentially expressed genes were annotated with Gene Ontology (GO) and analyzed with Kyoto encyclopedia of genes and genomes (KEGG) pathway using the Database for Annotation, Visualization and Integrated Discovery Bioinformatics Resources 6.7 [14] (http://david.abcc.ncifcrf.gov).
2.4. Quantitative Real-Time PCR (QPCR)
Six differentially expressed genes were selected for QPCR analysis, with glyceraldehydes-3-phosphate dehydrogenase (GAPDH) as a control, to verify the reliability of the array data. Each selected gene represented one effect of oxymatrine on liver fibrosis, alpha-1 type I collagen (COL1A1) for ECM deposition, transforming growth factor beta 1 (TGFβ 1), CTGF and platelet-derived growth factor alpha (PDGFA) for proliferative factors, early growth response 1 (EGR-1) and chemokine (C-X-C motif) ligand 2 (CXCL2) for proinflammatory mediators. The primers were delegated to Shanghai Sangon Biotech Corporation. Primer sequences are listed in Table 1. Six genes and GAPDH were amplified using a sequence detection system (ABI PRISM® 7900HT, ABI, Foster City, CA, USA). Each experiment
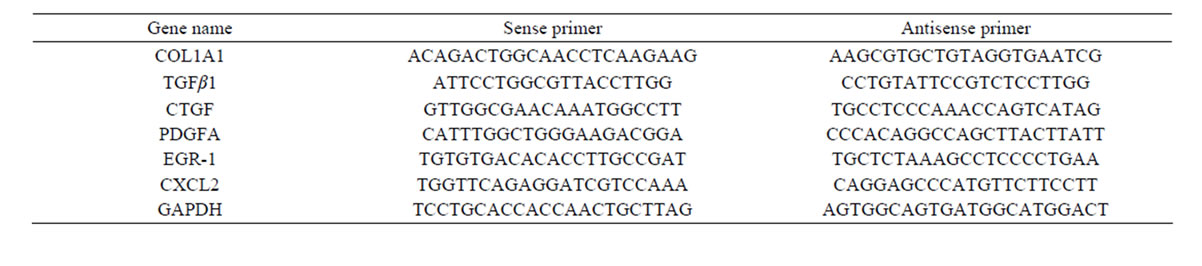
Table 1. Primer sequences for the genes to validate the microarray analysis by QPCR.
was done in triplicate. Relative gene expression was analyzed using the 2-ΔΔCT method.
3. RESULTS
3.1. Density Centrifugation Achieves High-Purity HSC
Isolation of HSC from normal rat liver usually results in at least 95% purity. Cells isolated by this method displayed fluorescent retinoid droplets. Culture-activated HSC exhibited myofibroblast-like appearance.
3.2. Effect of Oxymatrine on HSC Proliferation
MTT assay indicated that oxymatrine could significantly inhibit HSC proliferation dose-dependently compared with the control group (P < 0.05) (Table 2).
3.3. Microarray Analysis
cRNAs prepared from quiescent HSC and culture-activated HSC, were hybridized to a rat genome 430 2.0 array gene chips. Of the 28,700 genes represented therein, 4641 genes displayed at least a 1-fold increase or decrease in expression at the P < 0.01 level with a false discovery rate. Of these, 2702 genes were upregulated, while 1939 genes were downregulated in culture-activated HSC. Compared with the culture-activated HSC group, there were 56 genes downregulated markedly and 37 genes upregulated after oxymatrine treatment. The representative downregulated genes include alpha 2 type I procollagen,alpha-1 type I collagen, tissue inhibitor of metalloproteinase 1, interleukin 1 beta, early growth response 1, chemokine ligand 2, chemokine ligand 1, CTGF, TGFβ1. The representative upregulated genes include cytochrome P450 (family 17, subfamily a, polypeptide 1; family 2, subfamily c, polypeptide 7; family 2, subfamily d, polypeptide 4; subfamily IIC); D-amino-acid oxidase; phytanoyl-CoA 2-hydroxylase; thioredoxin reductase 2.
3.4. Gene Ontology Analysis
To elucidate the differential expression genes between quiescent HSC and culture-activated HSC, we examined
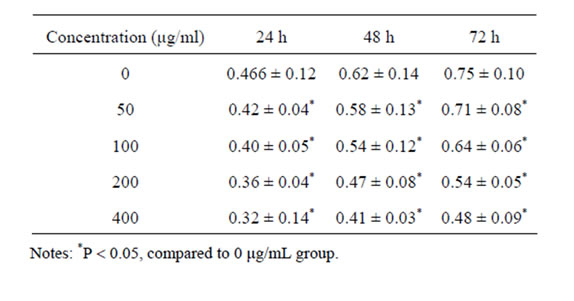
Table 2. Effect of oxymatrine on HSC proliferation.
the functional bias of 4641 differentially expressed transcripts according to Gene Ontology (GO) classifications. These 2702 upregulated transcripts were grouped into 794 GO based on biological process GO terms. The most enriched GO terms included response to wounding, wound healing and regulation of cell growth (Table 3). Analyses of GO indicated that there were 142 GO terms identified by cellular component classification, and 129 GO terms identified by molecular function classification. While, those 1939 downregulated transcripts were grouped into 605 GO based on biological process GO terms. The most enriched GO terms included oxidation reduction, carboxylic acid catabolic process, organic acid catabolic process, steroid metabolic process, cofactor metabolic process, coenzyme metabolic process (Table 4). Analyses of GO indicated that there were 82 GO terms identified by cellular component classification, and 183 GO terms identified by molecular function classification.
After oxymatrine treatment, these 56 downregulated transcripts were grouped into 56 GO based on biological process GO terms. The most enriched GO terms included response to wounding, inflammatory response, cell migration, cell motility, localization of cell, wound healing (Table 5). GO of the downregulated transcripts indicated that there were 19 GO terms identified by cellular component classification, and 11 GO terms identified by molecular function classification. Those 37 upregulated transcripts were grouped into 43 GO based on biological process GO terms. The most enriched GO terms included oxidation reduction, negative regulation of lipoprotein oxidation, regulation of lipoprotein oxidation, steroid metabolic process, regulation of lipase activity (Table 6). GO of the upregulated transcripts indicated that there were 13 GO terms identified by cellular component classification, and 9 GO terms identified by molecular function classification.
3.5. Kyoto Encyclopedia of Genes and Genomes Pathway Analysis
Analysis of KEGG pathways of these upregulated genes revealed many enrichment-related pathways including focal adhesion, ECM-receptor interaction, regulation of actin cytoskeleton, pathways in cancer, dilated cardiomyopathy, hypertrophic cardiomyopathy, TGF-β signaling pathway, chemokine signaling pathway, p53 signaling pathway, renal cell carcinoma, Wnt signaling pathway, leukocyte transendothelial migration, MAPK signaling pathway in HSC activation. The top 20 significantly perturbed pathways are listed in Table 7. Analysis of KEGG pathways of those downregulated genes revealed many enrichment-related pathways including drug metabolism, metabolism of xenobiotics by cytochrome P450, complement and coagulation cascades, retinol
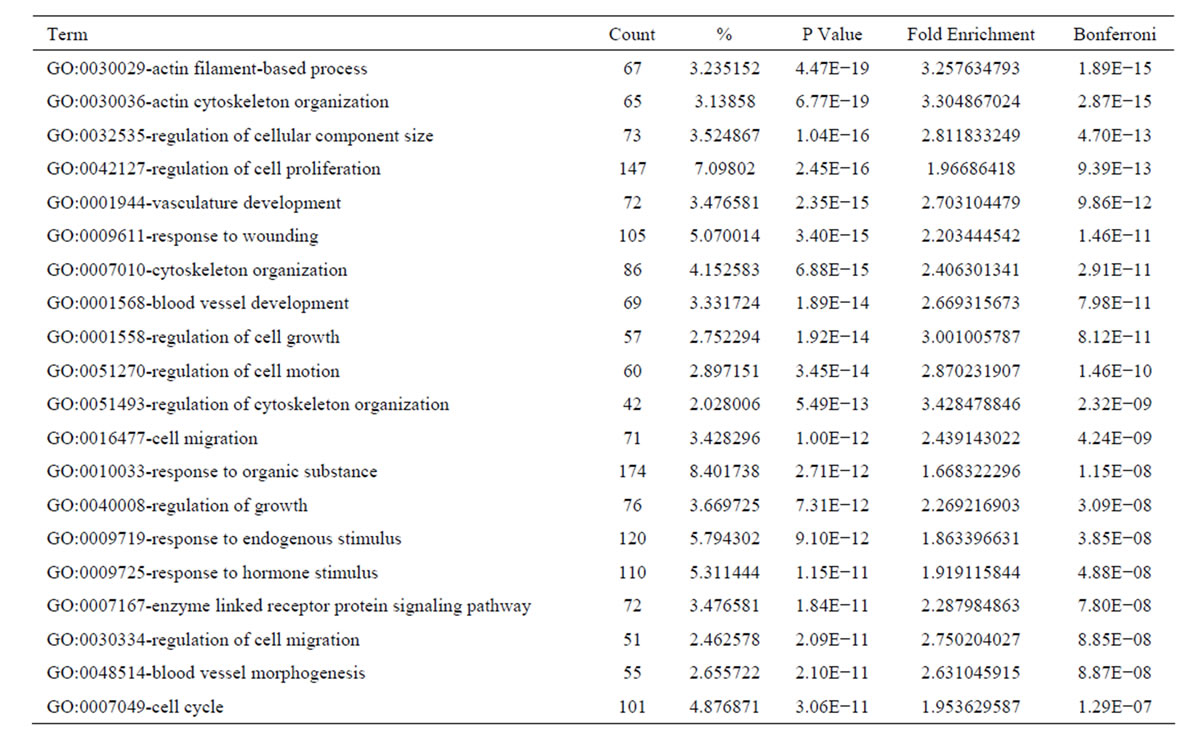
Table 3. Top 20 enriched GO terms of the upregulated genes in culture-activated HSC.

Table 4. Top 20 enriched GO terms of the downregulated genes in culture-activated HSC.
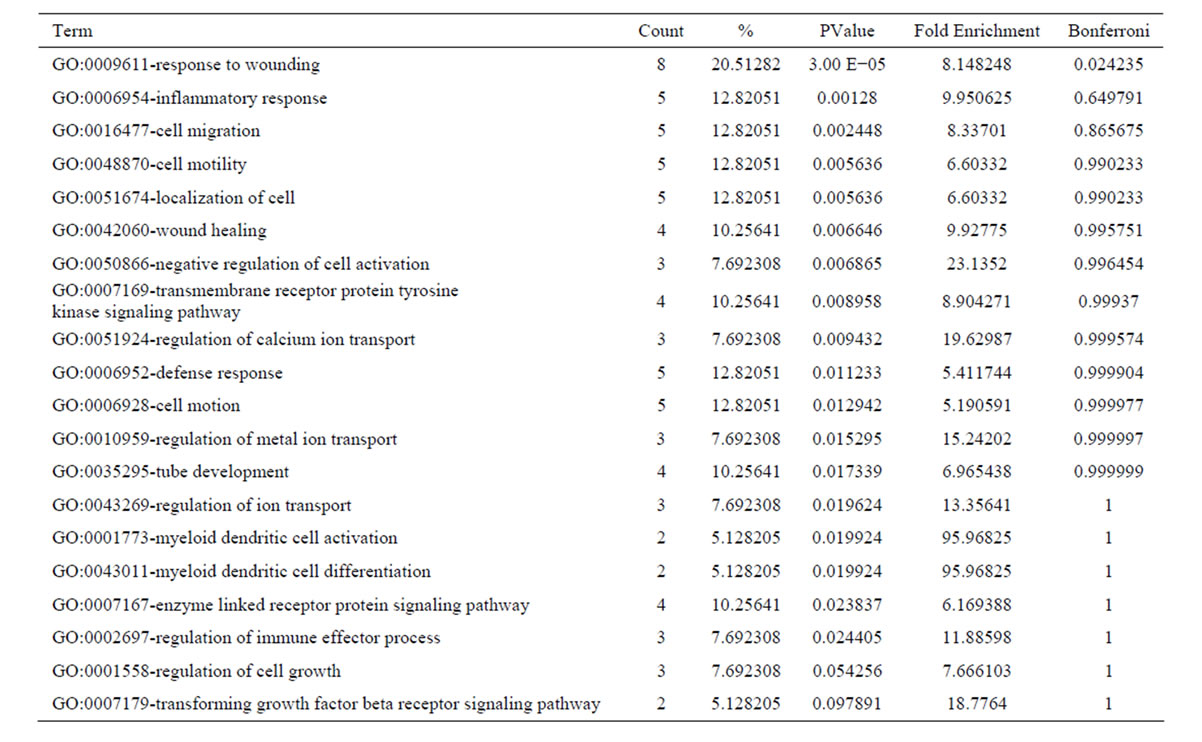
Table 5. Top 20 enriched GO terms of the downregulated genes following oxymatrine treatment.
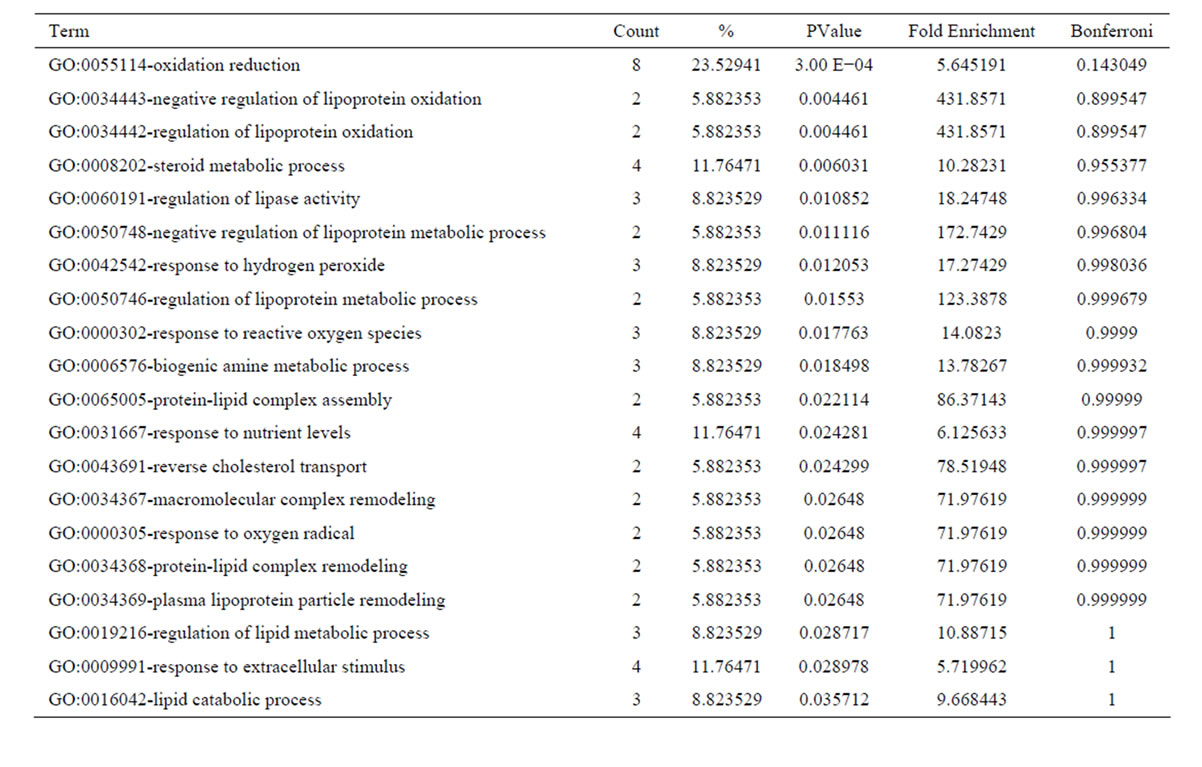
Table 6. Top 20 enriched GO terms of the upregulated genes following oxymatrine treatment.
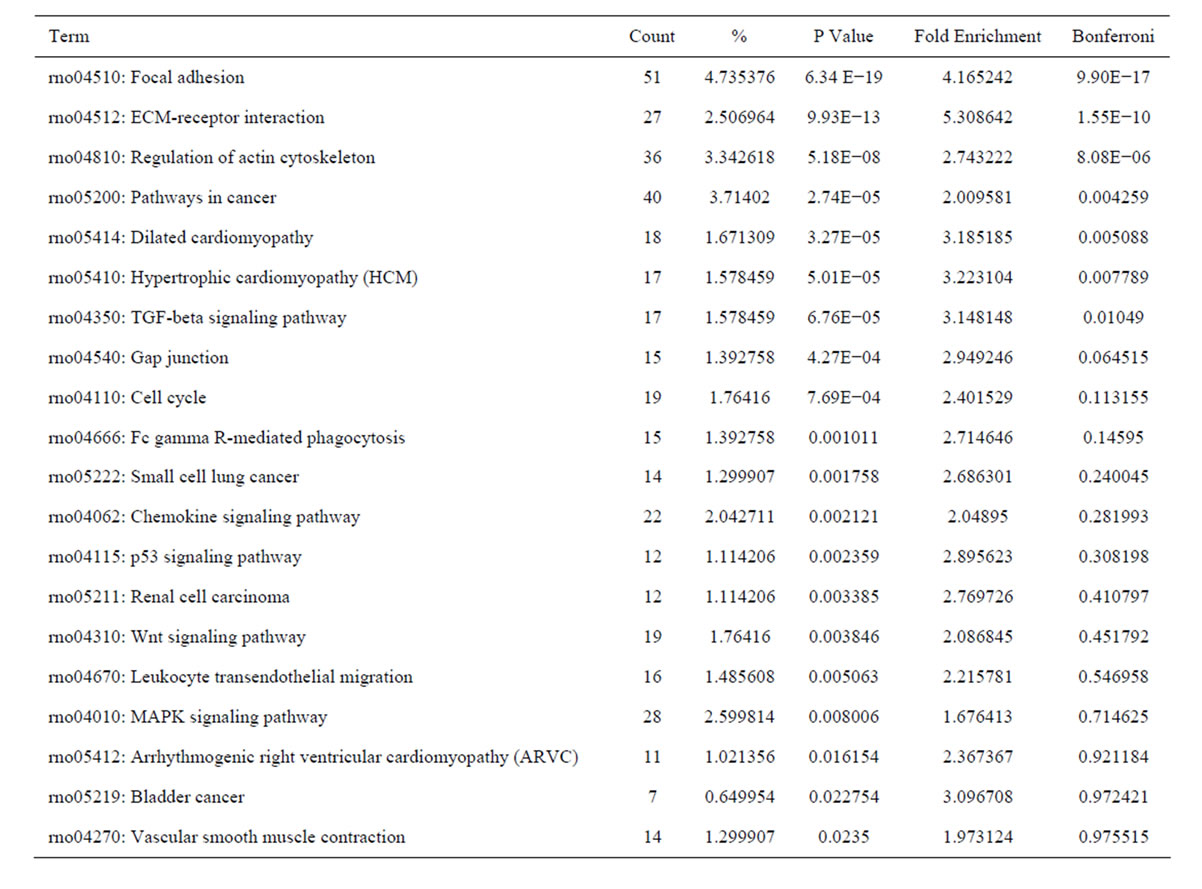
Table 7. The top 20 significantly perturbed pathways of the upregulated genes in culture-activated HSC.
metabolism, fatty acid metabolism, PPAR signaling pathway in HSC activation. The top 20 significantly perturbed pathways are listed in Table 8.
KEGG pathways analysis revealed that oxymatrine treatment could affect ECM-receptor interaction, focal adhesion, cytokine-cytokine receptor interaction, TGFbeta signaling pathway, MAPK signaling pathway and drug metabolism pathway. The significantly perturbed pathways are listed in Table 9.
3.6. Validation of Microarray Data
Changes in gene expression profiles observed in the microarrays were validated by QPCR analysis by using the same total RNAs analyzed in the cDNA microarray experiments. This verification confirmed that all the QPCR results were in agreement with the microarray data (Figure 1).
4. DISCUSSION
HSC activation is the key point of liver fibrosis. The molecular mechanisms underlying the activation of HSC are not fully understood. Inhibiting HSC activation is an important strategy for prevention and treatment of liver fibrosis.
We explored the mechanism of HSC activation with GO and KEGG, our results revealed that HSC activation was associated with these biological process GO terms including wound healing, regulation of cell growth, oxidation reduction. And the signaling pathways participating HSC activation included focal adhesion, ECM-receptor interaction, regulation of actin cytoskeleton, TGF-β signaling pathway, chemokine signaling pathway, p53 signaling pathway, Wnt signaling pathway, MAPK signaling pathway, and PPAR signaling pathway.
We aimed at exploring the potential targets of oxymatrine in the prevention of hepatic fibrosis with microarray and bioinformatics analyses. We found that the most enriched GO terms following oxymatrine treatment include response to wounding, inflammatory response, cell migration, cell motility, wound healing and oxidation reduction. KEGG pathways analysis revealed that oxymatrine treatment could affect ECM-receptor interaction, focal adhesion, cytokine-cytokine receptor interaction,
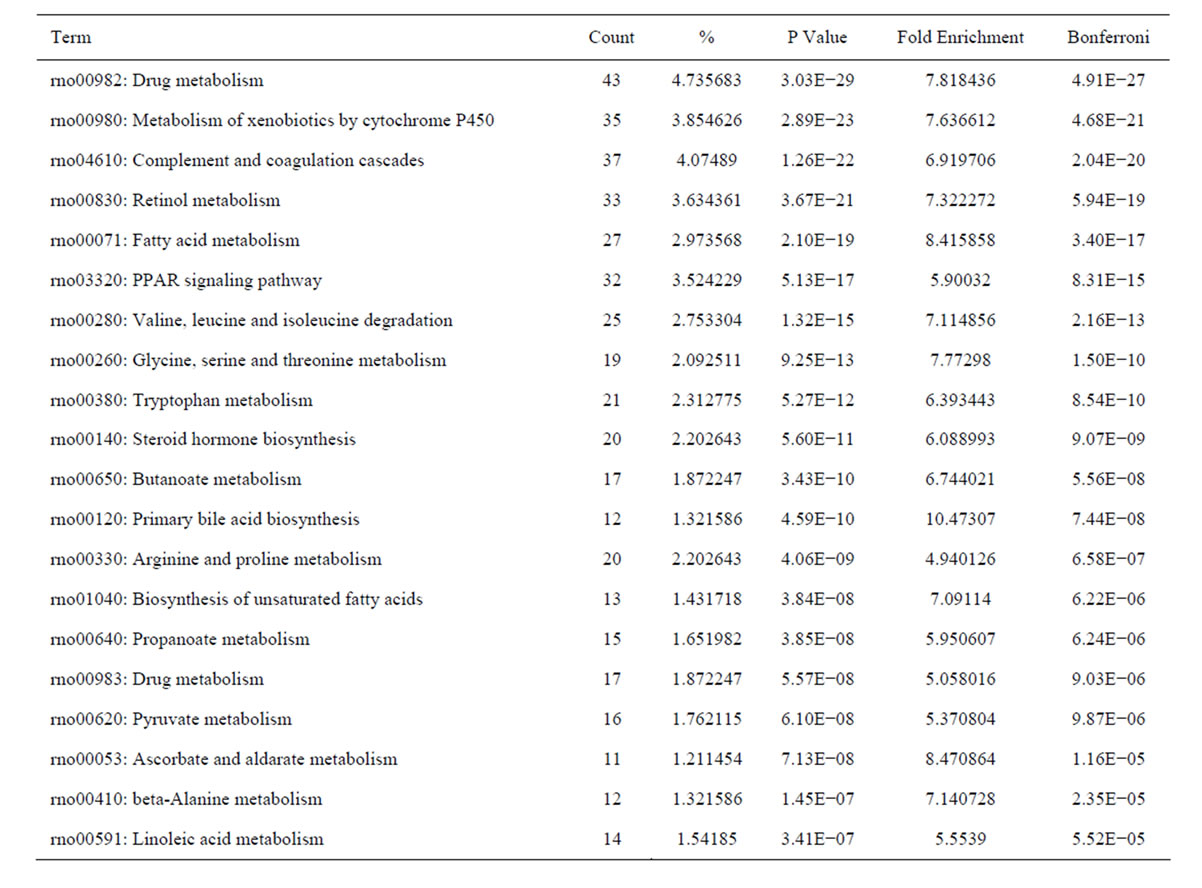
Table 8. The top 20 significantly perturbed pathways of the downregulated genes in culture-activated HSC.

Table 9. Signaling pathways related to the effects of oxymatrine on HSC.
TGF-beta signaling pathway, MAPK signaling pathway and drug metabolism pathway.
According to our results and previous reports, we speculate the mechanisms of oxymatrine in inhibiting liver fibrosis may include: 1) Inhibiting HSC proliferation: Our research showed oxymatrine could inhibit HSC proliferation in a dose-dependent manner. Oxymatrine has been demonstrated to prevent pig-seruminduced liver fibrosis in rats by inhibiting the activetion of HSC and synthesis of collagen [4]. 2) Antiinflammatory effects: Oxymatrine has been demonstrated to exhibit anti-inflammatory effects in models of heart, brain, liver and intestinal injury through blockade of inflammatory signaling [15]. In our study, we found oxymatrine could inhibit the expression of inflammatory factors such as EGR-1, interleukin-1, CXCL 2, CXCL1. 3) Affecting TGF-beta signaling pathway: Previous study demonstrated oxymatrine
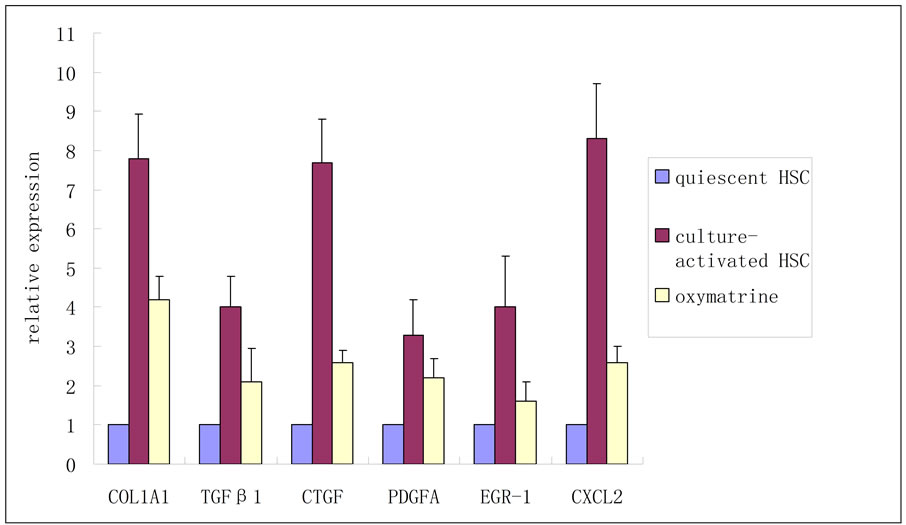
Figure 1. Validation of microarray data by QPCR.
could modulate the fibrogenic signal transduction of the TGF-β-Smad pathway [16]. Clinical studies have proved oxymatrine could lower the levels of TGF-β1 in chronic hepatitis B patients [17]. 4) Against lipid peroxidation: Oxidative stress is a major contributing factor to the onset of liver fibrosis. Mediators released from damaged hepatocytes, such as lipid peroxidation products, acetaldehyde as well as ROS (hydrogen peroxide, superoxide radical) are strong inducers of HSC activation. We found oxymatrine could affect the genes related to the biological oxidation, suggesting that oxymatrine might induce a decline of oxidative stress and lipid peroxidation, and thus inhibiting the activation of HSCs. Previous study proved that oxymatrine showed prophylactic and therapeutic effects in D-galactosamine-induced rat hepatic fibrosis, partly by protecting hepatocytes and suppressing fibrosis accumulation through acting against lipid peroxidation [18]. 5) Affecting MAPK signaling pathway: Previous study demonstrated that OM was effective in reducing the production and deposition of collagen in the liver tissue of experimental rats via the p38 mitogen-activated protein kinase signaling pathway [2].
In conclusion, our study revealed many genes implicated in HSC activation. Oxymatrine could inhibit HSC proliferation. The mechanism of oxymatrine was multilevel, multi-targeted and had multiple pathways. The intriguing findings of altered gene expression provide a molecular basis of oxymatrine in the treatment of liver fibrosis.
REFERENCES
- Luk, J.M., Wang, X., Liu, P., Wong, K.F., Chan, K.L., Tong, Y., Hui, C.K., Lau, G.K. and Fan, S.T. (2007) Traditional Chinese herbal medicines for treatment of liver fibrosis and cancer: From laboratory discovery to clinical evaluation. Liver International, 27, 879-890. doi:10.1111/j.1478-3231.2007.01527.x
- Deng, Z.Y., Li, J., Jin, Y., Chen, X.L. and Lü, X.W. (2009) Effect of oxymatrine on the p38 mitogen-activated protein kinases signaling pathway in rats with CCl4 induced hepatic fibrosis. Chinese Medical Journal (English), 122, 1449-1454.
- Wu, X.L., Zeng, W.Z., Jiang, M.D., Qin, J.P. and Xu, H. (2008) Effect of oxymatrine on the TGFbeta-Smad signaling pathway in rats with CCl4-induced hepatic fibrosis. World Journal of Gastroenterology, 14, 2100-2105. doi:10.3748/wjg.14.2100
- Shi, G.F. and Li, Q. (2005) Effects of oxymatrine on experimental hepatic fibrosis and its mechanism in vivo. World Journal of Gastroenterology, 11, 268-271.
- Wu, C.S., Piao, X.X., Piao, D.M., Jin, Y.R. and Li, C.H. (2005) Treatment of pig serum-induced rat liver fibrosis with Boschniakia rossica, oxymatrine and interferon-alpha. World Journal of Gastroenterology, 11, 122-126.
- Yu, X.H., Zhu, J.S., Yu, H.F. and Zhu, L. (2004) Immunomodulatory effect of oxymatrine on induced CCl4- hepatic fibrosis in rats. Chinese Medical Journal (English), 117, 1856-1858.
- Mao, Y.M., Zeng, M.D., Lu, L.G., Wan, M.B., Li, C.Z., Chen, C.W., Fu, Q.C., Wang, J.Y., She, W.M., Cai, X., Ye, J., Zhou, X.Q., Wang, H., Wu, S.M., Tang, M.F., Zhu, J.S., Chen, W.X. and Zhang, H.Q. (2004) Capsule oxymatrine in treatment of hepatic fibrosis due to chronic viral hepatitis: A randomized, double blind, placebo-controlled, multicenter clinical study. World Journal of Gastroenterology, 10, 3269-3273.
- Lin, M., Yang, L.Y., Li, W.Y., Peng, Y.P. and Zheng, J.K. (2009) Inhibition of the replication of hepatitis B virus in vitro by oxymatrine. Journal of International Medical Research, 37, 1411-1149. doi:10.1177/147323000903700515
- Cheng, Y., Ping, J., Xu, H.D., Fu, H.J. and Zhou, Z.H. (2006) Synergistic effect of a novel oxymatrine-baicalin combination against hepatitis B virus replication, alpha smooth muscle actin expression and type I collagen synthesis in vitro. World Journal of Gastroenterology, 12, 5153-5159.
- Liu, J., Manheimer, E., Tsutani, K. and Gluud, C. (2003) Medicinal herbs for hepatitis C virus infection: A Cochrane hepatobiliary systematic review of randomized trials. The American Journal of Gastroenterology, 98, 538-544. doi:10.1111/j.1572-0241.2003.07298.x
- Jian, Y.C., Li, W., He, Y., Jiang, M., Liu, Y.B. and Xiong, W.J. (2012) Effect of oxymatrine on hepatic gene expression profile in experimental liver fibrosis of rats. Chinese Journal of Integrative Medicine, 18, 445-450. doi:10.1007/s11655-012-1115-x
- Kang, Y.J. (2008) Herbogenomics: From traditional Chinese medicine to novel therapeutics. Experimental Biology and Medicine, 233, 1059-1065. doi:10.3181/0802-MR-47
- Weiskirchen, R. and Gressner, A.M. (2005) Isolation and culture of hepatic stellate cells. Methods in Molecular Medicine, 117, 99-113.
- Huang, da W., Sherman, B.T. and Lempicki, R.A. (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols, 4, 44-57. doi:10.1038/nprot.2008.211
- Guzman, J.R., Koo, J.S., Goldsmith, J.R., Mühlbauer, M., Narula, A. and Jobin, C. (2013) Oxymatrine prevents NF- κB nuclear translocation and ameliorates acute intestinal inflammation. Scientific Reports, 3, 1629. doi:10.1038/srep01629
- Wu, X.L., Zeng, W.Z., Jiang, M.D., Qin, J.P. and Xu, H. (2008) Effect of oxymatrine on the TGFbeta-Smad signaling pathway in rats with CCl4-induced hepatic fibrosis. World Journal of Gastroenterology, 14, 2100-2105. doi:10.3748/wjg.14.2100
- Shen, B.S. and Song, X.W. (2008) Effects of oxymatrine on serum cytokines and hepatic fibrotic indexes in patients with chronic hepatitis B. Zhongguo Zhong Xi Yi Jie He Zazhi, 28, 17-19.
- Yang, W., Zeng, M., Fan, Z., Mao, Y., Song, Y., Jia, Y., Lu, L., Chen, C.W., Peng, Y.S. and Zhu, H.Y. (2002) Prophylactic and therapeutic effect of oxymatrine on Dgalactosamine-induced rat liver fibrosis. Zhonghua Gan Zang Bing Za Zhi, 10, 193-196.
NOTES
*Contributed equally.
#Corresponding author.

