Journal of Biomedical Science and Engineering
Vol.5 No.11(2012), Article ID:24682,7 pages DOI:10.4236/jbise.2012.511082
Effect of Pleurotus ostreatus on hyperglycemia, DNA damage and chromosomes aberrations
![]()
Department of Biochemistry, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
Email: alalmalki@kau.edu.sa
Received 8 September 2012; revised 9 October 2012; accepted 20 October 2012
Keywords: Mushroom; Diabetes; Hyperglycemia; DNA Damage; Chromosome Aberrations and Sperm Alternations; Streptozotocin-Induced Diabetic Rats
ABSTRACT
One of the main health problems with high and markedly increased complications is diabetes. Despite several projects with preventative strategies and armories of medication, the arrangement of diabetes remains grossly unsatisfactory. Thus, it is vital to identify unfamiliar drugs or novel nutraceuticals for treating and preventing diabetes without side effects. The present study deals with scientific information on mushrooms with regards to its potential use as anti-diabetic active food. In addition to the anti-hyperglycemic action of mushrooms, the present study presents its effect on DNA damage, chromosome aberrations and sperm alternations in streptozotocininduced diabetic rats. These animals have been treated, for 30 days, with amaryl (as control treatment) (0.03 mg/kg·b·wt/dl), low-dose mushroom (100 mg/kg·b·wt/dl) and high-dose mushroom (200 mg/ kg·b·wt/dl). The glucose level GL of streptozotocininduced diabetic animals has been markedly improved by mushroom treatment; for example GL has decreases from 167.6 mg/dl down to 116.0 mg/dl for treatment with high-dose mushroom and 128.9 mg/dl for treatment with low-dose mushroom, comparing with amaryl treatment that decreases GL down to 92.6 mg/dl. But, the experimental results show that treatment with mushroom is better than treatment with amaryl in case of genetic changes (DNA fragmentation, disappear of some base pairs and chromosome aberrations. So, it is proposed that more close scientific attention be paid to precede more research of functional mushrooms for preventive and curative treatments for diabetes.
1. INTRODUCTION
Some Pleurotus ostreatus (mushrooms) have shown tendency to be effective for control of blood glucose and also for DNA damage and chromosomes aberrations without any side-effects. The present work demonstrates that mushrooms have promising role in the domain of repairing DNA damage and chromosomes aberrations. The published clinical and/or academic data are, however, still not sufficient enough to show how mushroom reduces hypoglycemic effects to be use as an authoritative drug or medical food (nutraceuticals). So, much scientific attention should be paid to the mushrooms that have effective medical functions and in particular curative functions of diabetes mellitus. Although chemical and biochemical hypoglycemic agents, e.g. insulin [1], troglitazone, repaglinide, tolbutamide and rosiglitazone are the backbone of classical-treatment of diabetes and are so efficient in controlling diabetes, they have hurtful side effects and fail to significantly alter the course of diabetic complications [2]. Mushrooms have been defined [3] as a macro fungus with special fruiting-bodies that could be epigeous and large enough to be seen and to be picked simply by naked hands [3]. Mushrooms constitute more than twenty thousand known species which are widely distributed all-over the globe; however, only about two thousands (10%) species are known and could be explored. Huge quantity of mushrooms species are unexplored and even undiscovered which would possibly be of certain medical interest [4]. For example, tremellafuciformis (snow fungus) is commonly used in Chinese sweet-cuisine and it is tasteless. Despite its unordinary nature, Tremella-fuciformis is highly valued for several medical benefits [5]. From its fruiting bodies, snow fungus show an important dose dependent hypoglycemic activity in normal mice and significant medical performance in streptozotocin induce diabetic mice [6]. The anti-diabetic activity of submerged culture of snow fungus, exo-polysaccharides has been reported [7]. Their data have suggested that exo-polysaccharides exhibits major hypoglycemic effect and even improve the medical activity of insulin [8]. This means that snow fungus has potential oral hypoglycemic effect and can be used as medical-food. Moreover, the administration of mushroom in drinking-water and the diet has highly opposed the hyper glycemia of streptozotocin diabetic mice [9]. Mushroom with “immune modulating” polysaccharides can be used as medical food, it can be used as drug in limited conditions and it can be used as nutraceutical (health-promoting food). Hokama et al. [10] have reported that many medical plants are an important source of strong antioxidants and have free radical scavenging activity [11]. Even many medical properties have been reported for reduction of blood glucose [6] and inhabitation of platelet aggregation [10]. In general, many physiological processes of hyperglycemia produce “oxygencenter” free radicals and other reactive oxygen species [12]. These reactive oxygen species can easily crush the effect of protective enzymes (antioxidant defense) and can cause high damage and even lethal cellular defects if membrane lipids, cellular proteins DNA and enzymes will be oxides [13]. As it is well known, the damage of nuclear component (or any part of its host) leads to diabetes mediated stress [14]. Consequently, the physical defects of cells will be stimulated to cause different singes of neurological deficiencies, carcinogenesis aging, heart disorders, and so on, while the germinal cell damage will yield infertility [14,15]. In addition, chromosomes aberrations, DNA damages and sperm abnormalities are the main signs in hyper-glycemic rates [14-17]. Natural and artificial antioxidant agents stimulate the different oxidation processes due to hyperglycemia. However, the use of artificial antioxidant results in potential risk, for example laws of some countries prohibits their use [13,18]. Thus, medical plants such as mushroom are considered as safer antioxidants [11,19,20]. Mushrooms, from these medical plants, strongly reduce the platelet aggregation [10], reduce the blood-cholesterol concentrations [21] and prevent induced liver per oxidation [22,23]. Several researches have shown the medical importance of mushroom, however, no much information are concerning to their anti hyper-glycemic agent. Thus, the aim of this work is to study the different effects of mushroom on streptozotocin-induced diabetic rats. In particular, chromosome aberrations, sperm abnormalities and DNA damage are closely studied.
2. METHODOLOGY, ANIMALS AND MATERIALS
2.1. Animals
Male albino rats of about 150 - 160 g have been housed under standard laboratory conditions and maintained on 12 hours light. These rats have been provided with water and pellet food ad libitum.
2.2. Preparation of Mushrooms
Mushroom stem-bodies have been purchased from local market in dried form. 5 g of these dried mushroom parts have been powdered and extracted with 100 ml of ethanol (95 percent). By vacuum distillation, the residue has been filtered then centered to a dry mass which has been used as mushroom extract. Low-level of this extract (100 mg/kg·b·wt) and high-level concentrations (200 mg/ kg·b·wt) have been used as it will be seen in the methodology. It is worth investigating this effect as a function of different concentrations; however, this will be the subject for future study.
2.3. Chemicals and Drugs
Kits of glucose oxidase peroxidase diagnostic enzyme and streptozotocin have been obtained from Sigma (USA, MO St. louis). Glimepiride tablets (amaryl), obtained from local pharmacies, have been ground to fine powder which dissolved in pure distilled-water. For continuous thirty days, it has been orally administrated at 0.03 mg/ kg·b·wt. This last value is equivalent to an acceptable dose for human (4 mg/kg) [8]. Here, standard treatment with glimepiride tablets has been used for comparison with mushroom treatment.
2.4. Diabetes Creation
For 24 hours, the experimental animals have been fasted, then injected with 65 mg/kg bodu (single dose) of freshly prepared streptozotocin (dissolved in citrate buffer PH4.5) to create diabetes [24]. The blood samples have been collected via retro-orbital venous plexus and serum glucose level have been estimated (just prior to killing the animals at the end of the experiment) by enzymatic glucose oxidase per-oxidase (GOD PAP) diagnostic kit [14, 25], the diabetes has been confirmed after 48 hours. The animals have been fasted for three hours then blood has been collected from orbital sinus.
2.5. Design of Set Up
25 male rats have been selected at random then divided into 5 groups each one has 5 experimental animals: 1) Control group, C-group (non-diabetic) 2) diabetic group 3) A-group which is lowand high-level amaryl treated diabetic animals 4) ML-group which is low-level mushroom treated diabetic animals, and 5) MH-group which is high-level mushroom treated diabetic animals. MLand MH-groups have been orally given 100 and 200 mg/ kg·b·wt/dl, respectively. After 30 days, at the experiment end, the rats have been sacrificed by cervical-dislocation to study molecular-genetic, cytogenetic and sperm studies.
2.6. Molecular-Genetic Studies
2.6.1. DNA Fragmentation
Immediately, after sacrificing the animals their livers have been collected and their tissues have been lysed in 0.5 ml of lysis buffer containing: 1 mM EDTA, 10 mM tris-HCl (pH 8), (0.2%) triton X 100 centerfuged at 10000 rpm for 20 minute at 4˚C. The pellets have been re-suspended in 0.5 ml of lysis buffer as it has been described by Gibb et al. [26] and the fragmented-DNA parts have been calculated from the following equation:

2.6.2. DNA Extraction
According to Sharma’s methodology [27], genomic DNA has been segregated from the animal’s liver and its concentration has been estimated using a spectrometer of 260 nm and 280 nm absorbance, respectively. The totality of isolated DNA has been test by electrophoresis in 0.8% agar rose gel using DNA molecular weight marker (Eurblio, Paris France). ISSR-analysis has been performed using three different primer that are procedure from integrated DNA technologies Inc (USA, CA, San Diego), based on core repeats anchored at the 3’ or 5’ end as it is shown in Table 1. Amplification reactions for ISSR-analysis have been used in final volume 25 μl containing 10xPCR buffer (50 mM KCl, 10 mM MgCl (pH9.0, 2 mM 2dNTPs, 10 mM primer, 50 ng DNA and 0.5 Taq Polymerase Promega, USA). ISSR has been performed using Zietkiewicz et al. [28] methodology [29] (Zietkiewicz et al. 1994). PCR products have been analyzed using 1.2% agar-rose gel electrophoresis and visualized with 10 ug/ul ethidium bromide staining. The sizes of fragments have been calculated based on a DNA ladder of 100 to 2000 bp (MBI, Fermentas). In fact, Agarose gel is one of several physical methods for demining the size of DNA. Using Electrophoresis technique, DNA is forced to migrate through a highly cross-linked agarose matrix in response to an electric field: The phosphates on DNA are negatively charged, and the molecule will move to the positive pole, “red one”. By this technique, one can separate the DNA bands of ISSR distinctly.
2.6.3. Chromosome Preparations
For the above mentioned five animal groups; animals femurs have been removed and the bone marrow cells have been aspirated from both femurs of each animal as

Table 1. Elementary sequences used for ISSR amplification.
it has been described elsewhere [28].
Cultures have been incubated, for one hour at 37 < T < 38˚C and the cells have been centrifuged for ten minute at 1000 r.p.m., then re-suspended in pre-warmed hypotonic solution at 37˚C for 20 minute. The samples have been centrifuged and fixed in cold “3:1 methanol-glacial acetic acid”. Each sample has been washed several times fixative and slides have been performed as shown elsewhere by Preston et al. [30]. Chromosome analyses have been carried out in 50 metaphase spreads.
2.6.4. Sperm Analysis
Using Wyrobec methodology [31,32], the epididimus excised and minced in about 8 ml of physiological saline solution, dispersed and filtered to exclude large tissue fragments. Spots have been prepared after staining the sperm with aqueous-Eosin Y. At least 3000 sperms per group have been assessed for morphological abnormalities which have been evaluated using Narayana method [32].
2.7. Statistical Analysis
SPSS-software has been used to carry out the statistical analysis and data have been analyzed using one way analysis variance; then Duncan’s-post-hot test has been used for comparison between different treatments in the same sex. The values are shown as “mean-value ± S.D.” and differences have been only considered if P < 0.05.
3. RESULTS
3.1. Blood Glucose Level
Data in Figure 1 show that:
1) Blood glucose level increases in diabetic rats than those of diabetic group.
2) Blood glucose level decreases in diabetic animals treated with amaryl.
3) Blood glucose level decreases in diabetic rats when treated with mushroom (MLand MH-groups).
4) These data are shown in Table 2.
3.2. DNA Fragmentation
The experimental data shown in Table 3 show that:
1) The rate of DNA fragmentation (for diabetic animals) increases comparing with those of controlling group.
2) The rate of DNA fragmentation (for diabetic animals treated with amaryl) decreases comparing with those of controlling group.
3) The rate of DNA fragmentation (for diabetic animals treated with mushroom) decreases comparing with those of controlling group.
4) Curiously, the data of high-dose and low-dose of

Figure 1. Effect of Mushroom treatment on blood glucose levels in diabetic rats after 30 treatment-days: 1 = Normal rats; 2 = Streptozotocin-induced diabetic rats treated with amaryl; 3 = Streptozotocin-induced diabetic rats treated high dose of mushroom; 4 = Streptozotocin-induced diabetic rats treated low dose of mushroom; 5 = Streptozotocin-induced diabetic rats and non-treated.
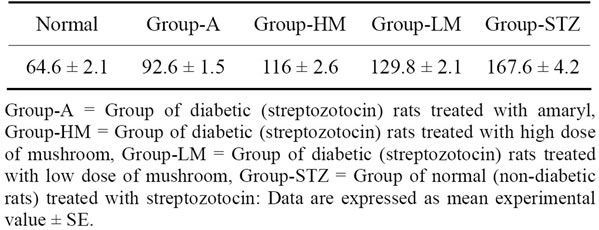
Table 2. Effect of Mushroom treatment on blood glucose levels (mg/dl) in diabetic rats after thirty treatment-days.

Table 3. Effect of different treatments on the rates of DNA fragmentation in streptozotocin-induced diabetic rats after thirty treatment-days.
mushroom treatment are nearly similar and no net difference between them (in the range of experimental error: P < 0.05).
3.3. ISSR PCR Analysis
As it has been mentioned, to detect the mushroom treatment efficiency, three ISSR primers are used: HB10, HB12 and HB14. The present experimental results show that for HB-10:
1) An equal four fragments which have difference of 150 bp fragment between data of control group and Agroup (Amaryle treated group).
2) The groups of animals treated with mushroom display four amplified fragments while 610 bp fragments have been disappeared comparing with control, amaryl-treated and diabetic groups.
For HB-12:
1) Six fragments in the control group and HM-group, whereas diabetic animals, amaryl samples and a group of rates treated with low mushroom concentration have lacked 230 bp fragments which are exist in each of controland HM-groups.
2) The groups of animals treated with mushroom display four amplified fragments while 610 bp.
For HB-14:
1) Eight fragments in the control group, while this primer revealed 4 and 5 fragments in each diabetic animals and amaryl samples respectively.
2) The groups of animals treated with mushroom differed from the control samples in 490, 290, 250 and 50 bp fragments for diabetic samples and 50 bp fragments for A-group. Also, samples treated with low and high concentrations of mushroom revealed an equal 7 fragments which are different from the control samples in only one fragment of 290 bp for HM-group.
The obtained data evidently observed that 490, 290, and 50 bp fragments which exist in mushroom group, they disappeared in diabetic rats. Also, the fragments have been disappeared comparing with control, amaryltreated and diabetic groups.
3) 490, 250 and 50 bp fragments are absent in A-group but they are present in HMand LM-groups.
4) The 290, 250 and 50 bp fragments (present in HMand LM-groups) disappear in diabetic group.
3.4. Chromosome Examination
Frequencies of structural and numerical chromosome aberrations are significantly (P < 0.05) increases in Dgroup (diabetic animals) than the control animals (Table 4). While amaryl-treated diabetic animals and mushroom-treated diabetic group decrease of most frequencies of structural and numerical chromosome aberrations than the diabetic animals (D-group).
It is worth noting that the differences between amaryltreated diabetic animals and mushroom-treated diabetic animals are significant (P < 0.05) and the diabetic rats treated with low or high concentrations of mushroom have low frequencies of most structural and numerical chromosome aberrations than the diabetic animals.
4. DISCUSSION
Mushroom has proved that it is an efficient exporter of main amino acids which contain antioxidant vitalities and many medicinal properties [23,33,34]. In particular, it contains levastatin which is a cholesterol-lowering drug used for correcting hypercholesterolemia [23,33]. In the present study, the medicinal-role of mushroom for anti-hyperglycemic drug, repair of DNA damage, chro-
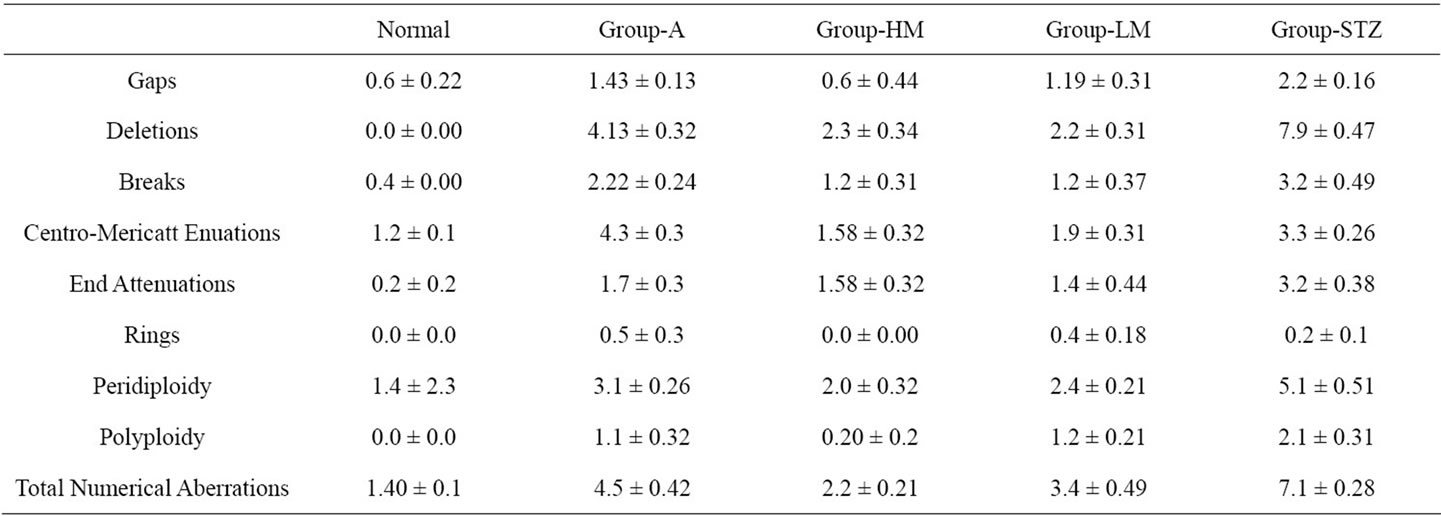
Table 4. Effect of different treatments on the frequency of chromosome aberrations in streptozotocin-induced diabetic rats after thirty treatment-days.

Figure 2. Effect of treatments with amaryl and mushroom on the rates of DNA fragmentation in streptozotocin-induced diabetic rats after thirty treatment-days: 1 = Normal rats; 2 = Streptozotocin-induced diabetic rats treated with amaryl; 3 = Streptozotocin-induced diabetic rats treated high dose of mushroom; 4 = Streptozotocin-induced diabetic rats treated low dose of mushroom; 5 = Streptozotocin-induced diabetic rats (nontreat rats).
mosome aberrations and sperm abnormalities. The blood glucose level has increased in streptozotocin diabetic rats rather than those of normal or un-diabetic. This situation is due to the cyto-toxic effect of the streptozotocin on pancreatic beta cells which results in hypo-insulin-emia and complete loss of glucose [35-37]. Other authors have reported abnormalities in pancreatic beta cells at about 10 - 16 weeks [37] or for 36 weak [35,36]; the data of the present work are in agreement with [14,17]. Moreover, the present experimental data (Figure 2) show that streptozotocin-diabetic rats have higher rates of DNA fragmentation compared to un-diabetic rats. Otton et al. [16] have reported that DNA fragmentationin lymphocytes collected from alloxan induced diabetic rats is found to be 81% compared to 45% of untreated (control) cells. In addition, the data of the present study (using ISSR PCR analysis) show that some fragments of bp have disappeared in streptozotocin diabetic rats even they have been present in control group. ISSR fragments data show that some base pair fragments of correcting hypercholesterolemia [23,33]. However, the DNA in hyperglycemic rats have been disappeared or deleted. The disappeared fragments of DNA in diabetes exist in normal group. Hyperglycemia conditions may be the reason of this loss. The experimental data of this study show, also, that hyperglycemic rats have important increase of chromosome aberrations compared to normal group which is in agreement with reference [17]. The sperm abnormalities in streptozotocin diabetic rats, of the present data, have significantly increased than the normal group (un-diabetic group) which is in fair agreement with Rabbani et al. [14].
DNA fragmentation, sperm abnormalities and loss of some base pair of DNA (Genetic alternations) is due to the effect of hyperglycemia [16]; several authors have reported that the overproduction of reactive oxygen species ROS which are side product of hyperglycemia attack the cell-membrane, nucleus and genetic parts leading to possible protein and DNA modifications [38,39] which leads, consequently, to sperm abnormalities and chromosome aberrations [14,16,40]. Thus, the DNA fragmentation and chromosome derangements, in the present data, could be a result of ROS. In addition, several damage path ways by the ROS such as accelerated formation of advanced glycation end production, polyol pathway, hexosamine pathway, protein kinase or lipid per-oxidation [14,15,41].
5. CONCLUSION
The present study shows that the treatment with mushroom could reduce the high blood glucose level but not as efficient as amaryl. However, treatment with mushroom has been more effective for decreasing the genetic alternations and sperm abnormalities in diabetic conditions than amaryl treatment.
![]()
![]()
REFERENCES
- Wild, S., Roglic, G., Green, A., Sicree, R. and King, H. (2004) Global prevalence of diabetes: Estimate for 2000 and projection of 2030. Diabetes Care, 27, 1047-1053.
- Li, W.L., Zheng, H.C., Bukuru, J. and De Kimpe, N. (2004) Natural medicines used in traditional Chinese medical system for therapy of diabetic mellitus. Journal of Ethnopharmacology, 92, 1-21.
- Chang S.T. and Miles P.G. (1992) Mushrooms biology a new discipline. Mycologist, 6, 64-65. doi:10.1016/S0269-915X(09)80449-7
- Hawksworth D.L. (2001) Mushrooms: The extent of unexplored potential. International Journal of Medical Mushrooms, 3, 333-337.
- Shu-Ting, C. and Philip, G.M. (2004) Tremella—Increased production by a mixed culture technique. In: Mushrooms: Cultivation, Nutritional Value Medical Effect and Environmental Impact, 2nd Edition, CRC Press, Boca Raton.
- Kiho, T., Tsujimura, Y., Sakushima, M., Usui, S. and Ukai, S. (1994) Polysaccharides in fungi. XXXIII. Hypoglycemic activity of an acidic polysaccharide (AC) Tremella fuciformis. Yakugaku zasshi, 114, 308-315.
- Cho, E.J., Hwang, H.J., Kim, S.W., Oh, J.Y., Baek. Y.M., Choi, J.W., et al. (2007) Hypoglycemic effects of exopolysaccharides produced by mycelia cultures of two different mushrooms Tremella fuciformis and Phellinus baumii in ob/ob mice. Applied Microbiology and Biotechnology, 75, 1257-1265.
- Nieszner, E., Posa, I., Kocsis, E., Pogatsa, G., Preda, I. and Koltai M.Z. (2002) Influence of different sulfonylureas on the size of myocardial infarction with and without ischemic preconditioning in rabbits. Experimental Clinical Endocrinology Diabetes, 110, 2002, 212-218.
- Gray, A.M. and Flatt, P.R. (1998) Insulin-releasing and insulin-like activity of Agaricus campestris (mushroom). Journal of Endocrinology, 157, 259-266.
- Hokama, Y. and Hokama, J.L. (1981) In vitro ihibitation of platelet aggregation with low Dalton compounds from aqueous dialysates of edible fungi. Research Communications in Chemical Pathology and Pharmacology, 31, 177-180.
- Jose, N. and Janardhanan, K.K. (2000) Antioxidant and antitumor activity of Pleurotus florida. Current Science, 79, 941-943.
- Szkudelski, T. (2001) The mechanism of alloxan and streptozotocin action in Beta cells of the rat pancreas. Physiological Research, 50, 537-546.
- Pihlanto, A. (2006) Anti-oxidative peptides derived from milk proteins. International Dairy Journal, 16, 2006, 1306-1314.
- Rabbani, S.I., Devi, K. and Khanam, S. (2009) Inhibitory effect of glimepiride on nicotinamide-streptozotocin induced nuclear damage and sperm abnormality in diabetic Wister rats. Indian Journal Experimental Biology, 47, 804-810.
- Valko, M., Leibfritz, D., Moncol, J., Croni, M.T., Mazur M. and Tesler, J. (2007) Free radicals and antioxidant in normal physiological function and human diseases. International Journal of Biochemistry and Cell Biology, 39, 2007, 44-84.
- Otton, R., Soriano, F.G., Verlengia, R. and Curi, R. (2004) Diabetes induces apoptosis in lymphocytes. Journal of Endocrinology, 182, 145-156. doi:10.1677/joe.0.1820145
- Abd El-Rahim, A.H., Radwan, H.A., Abd El-Moneim, O.M., Farag, I.M. and Nada, S.A. (2010) The influence of amaryl on genetic alterations and sperm abnormalities of rats with alloxan-induced hyperglycemia. Journal American Science, 6, 1739-1748.
- Tepe, G., Dietrich, T., Grafen, F., Brehme, U., Muschick, P. and Dinkelborg, L.M. (2005) Reduction of intimal hyperplasia with Re-188-labeled stents in a rabbit model at 7 and 26 weeks: An experimental study. Cardiovascular and Interventional Radiology, 28, 623-627.
- Wasser, S.P. and Weiss, A.L. (1999) Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: A modern perspective. Critical Reviews in Immunology, 19, 65-96.
- Ajith, T., Janardhanan, K.K., Antioxidant and anti-inflammatory activities of methanol extract of Phellinus rimosus (Berk) Pilat. Indian Journal of Experimental Biology, 39, 1166-1169.
- Aletor, V.A. (1995) Compositional studies on edible tropical species of mushrooms. Food Chemistry, 54, 265- 268.
- Lin, S., Zhu, P. and Liao, H. (1998) Blocking effect of edible mushroom beverages on the carbon tetrachloride induced rat liver lipid per-oxidation, Zhongguo Gonggong Weisheng Xuebao, 17, 15.
- Jayakumar, T., Ramesh, E. and Geraldine, P. (2006) Antioxidant activity of the oyster mushroom, Pleurotus ostreatus, on CCl(4)-induced liver injury in rats. Food and Chemical Toxicology, 44, 1989-1996.
- Tuzcu, M. and Baydas, G. (2006) Effect of melatonin and vitamin E on diabetes-induced learning and memory impairment in rats. European Journal of Pharmacology, 537, 106-110.
- Kuhad, A., Sethi, R. and Chopra, K. (2008) Lycopene attenuates diabetes-associated cognitive decline in rats. Life Sciences, 83, 128-134.
- Gibb, A., Taylar, D.D., Wan, T., Oconnor, D.M., Doering, D.L. and Gercel-Taylor, C. (1997) Apoptosis as a measure of chemo-sensitivity to cisplatin and taxol therapy in ovarian cancer cell lines. Gynecologic Oncology, 65, 13- 22
- Sharma, K.K., Lavanya, M. and Anjaiah, V. (2000) A method for isolation and purification of peanut genomic DNA suitable for analytical applications. Plant Molecular Biology Reporter, 18, 393-398.
- Williams, D.L., Harris, A., Williams, K.J., Brosius, M.J. and Lemods W. (1984) A direct bone marrow chromosome technique for acute lymphoblastic ISSR markers. International Journal of Agriculture and Biological Leukemia, Cancer Genetics Cytogenetics, 13, 239-257.
- Zietkiewicz, E., Rafalski, A. and Labuda, D. (1994) Genome finger-printing by simple repeat (SSR)-anchored polymerase chain reaction amplification. Genomics, 20, 176-183.
- Preston, R.J., Dean, B.D., Galloway, S., Holden, H., McFee, A.F. and Shelly, M. (1987) Mammalian in vivo cytogenetic assays: Analysis of chromosome aberrations in bone marrow cells. Mutation Research, 189, 157-165.
- Wyrobek, A.J. and Bruce, W.R. (1978) The induction of sperm shape-abnormalities in mice and humans. Chemical Mutagens, 5, 257-285.
- Narayana, K. (2008) An amino-glycoside antibiotic gentamycin induced oxidative stress, reduces antioxidant reserve and impairs spermatogenesis in rats. Journal of Toxicological Sciences, 33, 85-96.
- Endo, T.R. (1988) Induction of chromosomal structural changes by a chromosome of Aegilops cylindrical L in common wheat. Journal of Heredity, 79, 366-370.
- Mattila, P., Salo-Väänänen, P., Kӧnkӧ, K., Aro, H. and Jalava, T. (2002) Basic composition and amino acid contents of mushrooms cultivated in Finland. Journal of Agricultural and Food Chemistry, 50, 6419-6422. doi:10.1021/jf020608m
- Arulmozhi, D.K., Veeranjaneyulu, A. and Bodhankar, S.L. (2004) Neonatal streptozotocin-induced rat model of type 2 diabetes mellitus: A glance. Indian Journal of Pharmacology, 36, 217-221.
- Portha, B., Picolon, L. and Rosselin, G. (1979) A Chemical diabetes in the adult rats as the spontaneous evolution of neonatal diabetes. Diabetologia, 17, 371-377. doi:10.1007/BF01236272
- Weir, C.G., Clore, E.E., Zma-Chinsky, C.J. and BonnierWier, S. (1981) Islet secretion in a new experimental model for non-insulin-dependent diabetes. Diabetes Metabolism Reviews, 30, 590-595.
- Block, K.O., Zemel, R., Bloch, O.V., Grief, H. and Vardi, P. (2000) Streptozotocin and alloxan-based selection improve toxin resistance of insulin producing RINm Cells. International Journal of Experimental Diabetes Research, 1, 211-219. doi:10.1155/EDR.2000.211
- Rehman, A., Nourooz-Zadeh, J., Moller, W., Tritschler, H., Pereira, P. and Halliwell, B. (1999) Increased oxidative damage to all DNA bases in patients with type II diabetic mellitus. FEBS Letters, 448, 120-122. doi:10.1016/S0014-5793(99)00339-7
- Aydin, S., Aytac, E., Uzun, H., Altug, T., Mansur, B., Saygili, S., Buyukpinarbasili, N. and Sariyar, M. (2010) Effects of ganderma lucidum on obstructive jaundice-induced oxidative stress. Asian Journal of Surgery, 33, 173- 180.
- Piconi, L., Quagliaro, L. and Ceriello, A. (2003) Oxidative stress in diabetes. Clinical Chemistry and Laboratory Medicine, 41, 1144-1149.

