Materials Sciences and Applications
Vol.5 No.10(2014), Article ID:49393,15 pages
DOI:10.4236/msa.2014.510076
Nano-Sized Elements in Electrochemical Biosensors
Joanna Cabaj, Jadwiga Sołoducho*
Faculty of Chemistry, Wroclaw University of Technology, Wrocław, Poland
Email: joanna.cabaj@pwr.edu.pl, *jadwiga.soloducho@pwr.edu.pl
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 14 June 2014; revised 16 July 2014; accepted 30 July 2014
ABSTRACT
The emerging nanotechnology has opened novel opportunities to explore analytical applications of the fabricated nano-sized materials. Recent advances in nano-biotechnology have made it possible to realize a variety of enzyme electrodes suitable for sensing application. In coating miniaturized electrodes with biocatalysts, undoubtedly the most of the potential deposition processes suffer from the difficulty in depositing process and reproducible coatings of the active enzyme on the miniature transducer element. The promising prospects can concern to the obtaining of thin protein layers by using, i.e. electrochemical deposition, electrophoretic deposition as well as monolayer methods (Langmuir-Blodgett procedure, Layer-by-Layer—LbL). Many aspects dealing with deposition of enzyme by techniques employing electric field are considered, including surface charge of enzyme, and its migration under applied electric filed. The using of nanoscale materials (i.e. nanoparticles, nanowires, nanorods) for electrochemical biosensing has seen also explosive increase in recent years following the discovery of nanotubes. These structures offer a promise in the development of biosensing, facilitating the great improvement of the selectivity and sensitivity of the current methods. Finally, the perspectives in the further exploration of nanoscaled sensors are discussed.
Keywords:Enzymes, Immobilization, Nanobiosensors, Nanoparticles

1. Introduction
To date, there is an increasing necessity for mighty analytical tools with high sensitivity, fast response, selectivity, accuracy and low cost of production/operation. Notably, biosensors have found comprehensive adoption in the area of environmental control as well as pharmaceutics and medical diagnostics. Consequently, the main objective in biosensors design is the sufficient development of a biosurface, firmly sensitive and selective for a respective analyte, which may be able to generate measurable signals coupled to an adequate transducer.
Thus, many groups of researchers tend also to combine nanoparticles into the materials used for biosensors in order to improve the sensitivity of the system in potential sensing applications. Most recent studies show that biosensors composed with nanoparticles do take on rapid, simple, and accurate measurements, which offers exciting new opportunities for the development of biosensor capabilities. Owing to the emerging roles that nanoparticles are playing in the improvement of biosensors in recent years, it is necessary and meaningful for us to investigate the researches of nanoparticle-based biosensors from the point of view of management of technology. As a vital part of management of technology, grasping the latest development of technology and identifying the emerging characters can help us get competitive advantages in the future.
Several kinds of nanoparticles, including metal nanoparticles, oxide nanoparticles, semiconductor nanoparticles, and even nanodimensional conducting polymers have been used in biosensing systems. Owing to these unique properties, different kinds of nanoparticles always play different roles in different sensing systems. Generally, metal nanoparticles are always used as elements of “electronic wires”. Oxide nanoparticles are often applied to immobilize biomolecules, while semiconductor nanoparticles are often used as labels or tracers [1] .
The development of high performance and reliable miniaturized enzyme electrodes is a crucial objective worth pursuing in the nanosized biotechnology area. The miniaturization bids numerous advantages [2] . Miniaturized enzyme electrodes are useful in analysis of small sample volumes, hence practical if only small amounts of biological fluids are provided or, waste saving if larger quantities are available. These small systems can be also integrated into new technologies like microarray sensor or microfluidic systems. When used in vivo, miniaturized systems create less damage of the tissues and hence quick healing.
Certainly, there are several methods (i.e. Langmuir-Blodgett techniques, LbL) with which enzymes can be deposited including but not limited to entrapment and crosslinking [3] . But an important problem in the application of enzymatic proteins for the development of miniaturized electrodes is the difficulty in depositing uniform and reproducible layer coatings of enzymes on the transducer [4] .
Electrochemical and electrophoretic deposition are offered as techniques, which can employ electric field to produce apparently uniform and reproducible layer coatings of biocatalysts over very small areas. Electrochemical deposition is known for several decades, but by the contrast electrophoretic deposition is rather recent technique [5] . However, further development work needs to be done according to optimize the parameters for a broader use of especially in the fabrication of miniaturized enzyme systems [4] .
2. Immobilization of Enzymes in Miniaturized Systems
2.1. Electrochemical Deposition
A study of literature indicates that electrochemical deposition is one of the most techniques employed for enzyme immobilization, because of simple, cost effective apparatus. Electrochemical deposition can be employed to drive enzymes alone to deposit on the support, as well as with other components including i.e. collagen [6] , noble metal salts (Pt, Pd) [7] , monomers such as pyrrole, 1, 3-diaminobenzene [8] , some redox mediators (i.e. Prussian blue) or redox centers [9] , nanomaterials like carbon nanotubes and metal nanoparticles [10] . The final goal of all these efforts is to fabricate enzyme electrodes with appropriate characteristics in terms of preserved activity, enhanced kinetics and stability to fit with the specific application.
Generally, the activity of enzyme electrode prepared by electrochemical deposition depends primarily on thickness of the enzyme layers [11] .
The latter yields deposition of thin enzyme coatings because only enzymes present nearby vicinity of the electrode surface precipitate. Matsumoto et al. [12] observed that only few tens of nanometer are produced. The thickness of the enzyme coating can be increased to reach much thicker layers if i.e. a surfactant is added to enzyme solution prior deposition.
According to kinetics, it was observed, that enzymes deposited under electrochemical deposition may or may not keep similar kinetics as nativeproteins [13] [14] . This fact depends on the environment of the deposited enzyme coating and presence of other components. In case of stability, enzyme electrodes produced by electrochemical technique have usually moderate stabilities [4] .
2.2. Electrophoretic Deposition
Electrophoretic deposition is carried on from low conductivity aqueous solutions/suspensions (Figure 1). The technique requires high strength electric fields, which can reach several hundreds of volts to move the charged biocatalysts from bulk of the solution to the electrode. Both parameters of elevated zeta potential and high strength electric field yield significant migration of enzymatic proteins under electrophoretic deposition [4] . As a result, more enzymes reach the surface of the electrode and precipitate to form thick-deposited coatings [15] . It is weighty, that when high strength electric fields are employed in electrophoretic deposition, continuous direct current can no longer be utilized because it can induce heat and extensive water electrolysis [4] . This situation causes changes in temperature and pH in the environment of electrodes which maylowered the activity of the protein [16] . To avoid the problem it is possible to apply pulsed direct current and alternating current [4] .
Alternating current is no fixed anode or cathode but the polarization of each electrode changes continuously between the positive and negative signs. Due to the fact, that a negatively charged enzyme is subjected to asymmetrical alternating current signal, it should only oscillate in one location because the migration achieved during the first half cycle when one of electrodes is positively charged should be neutralized during the second half process when the other electrode becomes positively charged [4] . According to that, the migration of charged enzyme under symmetrical signals is zero. In results, only thin enzyme coatings can be deposited [5] [17] . Whereas, under unsymmetrical alternating current field is applied to negatively charged protein, large amount of enzymes accumulate nearby the surface of electrode and the enzymes precipitate to yield thick deposited layers. Furthermore, because alternating current fields generate the minimum of water electrolysis as well as heat, the maximum enzymes activity could be preserved after deposition [5] [17] . There is found a several enzymes (i.e. glucose oxidase, peroxidase), which have been successfully immobilized by this method [4] [5] [17] .
2.3. Thin Layer Methods
Adsorption of organic molecules on solid conducting supports to produce thin nanostructured films are one of the most employed architectures and represents an important approach in the field of nano-manipulation. Langmuir-Blodgett (LB) technique promotes a high control of the physical and chemical properties of nanostructured organic films and plays an important role in the production of miniaturized devices applicable as platforms for enzyme immobilization [18] .
Other pathways to prepare platforms based on nanomaterials, aiming the fabrication of electrochemical biosensors are dispersion in solvents, adsorption (e.g. LbL), formation of covalent bonding.
As well, the utilization of hybrid organic/inorganic thin films can, in a simple manner, be employed in solid conductor electrodes. The possibility to incorporate hybrids containing nanostructured materials for enhance electrochemical properties makes these techniques much attractive in the field of bionanoelectrochemistry [19] [20] .
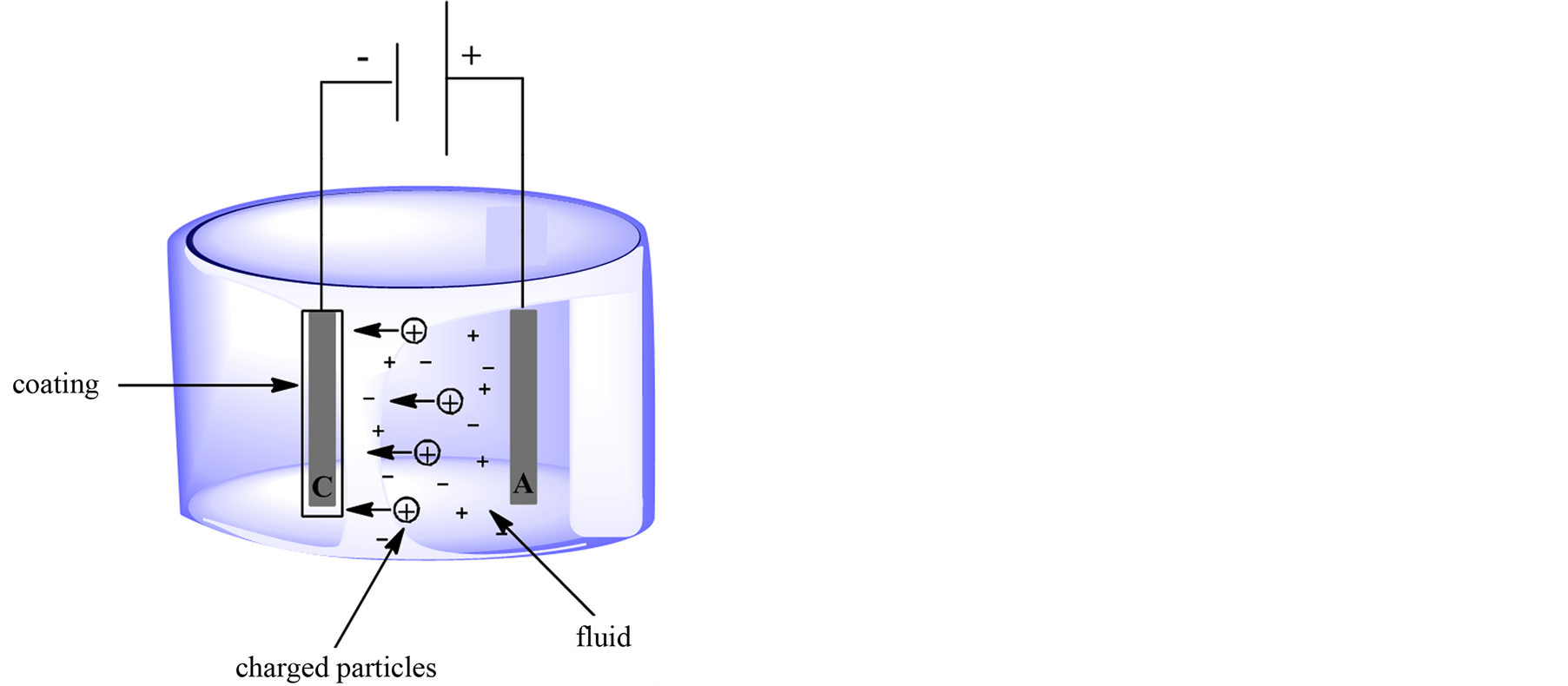
Figure 1. Electrophoretic coating.
The aim is to preserve the native enzyme molecular conformation and to arrange it in a suitable position for the molecular recognizing of an external molecule of a solution put on contact with the Langmuir-Blodgett device. As defended in a review by Girard-Egrot [21] , the successful incorporation of enzymes on a preformed Langmuir monolayer depends strongly on the methodology employed. The most one commonly used is the adsorption of the enzyme from the subphase, avoiding direct adsorption of the macromolecule present at the water surface. This strategy was used to produce electrochemical sensors containing i.e. phytic acid [22] , horseradish peroxidase [23] , hemoblogin [24] , and urease [25] , tyrosinase [26] , to detect a diversity of substances such as including phytic acid, hydrogen peroxide, glucose, choline, urea, and phenols.
In these types of sensors the sensor sensitization can be achieved by i.e. an amphiphilic heterocyclic semiconducting structures admixed into the film [27] or other, more sophisticated architectures have been developed in order to enhance the performance of LB-based enzyme electrochemical sensors. For instance, Sun et al. [28] used pyriduylthio-modified carbon nanotubes as Langmuir-Blodgett films to support hydrogenase added in a subsequent adsorption from solution.
Alternatively, a modern concept of self-assembly was introduced by Decher and co-workers [29] [30] at 90 decade as a low-cost and simple method to obtain nanostructured thin films under controlled conditions (pH, temperature, polyelectrolyte concentration, ionic strength, etc.). For this purpose, a large variety of materials for electrochemical sensing and biosensing can be obtained [31] -[33] . Basically, the processes of film fabrication by LbL technique (Figure 2) is governed by the adsorption of organic polyelectrolytes with opposite charges present on their molecular structure, in such a way that film roughness, thickness, porosity and morphology can be controlled at molecular level [34] . Important advantage in the use of LbL technique to construct biosensors is the possibility to incorporate organic/inorganic composite materials that contributes for the maximization of the biodevices electrochemical signal [35] . Also, it is important to emphasize that most hybrids based on nanomaterials has been utilized to detect electrochemical signal from biochemical reactions.
Proteins have also been used in LbL method to construct alternate multilayer of ceramic nanotubes (halloysite), spherical particles leading to an array of new ordered nanoparticles-tubules, which were applied to load co-enzymes (NAD) for the development of enzymatic nanoreactors. Decher and co-workers [36] also reported the use of protein/polyelectrolyte hybrid films via specific recognition. One of the main challenges is to maintain the integrity of the native protein structure to promote their utilization for technological applications.
Ram and co-workers [37] reported the utilization of LbL technique to produce nanostructured films of poly(ethylene imine) and poly(sodium polystyrene sulfonate), cholesterol oxidase and cholesterol esterase. The strong stability of multilayer films was also evaluated and contributes directly for the electrochemical properties of the film warranting the glucose oxidase immobilized on solid conductor supports remained active on the electrode surface.
3. Summary and Future Trends
Electrochemical nanobiosensors offer without doubts an important step toward development of selective, down to few target molecules sensitive biorecognition device for medical and security applications. In their case, very high amplification of signal could be reached, i.e. using high diameter carbon nanotubes filled with nanoparticles and their following electrochemical stripping.
The utilization of nano-manipulation techniques has also become an interesting approach to fabricate electrochemical devices with high specificity and molecular order. Moreover, the sensitivity and overall behavior of biosensors has grown rapidly as an outcome of incorporating different nanomaterials in their construction.
Electrochemical nanobiosensors consisting from single carbon nanotube are clear examples of future path of biosensor development. These strategies waits for exploration. There is high expectation that such devices will develop toward reliable point-of-care diagnostics of cancer and other diseases, and as tools for intra-operation pathological testing, proteomics and systems biology.
Acknowledgements
Authors are gratefully acknowledged for financial support of NCN-Grant no. 2012/05/B/ST5/00749 and Wrocław University of Technology.
References
- Luo, X., Morrin, A., Killard, A.J. and Smyth, M.R. (2006) Application of Nanoparticles in Electrochemical Sensors and Biosensors. Electroanalysis, 18, 319-326. http://dx.doi.org/10.1002/elan.200503415
- Dukhin, A.S. and Dukhin, S.S. (2005) Aperiodic Capillary Electrophoresis Method Using an Alternating Current Electric Field for Separation of Macromolecules. Electrophoresis, 26, 2149-2153. http://dx.doi.org/10.1002/elps.200410408
- Sheldon, R.A. and van Pelt, S. (2013) Enzyme Immobilisation in Biocatalysis: Why, What and How. Chemical Society Reviews, 42, 6223-6235. http://dx.doi.org/10.1039/c3cs60075k
- Amman, M. (2014) Electrochemical and Electrophoretic Deposition of Enzymes: Principles, Differences and Application in Miniaturized Biosensor and Biofuel Cell Electrodes. Biosensors and Bioelectronics, 58, 121-131.http://dx.doi.org/10.1016/j.bios.2014.02.030
- Ammam, M. and Fransaer, J. (2009) AC-Electrophoretic Deposition of Glucose Oxidase. Biosensors and Bioelectronics, 25, 191-197. http://dx.doi.org/10.1016/j.bios.2009.06.036
- Wang, S.S. and Vieth, W.R. (1973) Immobilization of Whole Cells in a Membraneous Form. Biotechnology and Bioengineering, 15, 93-115. http://dx.doi.org/10.1002/bit.260150108
- Johnston, D.A., Cardosi, M.F. and Vaughan, D.H. (1995) The Electrochemistry of Hydrogen Peroxide on Evaporated Gold/Palladium Composite Electrodes. Manufacture and Electrochemical Characterization. Electroanalysis, 7, 520-526. http://dx.doi.org/10.1002/elan.1140070603
- Gao, Z.Q., Binyamin, G., Kim, H.H., Barton, S.C., Zhang, Y.C. and Heller, A. (2002) Electrodeposition of Redox Polymers and Co-Electrodeposition of Enzymes by Coordinative Crosslinking. Angewandte Chemie International Edition, 41, 810-813. http://dx.doi.org/10.1002/1521-3773(20020301)41:5<810::AID-ANIE810>3.0.CO;2-I
- Ackermann, Y., Guschin, D.A., Eckhard, K., Shleev, S. and Schuhmann, W. (2010) Design of a Bioelectrocatalytic Electrode Interface for Oxygen Reduction in Biofuel Cells Based on a Specifically Adapted Os-Complex Containing Redox Polymer with Entrapped Trametes Hirsuta Laccase. Electrochemistry Communications, 12, 640-643.http://dx.doi.org/10.1016/j.elecom.2010.02.019
- Chiu, J.Y., Yu, C.M., Yen, M.J. and Chen, L.C. (2009) Glucose Sensing Electrodes Based on a Poly(3,4-Ethylenedioxythiophene)/Prussian Blue Bilayer and Multi-Walled Carbon Nanotubes. Biosensors and Bioelectronics, 24, 2015-2020. http://dx.doi.org/10.1016/j.bios.2008.10.010
- Almeida, N.F., Beckman, E.J. and Ataai, M.M. (1993) Immobilization of Glucose Oxidase in Thin Polypyrrole Films: Influence of Polymerization Conditions and Film Thickness on the Activity and Stability of the Immobilized Enzyme. Biotechnology and Bioengineering, 42, 1037-1045. http://dx.doi.org/10.1002/bit.260420904
- Matsumoto, N., Chen, X.H. and Wilson, G.S. (2002) Fundamental Studies of Glucose Oxidase Deposition on a Pt Electrode. Analytical Chemistry, 74, 362-367. http://dx.doi.org/10.1021/ac015536x
- Iwuoha, E.I., Saenzde Villaverde, D., Garcia, N.P., Smyth, M.R. and Pingarron, J.M. (1997) Reactivities of Organic Phase Biosensors. 2. The Amperometric Behaviour of Horseradish Peroxidase Immobilised on a Platinum Electrode Modified with an Electrosynthetic Polyaniline Film. Biosensors and Bioelectronics, 12, 749-761. http://dx.doi.org/10.1016/S0956-5663(97)00042-0
- Vidal, J.C., Garcia, E. and Castillo, J.R. (1999) In Situ Preparation of Overoxidized PPy/oPPD Bilayer Biosensors for the Determination of Glucose and Cholesterol in Serum. Sensors and Actuators B, 57, 219-226. http://dx.doi.org/10.1016/S0925-4005(99)00082-9
- Ammam, M. and Fransaer, J. (2010) Alternating Current Electrophoretic Deposition of Saccharomyces cerevisiae Cells and the Viability of the Deposited Biofilm in Ethanol Production. Electrochimica Acta, 55, 9125-9131.
- Ammam, M. and Fransaer, J. (2011) Effects of AC-Electrolysis on the Enzymatic Activity of Glucose Oxidase. Electroanalysis, 23, 755-763.
- Ammam, M. and Fransaer, J. (2010) Micro-Biofuel Cell Powered by Glucose/O2 Based on Electro-Deposition of Enzyme, Conducting Polymer and Redox Mediators: Preparation, Characterization and Performance in Human Serum. Biosensors and Bioelectronics, 25, 1474-1480. http://dx.doi.org/10.1016/j.bios.2009.11.001
- Schnorr, J.M. and Swager, T.M. (2011) Emerging Applications of Carbon Nanotubes. Chemistry of Materials, 23, 646-657. http://dx.doi.org/10.1021/cm102406h
- Baughman, R.H., Zakhidov, A.A. and de Heer, W.A. (2002) Carbon Nanotubes—The Route toward Applications. Science, 297, 787-792. http://dx.doi.org/10.1126/science.1060928
- Hrapovic, S., Liu, Y., Male, K.B. and Luong, J.H.T. (2004) Electrochemical Biosensing Platform Using Platinum Nanopartyicles and Carbon Nanotubes. Analytical Chemistry, 76, 1083-1088. http://dx.doi.org/10.1021/ac035143t
- Girard-Egrot, A.P., Godoy, S. and Blum, L.J. (2005) Enzyme Association with Lipidic Langmuir-Blodgett Films: Interests and Applications in Nanobioscience. Advances in Colloid and Interface Science, 116, 205-225. http://dx.doi.org/10.1016/j.cis.2005.04.006
- Caseli, L., Moraes, M.L., Zucolotto, V., Ferreira, M., Nobret, T.M., Zaniquelli, M.E.D., Pereira, U. and Oliveira Jr., O.N. (2006) Fabrication of Phytic Acid Sensor Based on Mixed Phytase-Lipid Langmuir-Blodgett Films. Langmuir, 22, 8501-8508. http://dx.doi.org/10.1021/la061799g
- Schmidt, T.F., Caseli, L., Viitala, T. and Oliveira Jr., O.N. (2008) Enhanced Activity of Horseradish Peroxidase in Langmuir-Blodgett Films of Phospholipids. Biochimica et Biophysica Acta, 1778, 2291-2297. http://dx.doi.org/10.1016/j.bbamem.2008.05.012
- Yin, F., Kafi, A.K.M., Shin, H.K. and Kwon, Y.S. (2005) Human Serum Albumin-Octadecylamine Langmuir-Blodgett Film Formed by Spreading Human Serum Albumin Solution Directly on Subphase’s Interface Covered with a Layer of Octadecylamine. Thin Solid Films, 488, 223-229. http://dx.doi.org/10.1016/j.tsf.2005.04.030
- Caseli, L., Nobre, T.M., Zaniquelli, M.E.D., Zucolotto, V. and Oliveira Jr., O.N. (2008) Using Phospholipid Langmuir and Langmuir-Blodgett Films as Matrix for Urease Immobilization. Journal of Colloid and Interface Science, 319, 100-108. http://dx.doi.org/10.1016/j.jcis.2007.12.007
- Apetrei, C., Alessio, P., Constantino, C.J.L., de Saja, J.A., Rodriguez-Mendez, M.L., Pavinatto, F.J., Fernandes, E.G.R., Zucolotto, V. and Oliveira Jr., O.N. (2011) Biomimetic Biosensor Based on Lipidic Layers Containing Tyrosinase and Lutetium Bisphthalocyanine for the Detection of Antioxidants. Biosensors and Bioelectronics, 26, 2513-2519. http://dx.doi.org/10.1016/j.bios.2010.10.047
- Cabaj, J., Chyla, A., Jedrychowska, A., Olech, K. and Soloducho, J. (2012) Detecting Platform for Phenolic Compounds-Characteristic of Enzymatic Electrode. Optical Materials, 34, 1677-1681. http://dx.doi.org/10.1016/j.optmat.2012.02.042
- Sun, Q., Zorin, N.A., Chen, D., Chen, M., Lieu, T.X., Myake, J. and Qian, D.J. (2010) Langmuir-Blodgett Films of Pyridyldithio-Modified Multiwalled Carbon Nanotubes as a Support to Immobilize Hydrogenase. Langmuir, 26, 10259-10265. http://dx.doi.org/10.1021/la100432t
- Decher, G. and Hong, J.D. (1991) Buildup of Ultrathin Multilayer Films by a Self-Assembly Process: II. Consecutive Adsorption of Anionic and Cationic Bipolar Amphiphiles and Polyelectrolytes on Charged Surfaces. Berichte der Bunsengesellschaft für physikalische Chemie, 95, 1430-1434. http://dx.doi.org/10.1002/bbpc.19910951122
- Decher, G. (1997) Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science, 277, 1232-1237. http://dx.doi.org/10.1126/science.277.5330.1232
- Siqueira Jr., J.R., Gasparoto, L.H.S., Crespilho, F.N., Carvalho, A.J.F., Zucolotto, V. and Oliveira Jr., O.N. (2006) Physicochemical Properties and Sensing Ability of Metallophthalocyanines/Chitosan Nanocomposites. Journal of Physical Chemistry B, 110, 22690-22694. http://dx.doi.org/10.1021/jp0649089
- Jeong, R.A., Hwang, J.Y., Joo, S., Chung, T.D., Vark, S.P., Kang, S.K., Lee, W.Y. and Kim, H.C. (2003) In Vivo Calibration of the Subcutaneous Amperometric Glucose Sensors Using a Non-Enzyme Electrode. Biosensors and Bioelectronics, 19, 313-319. http://dx.doi.org/10.1016/S0956-5663(03)00219-7
- Jie, G., Liu, B., Pan, H., Zhu, J.J. and Chen, H.Y. (2007) CdS Nanocrystal-Based Electrochemiluminescence Biosensor for the Detection of Low-Density Lipoprotein by Increasing Sensitivity with Gold Nanoparticle Amplification. Analytical Chemistry, 79, 5574-5581. http://dx.doi.org/10.1021/ac062357c
- Shi, G.Y., Qu, Y.H., Zhai, Y.Y., Liu, Y., Sun, Z.Y., Yang, J.G. and Jin, L.T. (2007) {MSU/PDDA}n LBL Assembled Modified Sensor for Electrochemical Detection of Ultratrace Explosive Nitroaromatic Compounds. Electrochemistry Communications, 9, 1719-1724. http://dx.doi.org/10.1016/j.elecom.2007.03.019
- Hou, Y., Cheng, Y., Hobson, T. and Liu, J. (2010) Design and Synthesis of Hierarchical MnO2 Nanospheres/Carbon Nanotubes/Conducting Polymer Ternary Composite for High Performance Electrochemical Electrodes. Nano Letters, 10, 2727-2733. http://dx.doi.org/10.1021/nl101723g
- Cassier, T., Lowack, K. and Decher, G. (1998) Layer-by-Layer Assembled Protein/Polymer Hybrid Films: Nasnoconstruction via Specific Recognition. Supramolecular Science, 5, 309-315. http://dx.doi.org/10.1016/S0968-5677(98)00024-8
- Ram, K., Bertoncello, M.P., Ding, H., Paddeu, S. and Nicolini, C. (2001) Cholesterol Biosensors Prepared by Layerby-Layer Technique. Biosensors and Bioelectronics, 16, 849-856. http://dx.doi.org/10.1016/S0956-5663(01)00208-1
- Musameh, M., Wang, J., Merkoci, A. and Lin, Y.H. (2002) Low-Potential Stable NADH Detection at Carbon-NanotubeModified Glassy Carbon Electrodes. Electrochemistry Communications, 4, 743-746. http://dx.doi.org/10.1016/S1388-2481(02)00451-4
- Dequaire, M., Degrand, C. and Limoges, B. (2000) An Electrochemical Metalloimmunoassay Based on a Colloidal Gold Label. Analytical Chemistry, 72, 5521-5528. http://dx.doi.org/10.1021/ac000781m
- Sadik, O.A., Aluoch, A.O. and Zhou, A.L. (2009) Status of Biomolecular Recognition Using Electrochemical Techniques. Biosensors and Bioelectronics, 24, 2749-2765. http://dx.doi.org/10.1016/j.bios.2008.10.003
- Zhang, S., Wang, N., Yu, H., Niu, Y. and Sun, C. (2005) Tailoring the Surface Potential of Gold Nanoparticles with Self-Assembled Monolayers with Mixed Functional Groups. Biochemistry, 67, 15-22.
- Yogeswaran, U. and Chen, S.M. (2008) A Review on the Electrochemical Sensors and Biosensors Composed of Nanowires as Sensing Material. Sensors, 8, 290-313. http://dx.doi.org/10.3390/s8010290
- Ajayan, P.M. (1999) Nanotubes from Carbon. Chemical Reviews, 99, 1787-1800. http://dx.doi.org/10.1021/cr970102g
- Lynam, C., Gilmartin, N., Minett, A.I., O’Kennedy, R. and Wallace, G. (2009) Carbon Nanotube-Based Transducers for Immunoassays. Carbon, 47, 2337-2343. http://dx.doi.org/10.1016/j.carbon.2009.04.017
- Zhao, Y.D., Zhang, W.D., Chen, H., Luo, Q.F. and Li, S.F.Y. (2002) Direct Electrochemistry of Horseradish Peroxidase at Carbon Nanotube Powder Microelectrode. Sensors and Actuators B, 87, 168-172. http://dx.doi.org/10.1016/S0925-4005(02)00232-0
- Yamamoto, K., Shi, G., Zhou, T.S., Xu, F., Xu, J.M., Kato, T., Jind, J.Y. and Jin, L.T. (2003) Study of Carbon Nanotubes-HRP Modified Electrode and Its Application for Novel On-Line Biosensors. Analyst, 128, 249-254. http://dx.doi.org/10.1039/b209698f
- Wang, J.X., Li, M.X., Shi, Z.J., Li, N.Q. and Gu, Z.N. (2002) Direct Electrochemistry of Cytochrome c at a Glassy Carbon Electrode Modified with Single-Wall Carbon Nanotubes. Analytical Chemistry, 74, 1993-1997. http://dx.doi.org/10.1021/ac010978u
- Davis, J.J., Coles, R.J., Allen, H. and Hill, O. (1997) Protein Electrochemistry at Carbon Nanotube Electrodes. Journal of Electroanalytical Chemistry, 440, 279-282.
- Guiseppi-Elie, A., Lei, C.H. and Baughman, R.H. (2002) Direct Electron Transfer of Glucose Oxidase on Carbon Nanotubes. Nanotechnology, 13, 559-564. http://dx.doi.org/10.1088/0957-4484/13/5/303
- Dechakiatkrai, C., Chen, J., Lynam, C., Min, S.K., Kim, S.J., Phanichphant, S. and Wallace, G.G. (2008) Direct Ascorbic Acid Detection with Ferritin Immobilized on Single-Walled Carbon Nanotubes. Electrochemical and SolidState Letters, 11, K4-K6. http://dx.doi.org/10.1149/1.2795834
- Chen, R.J., Zhang, Y.G., Wang, D.W. and Dai, H.J. (2001) Noncovalent Sidewall Functionalization of Single-Walled Carbon Nanotubes for Protein Immobilization. Journal of the American Chemical Society, 123, 3838-3839. http://dx.doi.org/10.1021/ja010172b
- Rubianes, M.D. and Rivas, G.A. (2003) Carbon Nanotubes Paste Electrode. Electrochemistry Communications, 5, 689-694. http://dx.doi.org/10.1016/S1388-2481(03)00168-1
- Perez, B., Pumera, M., del Valle, M., Merkoci, A. and Alegret, S. (2005) Glucose Biosensor Based on Carbon Nanotube Epoxy Composites. Journal of Nanoscience and Nanotechnology, 5, 1694-1698. http://dx.doi.org/10.1166/jnn.2005.400
- Pumera, M., Merkoci, A. and Alegret, S. (2006) Carbon Nanotube-Epoxy Composites for Electrochemical Sensing. Sensors and Actuators B, 113, 617-622. http://dx.doi.org/10.1016/j.snb.2005.07.010
- Wang, J. and Musameh, M. (2004) Carbon Nanotube Screen-Printed Electrochemical Sensors. Analyst, 129, 1-2. http://dx.doi.org/10.1039/b313431h
- Sanchez, S., Pumera, M., Cabruja, E. and Fabregas, E. (2007) Carbon Nanotube/Polysulfone Composite Screen-Printed Electrochemical Enzyme Biosensors. Analyst, 132, 142-147. http://dx.doi.org/10.1039/b609137g
- Armada, M.P.G., Losada, J., Cuadrado, I., Alonso, B., Gonzalez, B., Casado, C.M. and Zhang, J.B. (2004) Preparation of Biosensors Based in a Siloxane Homopolymer with Interacting Ferrocenes for the Amperometric Detection of Peroxides. Sensors and Actuators B, 101, 143-149. http://dx.doi.org/10.1016/j.snb.2004.02.043
- Liu, G.D. and Lin, Y.H. (2006) Amperometric Glucose Biosensor Based on Self-Assembling Glucose Oxidase on Carbon Nanotubes. Electrochemistry Communications, 8, 251-256. http://dx.doi.org/10.1016/j.elecom.2005.11.015
- Liu, G.D. and Lin, Y.H. (2006) Carbon Nanotube-Templated Assembly of Protein. Journal of Nanoscience and Nanotechnology, 6, 948-953. http://dx.doi.org/10.1166/jnn.2006.133
- Qu, F.L., Yang, M.H., Jiang, J.H., Shen, G.L. and Yu, R.Q. (2005) Amperometric Biosensor for Choline Based on Layer-by-Layer Assembled Functionalized Carbon Nanotube and Polyaniline Multilayer Film. Analytical Biochemistry, 344, 108-114.http://dx.doi.org/10.1016/j.ab.2005.06.007
- Zhao, H.T. and Ju, H.X. (2006) Multilayer Membranes for Glucose Biosensing via Layer-by-Layer Assembly of Multiwall Carbon Nanotubes and Glucose Oxidase. Analytical Biochemistry, 350, 138-144. http://dx.doi.org/10.1016/j.ab.2005.11.034
- Yu, X., Chattopadhyay, D., Galeska, I., Papadimitrakopoulos, F. and Rusling, J.F. (2003) Peroxidase Activity of Enzymes Bound to the Ends of Single-Wall Carbon Nanotube Forest Electrodes. Electrochemistry Communications, 5, 408-411. http://dx.doi.org/10.1016/S1388-2481(03)00076-6
- Patolsky, F., Weizmann, Y. and Willner, I. (2004) Long-Range Electrical Contacting of Redox Enzymes by SWCNT Connectors. Angewandte Chemie International Edition, 43, 2113-2117. http://dx.doi.org/10.1002/anie.200353275
- Pumera, M., Sanchez, S., Ichinose, I. and Tang, J. (2007) Electrochemical Nanobiosensors. Sensors and Actuators B, 123, 1195-1205. http://dx.doi.org/10.1016/j.snb.2006.11.016
- Cui, Y., Wei, Q.Q., Park, H.K. and Lieber, C.M. (2001) Functional Nanoscale Electronic Devices Assembled Using Silicon Nanowire Building Blocks. Science, 293, 1289-1292.
- Zhou, X.T., Hu, J.Q., Li, C.P., Ma, D.D.D., Lee, C.S. and Lee, S.T. (2003) Silicon Nanowires as Chemical Sensors. Chemical Physics Letters, 369, 220-224. http://dx.doi.org/10.1016/S0009-2614(02)02008-0
- Shao, M.W., Yao, H., Zhang, M.L., Wong, N.B., Shan, Y.Y. and Lee, S.T. (2005) Fabrication and Application of Long Strands of Silicon Nanowires as Sensors for Bovine Serum Albumin Detection. Applied Physics Letters, 87, Article ID: 183106. http://dx.doi.org/10.1063/1.2123393
- Shao, M.W., Shan, Y.Y., Wong, N.B. and Lee, S.T. (2005) Silicon Nanowire Sensors for Bioanalytical Application: Glucose and Hydrogen Peroxide Detectionadv. Advanced Functional Materials, 15, 1478-1482. http://dx.doi.org/10.1002/adfm.200500080
- Janata, J. and Blackburn, G.F. (1984) Immunochemical Potentiometric Sensors. Annals of the New York Academy of Sciences, 428, 286-292. http://dx.doi.org/10.1111/j.1749-6632.1984.tb12304.x
- Janata, J. (1989) Principles of Chemical Sensors. Plenum Press, New York.
- Klabunde, K.J. (2001) Introduction to Nanotechnology. In: Klabunde, K.J., Ed., Nanoscale Materials in Chemistry, John Wiley and Sons, New York.
- Du, J., Yu, X.P. and Di, J.W. (2012) Comparison of the Direct Electrochemistry of Glucose Oxidase Immobilized on the Surface of Au, CdS and ZnS Nanostructures. Biosensors and Bioelectronics, 37, 88-93. http://dx.doi.org/10.1016/j.bios.2012.04.044
- Zheng, B.Z., Xie, S.P., Qian, L., Yuan, H.Y., Xiao, D. and Choi, M.M.F. (2011) Gold Nanoparticles-Coated Eggshell Membrane with Immobilized Glucose Oxidase for Fabrication of Glucose Biosensor. Sensors and Actuators B, 152, 49-55. http://dx.doi.org/10.1016/j.snb.2010.09.051
- Hanefeld, U., Gardossi, L. and Magner, E. (2009) Understanding Enzyme Immobilization. Chemical Society Reviews, 38, 453-468. http://dx.doi.org/10.1039/b711564b
- Brogan, K.L., Wolfe, K.N., Jones, P.A. and Schoenfisch, M.H. (2003) Direct Oriented Immobilization of F(ab’) Antibody Fragments on Gold. Analytica Chimica Acta, 496, 73-80. http://dx.doi.org/10.1016/S0003-2670(03)00991-7
- Rao, S.V., Anderson, K.W. and Bachas, L.G. (1998) Fundamental Review, Oriented Immobilization of Proteins. Microchimica Acta, 128, 127-143. http://dx.doi.org/10.1007/BF01243043
- Liu, Z.M., Liu, J., Shen, G.L. and Yu, R.Q. (2006) A Reagentless Tyrosinase Biosensor Based on 1,6-Hexanedithiol and Nano-Au Self-Assembled Monolayers. Electroanalysis, 18, 1572-1577. http://dx.doi.org/10.1002/elan.200603512
- Nakanishi, K., Sakiyama, T., Kumada, Y., Immamura, K. and Imanaka, H. (2008) Recent Advances in Controlled Immobilization of Proteins onto the Surface of the Solid Substrate and Its Possible Application to Proteomics. Current Proteomics, 5, 161-175. http://dx.doi.org/10.2174/157016408785909622
- Snabe, T., Rode, G.A., Neves-Petersen, M.T., Buus, S. and Petersen, S.B. (2006) Oriented Coupling of Major Histocompatibility Complex (MHC) to Sensor Surfaces Using Light Assisted Immobilization Technology. Biosensors and Bioelectronics, 21, 1553-1559. http://dx.doi.org/10.1016/j.bios.2005.06.010
- Madoz-Gúrpide, J., Abad, J.M., Fernández-Recio, J., Vélez, M., Vázquez, L., Gómez-Moreno, C. and Fernández, V.M. (2000) Modulation of Electroenzymatic NADPH Oxidation through Oriented Immobilization of Ferredoxin: NADP+ Reductase onto Modified Gold Electrodes. Journal of the American Chemical Society, 122, 9808-9817. http://dx.doi.org/10.1021/ja001365m
- Ha, T.H., Jeong, J.Y. and Chung, B.H. (2005) Immobilization of Hexa-Arginine Tagged Esterase onto Carboxylated Gold Nanoparticles. Chemical Communications, 48, 3959-3961. http://dx.doi.org/10.1039/b504184h
- Brown, K.R., Fox, A.P. and Natan, M.J. (1996) Morphology-Dependent Electrochemistry of Cytochrome c at Au Colloid-Modified SnO2 Electrodes. Journal of the American Chemical Society, 118, 1154-1157. http://dx.doi.org/10.1021/ja952951w
- Lin, J., Qu, W. and Zhang, S. (2007) Disposable Biosensor Based on Enzyme Immobilized on Au-Chitosan-Modified Indium Tin Oxide Electrode with Flow Injection Amperometric Analysis. Analytical Biochemistry, 360, 288-293. http://dx.doi.org/10.1016/j.ab.2006.10.030
- Tangkuaram, T., Ponchio, C., Kangkasomboon, T., Katikawong, P. and Veerasai, W. (2007) Design and Development of a Highly Stable Hydrogen Peroxide Biosensor on Screen Printed Carbon Electrode Based on Horseradish Peroxidase Bound with Gold Nanoparticles in the Matrix of Chitosan. Biosensors and Bioelectronics, 22, 2071-2078. http://dx.doi.org/10.1016/j.bios.2006.09.011
- Shumyantseva, V.V., Carrara, S., Bavastrello, V., Jason Riley, D., Bulko, T.V., Skryabin, K.G., Archakov, A.I. and Nicolini, C. (2005) Direct Electron Transfer between Cytochrome P450scc and Gold Nanoparticles on Screen-Printed Rhodium-Graphite Electrodes. Biosensors and Bioelectronics, 21, 217-222. http://dx.doi.org/10.1016/j.bios.2004.10.008
- Abad, J.M., Velez, M., Santamaria, C., Guisan, J.M., Matheus, P.R., Vazquez, L., Gazaryan, I., Gorton, L., Gibson, T. and Fernandez, V.M. (2002) Immobilization of Peroxidase Glycoprotein on Gold Electrodes Modified with Mixed Epoxy-Boronic Acid Monolayers. Journal of the American Chemical Society, 124, 12845-12853. http://dx.doi.org/10.1021/ja026658p
- Challa, S.S.R.K. (2010) Nanocomposites: Nanomaterials for the Life Sciences. Wiley-VCH, Weinheim.
- Yang, W.W., Wang, J.X., Zhao, S., Sun, Y.Y. and Sun, C.Q. (2006) Multilayered Construction of Glucose Oxidase and Gold Nanoparticles on Au Electrodes Based on Layer-by-Layer Covalent Attachment. Electrochemistry Communications, 8, 665-672. http://dx.doi.org/10.1016/j.elecom.2005.11.014
- Xiao, Y., Patolsky, F., Katz, E., Hainfeld, J.F. and Willner, I. (2003) “Plugging into Enzymes”: Nanowiring of Redox Enzymes by a Gold Nanoparticle. Science, 299, 1877-1881. http://dx.doi.org/10.1126/science.1080664
- Gao, F.X., Yuan, R., Chai, Y.Q., Chen, S.H., Cao, S.R. and Tang, M.Y. (2007) Amperometric Hydrogen Peroxide Biosensor Based on the Immobilization of HRP on Nano-Au/Thi/Poly (p-Aminobenzene Sulfonic Acid)-Modified Glassy Carbon Electrode. Journal of Biochemical and Biophysical Methods, 70, 407-413. http://dx.doi.org/10.1016/j.jbbm.2006.09.007
- Yang, G., Yuan, R. and Chai, Y.Q. (2008) A High-Sensitive Amperometric Hydrogen Peroxide Biosensor Based on the Immobilization of Hemoglobin on Gold Colloid/L-Cysteine/Gold Colloid/Nanoparticles Pt-Chitosan Composite Film-Modified Platinum Disk Electrode. Colloids and Surfaces B, 61, 93-100. http://dx.doi.org/10.1016/j.colsurfb.2007.07.014
- Tang, L., Zeng, G.M., Shen, G.L., Li, Y.P., Zhang, Y. and Huang, D.L. (2008) Rapid Detection of Picloram in Agricultural Field Samples Using a Disposable Immunomembrane-Based Electrochemical Sensor. Environmental Science & Technology, 42, 1207-1212. http://dx.doi.org/10.1021/es7024593
- Turner, M., Golovko, V.B., Vaughan, O.P., Abdulkin, P., Berenguer-Murcia, A., Tikhov, M.S., Johnson, B.F. and Lambert, R.M. (2008) Selective Oxidation with Dioxygen by Gold Nanoparticle Catalysts Derived from 55-Atom Clusters. Nature, 454, 981-983.http://dx.doi.org/10.1038/nature07194
NOTES

*Corresponding author.

