Advances in Bioscience and Biotechnology
Vol. 3 No. 7A (2012) , Article ID: 25127 , 8 pages DOI:10.4236/abb.2012.327122
Radio-protective response on the environmental pollutant induced oxidative stress
![]()
1Drug Radiation Research Department, National Center for Radiation Research and Technology, Atomic Energy Authority, Cairo, Egypt
2Biochemistry Department, Faculty of Science, Ain Shams University, Cairo, Egypt
Email: *drolagharib@yahoo.com
Received 23 August 2012; revised 28 September 2012; accepted 11 October 2012
Keywords: Radio Protective Response; Low Dose Radiation; TCE; Oxidative Stress
ABSTRACT
The purpose of this study was to investigate the protective effect of low dose radiation on TCE induced oxidative damage in rats. The oxidative damage of both liver and kidney was assessed by serum alkaline phosphatase (ALP), Gamma Glutamyl Trans-Peptidase (GGTP), Alanine and Aspartate Amino Transferase (ALT & AST) activities in addition concentrations of cholesterol, high density lipoprotein-cholesterol (HDL-c), low density lipoprotein-cholesterol (LDL-c), Triacyglycerols (TGs), urea and creatinine were analyzed. Liver lipid peroxidation (MDA), Nitric Oxide (NO), reduced glutathione (GSH) levels, and activities of both Super-Oxide Dismutase (SOD) and Glutathione Peroxidase (GSH-Px) were measured. Results: TCE administration increase serum ALP, GGTP, ALT, AST activities, cholesterol, triacylglycerols, LDL-c, urea and creatinine concentrations, besides liver MDA and NO, whereas it decreased SOD, GSH-Px activities, GSH level in liver, HDL-c in serum. Low dose of gamma rays (0.5 Gy) exposure significantly improved lipid peroxidation and oxidative injury induced by TCE. Conclusion: The study indicates that treatment with low dose of gamma rays ameliorate harmful effects induced by TCE taking in consideration the effect of gamma radiation as a stimulant of radical detoxification.
1. INTRODUCTION
The concept of radiation hormesis is usually applied to physiological benefits from low radiation dose ranging from 1 - 50 cGy. It is widely believed that radiation biology in the future will be focused on bimolecular and genetic implications, problems such as radiation hormesis and radio-protective response [1]. In the low-dose region, radiation exerts protection against other challenges involving radicals and thus causes a net beneficial effect by temporarily shielding the hit cell against radicals produced through endogenous processes. Low dose of radiation has been observed to stimulate the radical detoxification system, enhancement of DNA repair rates and induce immune competence that associate with an increase in number of cytotoxic lymphocytes, even causing a reduction of the incidence of metastatic cancer [2] (Figure 1).
It can be concluded that low dose preirradiation possibly cancel the induction of thymic lymphoma by 2 Gy challenge dose [3]. Moreover, Azzam, suggested that a single low dose at background or occupational exposure level may reduce cancer risk [4], while Yonezawa and his colleagues indicated that pre-irradiated mice with 50 cGy of X rays increased the survival rats about 70% after subsequent lethal high dose irradiation [5]. According to Manno, Yamoaka, Kojima and their colleagues and Feinendegen, pre-irradiated animals with low dose may enhance the activities of SOD, GSH-Px [2,6-8], while Nomura and Yomaoka, suggested that low dose radiation reduced oxidative damage induced by CCl4 in mouse liver, this by increasing the endogenous antioxidant in animal tissues [9]. Thus suppression in oxidative injury by endogenous and exogenous antioxidant has proven effective. Kojima and his colleagues found that liver GSH level in male mice increased 2 hr after irradiation with 60 cGy of gamma rays, reduced a maximum at around 4 hr and returned almost to the control level by 12 hr [10].
Trichloroethylene (TCE), which is an organic unsaturated solvent used as solvent to remove grease from metal parts, it is an ingredient in adhesive, paint and spot removers [11]. Also TCE is known to be a rodent carcinogen and neuro-toxicant [12]. TCE undergoes me-

Figure 1. Low-dose induced adaptive protection scheme of duration of protection.
tabolism by two ways cytochrome P450 dependent oxidation and conjugation with glutathione (GSH). The initial step in GSH conjugate pathway occurs primarily in liver with subsequent metabolism by GGTP. Metabolites are directed to kidney [13] (Figure 2). According to Bull, Three metabolites of TCE; chloral hydrate (CH), dichloroacetate (DCA), and trichloroacetate (TCA) appear capable of inducing liver tumors in mice, but DCA is active in rats as well [14]. Moreover, TCE also increased lipid peroxidation [15]. On the other hand, Chen and his colleagues stated that in human lung cancer cells, GSH plays a vital role in protection of TCE and PERC induced oxidative stress and apoptosis, which may be mediated through a P53 dependent pathway [16]. In study, we tested whether exposure to low dose radiation (0.5 Gy) after administration of TCE ameliorate oxidative damage induced by environmental pollutant.
2. MATERIALS AND METHODS
2.1. Chemicals
All chemicals were obtained from Sigma Chemical (USA). Kits used in this experiments were purchased from Bio-Diagnostics (UK).
2.2. Radiation Process
A single dose whole body irradiation (0.5 Gy) was performed with rats, using gamma rays by Cesium 137 irradiation unit, National Center for Radiation Research and Technology (NCRRT), with the dose rate 0.912 rad/sec. The radiation process of animals has been carried out at the central position of the sample chamber using a special designed polyethylene plates with a polyethylene cover. This place is actually calibrated using alanine dosimeter relative to a primary standard.
2.3. Animals and Treatment
Male Wistar rats, each weighing 150 - 180 gm, were pur-
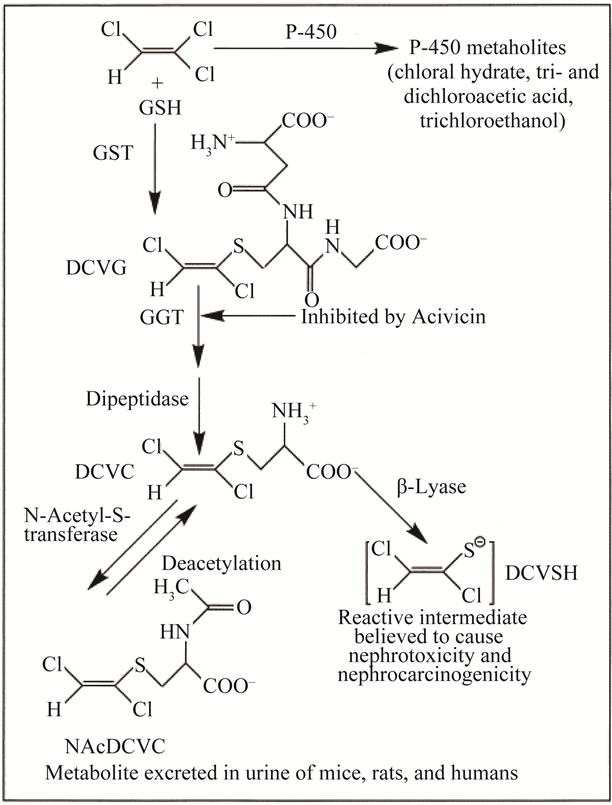
Figure 2. Scheme for metabolism of TCE by GST pathway. Β-lyase: cysteine conjugate Β-lyase, DCVG: S-(1,2-dichlorovinyl) glutathione. DCVC: S-(1, 2-dichlorovinyl)-L-cysteine, NAcDCVC: N-acetyl-S-(1,2-dichlorovinyl)-l-cysteine.
chased from the animal breeding unit of the National research centre, Giza, Egypt. Rats were housed under appropriate conditions of controlled humidity, temperature and light. The animals were allowed free access to water and fed a standard pellet rat diet. The rats were acclimatized in the animal facility of the National Centre for Radiation Research and Technology (NCRRT)— Atomic Energy Authority, Cairo, Egypt for at least one week before subjecting them to experimentation. The study was conducted accordance with the guidelines set by the European Economic Community (EEC) regulations (Revised Directive 86/609/EEC) and approved by the Ethical Committee at NCRRT. Rats were segregated into groups of six animals each. Each group was subjected to one of the following treatments described in the next sections.
Control animals.
Irradiated group: The animals in IRR group were irradiated with a shot dose of 0.5 Gy.
Trichloroethylene administered group: Animals in TCE group were administered interperitoneally with a single dose of TCE (500 mg/Kg b.wt) [17].
Curative group: Animals in TCE + IRR group were administered interperitoneally with a single dose of TCE (500 mg/Kg b.wt) after 30 min. the animals were exposed to 0.5 Gy γ-ray as a single shot dose. Animal groups were sacrificed 1 & 3 days post TCE administration and/or radiation exposure, blood and liver samples were collected for biochemical analysis.
2.4. Biochemical Analysis
The blood samples were collected directly from the animals by heart puncturing. The blood samples were centrifuged using universal 16R/ Germany centrifuge at 3000 rpm for 15 min. and the clear serum were collected and stored in a refrigerator. The activities of ALP, GGTP, ALT and AST as well as concentrations of cholesterol, HDL-c, LDL-c, Triacylglycerols, urea and creatinine in serum were analyzed. Liver was excised from the rats and the homogenization was carried out using a homogenizer (universal laboratory AID type MPW-309, Poland). One portion used to analyze MDA, NO and GSH levels as well as SOD and GSH-Px activities. ALP activity was assayed according to Ellis and his colleagues [18]. The quantitative determination of GGTP activity was done using kit according the method of Szasz et al. [19]. ALT and AST activities were determined by kits according to the method of Reitman and Frankel [20]. LDL-c concentration was determined according to the method of Castelli [21], while HDL-c level was estimated according to Lopes et al. [22]. Cholesterol concentration was determined according to the method of Meiattini et al. [23]. Triacylglycerols concentration was determined according to the method of Bucolo and David [24]. Urea content present in the serum sample was determined according to the method of Patton and Crouch [25]. Creatinine content was determined according to the method of Folin [26]. MDA was assayed by the method of Yoshioka, et al. [27]. NO concentration was measured by using Moshage, et al. [28]. Reduced GSH was determined using the method of Ellman [29]. Superoxide dismutase activity was estimated by the method of Marklund and Marklund [30]. The activity of GSH-Px was measured according to the method of Gross et al. [31]. All biochemical assays were performed with a Heλios UV/VIS spectrophotometer (Thermo Spectronic, UK).
2.5. Atomic Absorption Analysis
Liver tissues were digested with a mixture of conc. HNO3 and H2O2 (5:1). Digestion was done by using micro-wave sample preparation Labstation, MLS-1200 MEGA, Italy. Then digested samples were diluted with deionized water to a fixed volume [32]. Selected elements (Fe, Cu, Zn, and Mn) were estimated quantitatively by atomic absorption solar system Unicam 939, UK (Hallow cathode lamps in air acetylene flame) [33]. Concentration of elements in tissues was calculated by using calibration curve prepared from their stock solution (1 mg). The concentration of elements per grams wet tissues could be determined by the following equation [34]:
Conc. (μg/g) = [Conc.(μg/ml)/sample weight] × Dil. Factor.
Con. (μg/g): Concentration of the element per grams wet tissues.
Conc. (μg/ml): Concentration of the sample.
Dil. Factor: Dilution factor of the sample.
2.6. Statistical Analysis
To assess the significant level of influence caused by low dose radiation in TCE administered rats, one way analysis of variances (ANOVA) followed by Tukey’s multiple comparison test was used. Data represents means values and standard deviation of at least three independent experiments. Statistical analysis was performed by using Graph-Pad software, San Diago, CA, USA) Differences were considered statistically significant when the P value was less than 0.05.
3. RESULTS
This study was carried to evaluate the liver and kidney function tests, lipid profile in serum and some enzymatic and non-enzymatic antioxidant such as SOD, GSH-Px activities as well as reduced GSH, MDA and NO concentration in liver homogenate, besides certain trace elements (Fe, Cu, Zn and Mn) in liver after radiation treatment to improve the toxic effects of trichloroethylene in male albino rats.
3.1. Liver Enzymes (Table 1)
TCE treated group showed a highly significant increase (p ≤ 0.001) in all tested serum liver enzymes (ALP, GGTP, AST and ALT) comparing to control group after 1 and 3 days of treatment. Results of TCE and IRR group showed a significant increase (p ≤ 0.05) in serum GGTP activity on the 1st exposure day by 16.63% with respect to the control level (Table 1), accompanied with a highly significant increase in serum ALT activity throughout the test period, the percentage change of this increase recording 11.82% and 14.15% on the 1st and 3rd post exposure days respectively (Table 1) as compared to the control group. In addition, all serum liver enzymes showed a highly significant decreased with respect to the TCE treated group (Table 1).
3.2. Lipid Profile
Results of TCE treated group showed a highly significant elevation (P ≤ 0.001) in concentrations of cholesterol, TGs, and LDL-c (Table 2) while HDL-c showed a highly significant decline after 1 and 3 days of TCE administra-
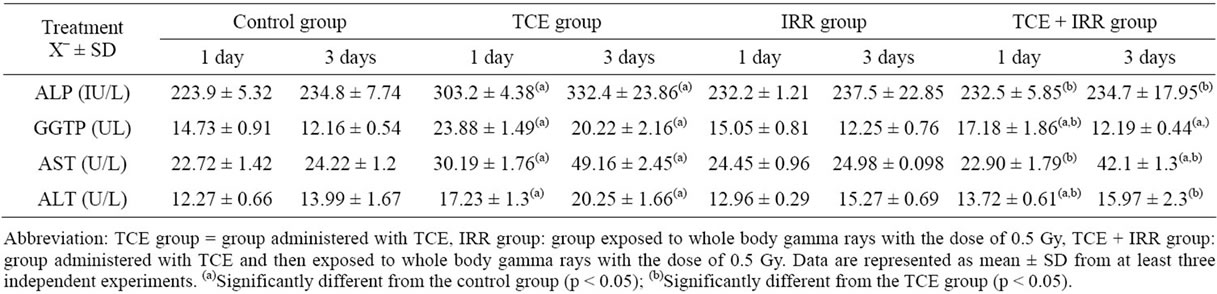
Table 1. Effect of low dose of radiation treatment on serum ALP (IU/L), GGTP, AST and ALT (UL) activities of rats received trichloroethylene (TCE).
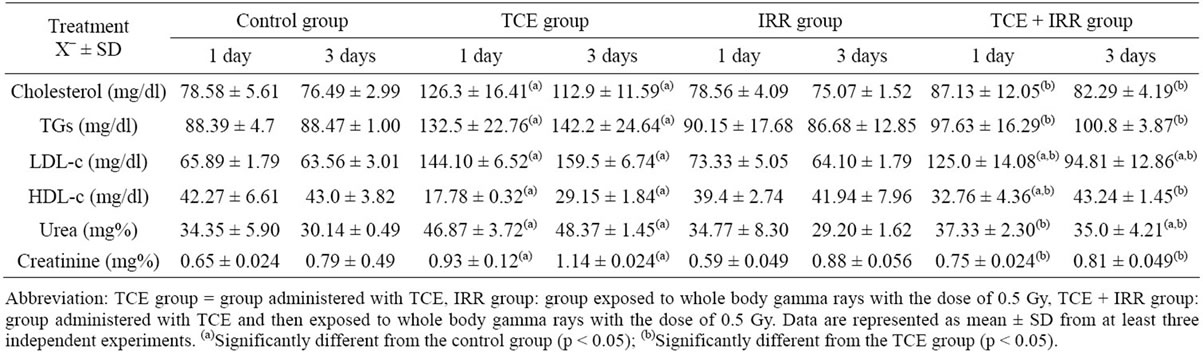
Table 2. Effect of low dose of radiation treatment on serum cholesterol, TGs, LDL-c, HDL-c (mg/dl), urea (mg%) and creatinine (mg%) concentrations of rats received trichloroethylene (TCE).
tion, the percentage change of this decline recorded 58% and 32% respectively as compared to the normal control levels (Table 2). In response to the group of animals exposed to 0.5 Gy γ-rays, levels of TGs, cholesterol, HDLc and LDL-c showed non-significant change throughout the experimentation period as compared to the normal control group.
TCE irradiation group results in a highly significant decrease (p ≤ 0.001, –22.49%) in HDL-c after 1 day of radiation exposure; accompanied with a highly significant increase (p ≤ 0.001) in LDL-c after 1 and 3 days of treatment, recorded 90% and 49% respectively comparing to control group (Table 2).
3.3. Kidney Function
Table 2 illustrated a highly significant increase in urea and creatinine levels in TCE administered group after 1st and 3rd days of TCE administration compared with control group. In response to radiation exposure no significant difference in urea and creatinine levels was notice throughout the experimentation time comparing to control group. On the other hand, in group of animals treated with low dose of γ-ray (0.5 Gy) after TCE administration, the elevation in both urea and creatinine on the 1st days and 3rd days were ameliorated till became insignificant as compared with the control group except in urea level after 3 days, while at the same corresponding time, urea level showed a significant increase (p ≤ 0.05, 16%) with respect to normal control level. Urea and creatinine reflected a highly significant decrease (p ≤ 0.001) throughout the experimentation period with respect to TCE administered group (Table 2).
3.4. Liver Homogenates
Data given in Table 3 showed that administration of TCE solely revealed reduction (P ≤ 0.001) in reduced GSH as well as activities of antioxidant scavenger enzymes GSH-Px and SOD. This reduction was accompanied with significant elevation in concentration of liver MDA, which reached 52% and 32% on the 1st and 3rd days of TCE administration, corresponding to 20% and 24% in NO concentration respectively. However, exposing animals to 0.5 Gy gamma rays resulted in non-significant changes comparing to control group after 1 and 3 days of radiation exposure (Table 3).
As shown in Table 3; treatment with 0.5 Gy gamma rays after TCE administration markedly ameliorate the changes in all parameters at all time intervals as compared to normal control levels except MDA (3 days) and NO (1 day) showed a highly significant increase. Comparing these results with those of TCE administered group, revealed a highly significant increase in GSH-Px

Table 3. Effect of low dose of radiation treatment on liver SOD (U/g tissue) and GSH-Px (mg/min./g tissue) activities as well as reduced GSH (mg/g tissue), MDA (μmol/g tissue) and NO (μg/g tissue) of rats received trichloroethylene (TCE).
activity and reduced GSH after 1 and 3 days of radiation treatment and the percentage change of this increase recorded (58% and 35.7%) and (34% and 59.6%) respectively with respect to TCE administered group (Table 3). However, the same group of animals showed non-significant change in SOD and NO levels on the 1st day that accompanied with significant increase on the 3rd day (Table 3). On the other hand MDA concentration showed nonsignificant decrease on the 1st day that became significant on the 3rd day of TCE and radiation exposure group (p ≤ 0.05) (Table 3).
3.5. Trace Element
The data presented in Figures 3 and 4 illustrated that TCE injection progressively elevated the level in Fe and Cu concentration reached its maximum on the 3rd post exposure day (p ≤ 0.001, 45% and p ≤ 0.01, 30% respectively). Meanwhile, an initial decrease was shown in Zn and Mn levels recorded a highly significant decrease (p ≤ 0.001, –28%) in case of Mn and became significantly increase for both elements (Zn = 29.5% and Mn = 22%) after 3 days of TCE treatment compared with control level (Figures 3 and 4).
As shown in Figures 3 and 4, exposure to 0.5 Gy γ- rays after TCE administration significantly ameliorated the levels of Zn and Mn during the experimentation period. Comparing Cu and Fe concentration to those of the normal control group a highly significant increase was recorded p ≤ 0.001, 48% and p ≤ 0.05, 23% respectively on the 3rd post exposure day (Figures 3 and 4).
4. DISCUSSION
Liver injury initiated by hepato-toxicant such as TCE progress even after the offending has been estimated from the body [35]. Following exposure to a toxic dose of TCE, injury was initiated via free radicals generation. The present study revealed that the injection of TCE (500 mg/kg b.w) resulted in elevation in levels of ALT, AST, GGTP, and ALP on the 1st and 3rd days post TCE injection. According to Anand and his colleagues, Pratt and Kaplan and Lawrence and his colleagues, this elevation may be due to the generation of free radical induced damage in liver function [36-38]. Moffalt and Denizeau, found that higher dose of Zn accompanied with higher serum activities of transaminease and alkaline phosphatase, which is in agreement with the present study [39]. Moreover, the defect in LDL receptor or oxidized cholesterol toxicity caused by free radical exposure [40] could be affected the level of lipid profile parameters that recorded in the present study. High triacylglycerols level in the blood may be a result of oxidative stress that occurred mainly due to TCE administration. The major metabolic consequence of oxidative stress in insulin resistance may be strongly associated with the deposition of triacylglycerols in the liver [41]. The increase in nitric oxide concentration after one and three days of TCE administration due to rapid reaction of NO with superoxide anion to form peroxinitrite, that may be cytotoxic by itself or easily decompose to the highly reactive, toxic hydroxyl radical and nitrogen dioxide [42]. In addition, the present study confirms the findings of Goel., Khan and their colleagues and Gharib that TCE significantly increased urea and creatinine in rats [15,43,44]. This increase could be attributed to the depletion of GSH that enhance utilization of protein thereby increasing the urea level that is accompanied by an increase in creatinine level suggested by Mostafa [45]. On the other hand, Fletcher and his colleagues concluded that the deposition of iron in liver cells may lead to increase in lipid concentration associated with a decrease in both SOD activity and reduced GSH, which is in agreement with the present study [46], these results also could be attributed to the decreasing of the antioxidant defence system and
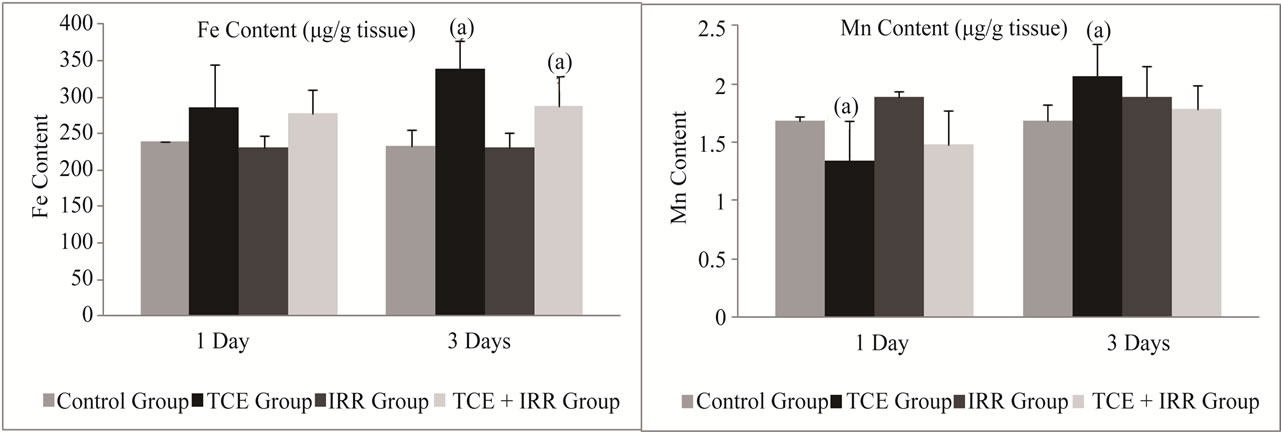
Figure 3. Effect of low dose of gamma radiation treatment on liver Fe and Mn content (μg/g tissue) in rats received trichloroethylene (TCE). Abbreviation: TCE group = group administered with TCE, IRR group: group exposed to whole body gamma rays with the dose of 0.5 Gy, TCE + IRR group: group administered with TCE and then exposed to whole body gamma rays with the dose of 0.5 Gy. Data are represented as mean ± SD from at least three independent experiments. (a)Significantly different from the control group (p < 0.05); (b)Significantly different from the TCE group (p < 0.05).
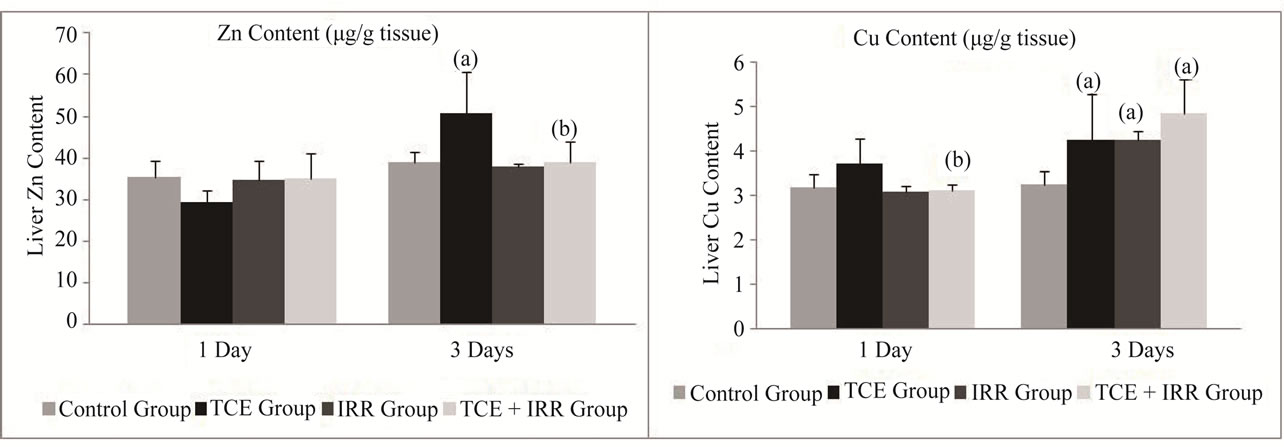
Figure 4. Effect of low dose of gamma radiation treatment on liver Zn and Cu contents (μg/g tissue) in rats received trichloroethylene (TCE). Abbreviation: TCE group = group administered with TCE, IRR group: group exposed to whole body gamma rays with the dose of 0.5 Gy, TCE + IRR group: group administered with TCE and then exposed to whole body gamma rays with the dose of 0.5 Gy. Data are represented as mean ± SD from at least three independent experiments. (a)Significantly different from the control group (p < 0.05); (b)Significantly different from the TCE group (p < 0.05).
elevation of free radical [9,16,47,48]. Moreover, the decrease in liver GSH induced by TCE administration is accompanied by a remarkable increase of GGTP activity could be explained through the fact that GGTP acts in transferring the γ-glutamyl group of GSH to amino acids through γ-glutamyl cycle [49]. The present study showed a highly significant increase of Cu and Zn 3 days post TCE injection, while Mn showed a highly significant decrease on the first day accompanied by an increase on the 3rd day post TCE treatment. These results can be explained by Dashti and his colleagues who mentioned that the increase in Zn and Cu content could be due to the severity of cellular damage observed microscopically in the liver [50]. Moreover, Keen and his colleagues stated that Mn is eliminated from the body mainly in the bile. Thus, the impaired liver function may lead to decrease Mn secretion [51]. Low dose gamma radiation improved the toxicity of TCE in the present study. According to Kojima and his colleagues, the elevation in GSH content in liver by low dose gamma rays might be the complex response of the cells to protect themselves against the additional oxidative injury [8]. Also, Pathak and his colleagues mentioned that exposure to low dose of gamma radiation stimulate the enzymatic SOD, GR and GSH-Px and non enzymatic defense GSH which enhancement the endogenous antioxidant machinery and protecting the organs from the damage caused by oxidative stress [52].
Based on the results of the present study the effect of low dose of radiation improves the toxicity induced by TCE administration since it acts as antioxidant through the induction of antioxidant enzymes of the liver and kidney.
REFERENCES
- Macklis, R.M. and Bresford, B. (1991) Radiation hormesis. Journal of Nuclear Medicine, 32, 350-359.
- Feinendegen, L.F. (2005) Evidence for beneficial low level radiation effects and radiation hormesis. British Journal of Radiology, 78, 3-7. doi:10.1259/bjr/63353075
- Bhattacharjee, D. (1996) Role of radio-adaptation on radiation-induced thymic lymphoma in mice. Mutation Research, 358, 231-235. doi:10.1016/S0027-5107(96)00125-X
- De Azzam, E.I., Toledo, S.M., Raaphorst, G.P. and Mitchel, R.E.J. (1996) Low dose ionizing radiation decreases the frequency of neoplastic transformation to a level below the spontaneous rate in C3H 10T 1/2 cells. Radiation Research, 146, 369-373. doi:10.2307/3579298
- Yonezawa, M., Misonoh, J. and Hosokawa, Y. (1996) Two types of X-ray induced radio-resistance in mice, presence of 4 dose ranges with distinct biological effects. Mutation Research, 358, 237-243. doi:10.1016/S0027-5107(96)00126-1
- Manno, M., Bertazzon, A., Burlina, L. and Galzigna, L. (1985) Interaction of low doses of ionizing radiation and carbon tetrachloride on liver superoxide dismutase and glutathione peroxidase in mice. Enzyme, 34, 107-112.
- Yamaoka, K., Kojima, S., Takahashi, M., Nomura, T. and Iriyama, K. (1998) Change of glutathione peroxidase synthesis along with that of super oxide dismutase synthesis in mice spleens after low-dose X-ray irradiation. Biochimica et Biophysica Acta, 1381, 265-270. doi:10.1016/S0304-4165(98)00021-X
- Kojima, S., Matuski, O. and Nourma, T. (1997) Effect of small doses of gamma ray on glutathione synthesis in mouse. International Conference on low doses of ionizing radiation Biology Effect and regulatory Control, Sevillr, 17-21.
- Nomura, T. and Yamaoka, K. (1999) Low-dose γ-ray irradiation reduces oxidative damage induced by CCl4 in mouse liver. Free Radical Biology & Medicine, 27, 1324- 1333.
- Kojima, S., Matsumori, S., Ishida, H. and Yamaoka, K. (2000) Possible role of elevation of glutathione in the acquisition of enhanced proliferation of mouse splenocytes exposed to small dose gamma-rays. International Journal of Radiation Biology, 76, 1641-1647. doi:10.1080/09553000050201136
- Agency for Toxic Substance and Disease Registry (ATSDR) (2003) Managing hazardous materials incidents Vol. III. Medical management guidelines for acute chemical exposures: Trichloroethylene (TCE). US Department of Health and Human Services, Public Health Service, Atlanta.
- Boyes, W.K., Bushnell, P.J., Crofton, K.M., Evans, M. and Simmons, J.E. (2000) Neurotoxic and pharmacokinetic response to trichloroethylene as a function of exposure scenario. Environmental Health Perspectives, 108, 317-322. doi:10.1289/ehp.00108s2317
- Lash, L.H. and Parker, J.C. (2001) Hepatic and renal toxicities associated with trichloroethylene. Pharmacological Reviews, 53, 177-208.
- Bull, R.J. (2000) Mode of action of liver tumor induction by trichloroethylene and its metabolites, trichloroacetate and dichloroacetate. Environmental Health Perspectives, 108, 241-259. doi:10.1289/ehp.00108s2241
- Gharib, O.A. (2009) Effects of Kombucha on oxidative stress induced nephrotoxicity in rats. Chinese Medicine, 4, 23. doi:10.1186/1749-8546-4-23
- Chen, S.J., Wang, J.L., Chen, J.H. and Huang, R.N. (2002) Possible involvement of glutathione and P53 in trichloroethylene and percholoroethylene. Induced lipid peroxidetion and apoptosis in human lung cancer cells. Free Radical Biology & Medicine, 33, 464-472.
- Torasson, M., Clark, J., Dankovic, D., Mathias, P., Skaggs, S., Walker, C. and Werren, D. (1999) Oxidative stress and DNA damage in Fischer rats following acute exposure to trichloroethylene or perchloroethylene. Toxicology, 138, 43. doi:10.1016/S0300-483X(99)00083-9
- Ellis, G., Belfield, A. and Goldberg, D.M. (1971) Colorimetric determination of acid phosphatase activity using adenosine 3’-monophosphate as substrate. Journal of Clinical Pathology, 24, 493-500. doi:10.1136/jcp.24.6.493
- Szasz, G., Weimann, G., Stahler, O., Wahefedl, A.W. and Persijn, J.P. (1974) New substances for measuring gamma-glutamyl transpeptidase activity. Journal of Clinical Chemistry & Clinical Biochemistry, 12, 228-232.
- Retiman, S. and Frankel, S. (1957) A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. American Journal of Clinical Pathology, 28, 56-63.
- Castelli, W.P. (1986) Triglyceride tissue: A view from Framingham. American Heart Journal, 112, 432-437. doi:10.1016/0002-8703(86)90296-6
- Lopes, V.M.F, Stone, P., Ellis, S. and Colwell, J.A. (1977) Cholesterol determination in high density lipoproteins separated by three different methods. Clinical Chemistry, 23, 882-884.
- Meiattini, F., Prencipe, L., Bardelli, F., Giannini, G. and Tarli, P. (1978) The 4-hydroxybenzoate/4-amino phenazone chromogentic system used in the enzymic determination of serum cholesterol. Clinical Chemistry, 24, 2161- 2165.
- Bucolo, G. and David, H. (1973) Quantitative determination of serum triglyceride by the use of enzymes. Clinical Chemistry, 19, 476-482.
- Patton, C.J. and Crouch, S.R. (1977) Spectrophotometric and kinetics investigation of the Berthelot reaction for the determination of ammonia. Analytical Chemistry, 49, 464-469. doi:10.1021/ac50011a034
- Folin, O.Z. (1943) Micro Method for the determination of non-protein nitrogen, in laboratory manual of biological chemistry with supplement. 5th Edition, Appleton-Century Co. Inc., New York, 271.
- Yoshioka, T., Kawada, K., Shimada, T. and Movi, M. (1979) Lipid peroxidation in maternal and cord blood and protective mechanism against activated oxygen toxicity in the blood. American Journal of Obstetrics & Gynecology, 135, 372-376.
- Moshage, H., Kok, B., Huizenga, J.R. and Jansen, P.L.M. (1995) Nitrie and nitrate determination in plasma a critical evaluation. Clinical Chemistry, 41, 892-896.
- Ellman, G.C. (1959) Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics, 82, 70-77. doi:10.1016/0003-9861(59)90090-6
- Marklund, S. and Marklund, G. (1974) Involvement of the super oxide anion radical in the antioxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry, 47, 469-474. doi:10.1111/j.1432-1033.1974.tb03714.x
- Gross, R.T., Bracci, R., Rudolph, N., Schroeder, E. and Kochen, J.A. (1967) Hydrogen peroxide toxicity and detoxification in the erythrocytes of newborn infants. Blood, 29, 481-493.
- IAEA (1980) Elemental analysis of biological materials. Current problem and techniques with special reference to trace elements. International Atomic Energy Agency, IAEA, Vienna. Technical Reports Series, 379.
- Kingstone, H.M. and Jassie, L. (1988) Introduction to microwave sample preparation. Theory and practice. American Chemical Socity Professional Reference Book, Washington DC, 263.
- Gregus, Z. and Klaassen, C.D. (1986) Disposition of metals in rats: A comparative study of fecal urinary and billary excretion and tissue distribution of eighteen metals. Toxicology and Applied Pharmacology, 85, 24-38. doi:10.1016/0041-008X(86)90384-4
- Ramaiah, S.K., Apte, U.M. and Mehenadale, H.M. (2001) Cytochrome P450 induction increases thioacetamide liver injury in dietrestricted rats. Drug Metabolism and Disposition, 29, 1088-1095.
- Anand, S.S., Mumtaz, M.M. and Mehendale, H.M. (2005) Dose dependent liver regeneration in chloroform trichloroethylene and allyl alcohol ternary mixture hepatotoxicity in rats. Archives of Toxicology, 79, 671-682. doi:10.1007/s00204-005-0675-3
- Pratt, D.S. and Kaplan, M.M. (2002) Evaluation of liver function. In: Braunwald, E., et al., Eds., Harrison’s Principles of Internal Medicine, McGraw-Hill, New York, 1711-1715.
- Lawerence, B.P., Will, Y., Reed, D.J. and Kerkvliet, N.I. (2000) Gamma-glutamyl transpeptidase knockout mice as a model for understanding the consequence of diminished glutathione on T cell dependent immune response. European Journal of Immunology, 30, 1902-1910. doi:10.1002/1521-4141(200007)30:7<1902::AID-IMMU1902>3.0.CO;2-A
- Moffatt, P., Plaa, G.L. and Denizeau, F. (1996) Rat heaptocytes with elevated metallothionein expression are resistant to N-methyl-N#-nitro-Nnitrosoguanidine cytotoxicity. Toxicology and Applied Pharmacology, 136, 200- 207. doi:10.1006/taap.1996.0025
- Chisolm, G.M., Hazen, S.L., Fox, P.L. and Cathcart, M.K. (1999) The oxidation of lipoprotein by monocytes macrophagaes Biochemical and Biological mechanism. The Journal of Biological Chemistry, 274, 5959-5962. doi:10.1074/jbc.274.37.25959
- Browning, J.D. and Horton, J.D. (2004) Molecular mediators of hepatic steatosis and liver injury. The Journal of Clinical Investigation, 114, 147-152.
- Ohshima, H. and Bartsch, H. (1994) Chronic infection and inflammatory processes as cancer risk factors: Possible role of nitric oxide in carcinogenesis. Mutation Research, 305, 253-264. doi:10.1016/0027-5107(94)90245-3
- Goel, S.K., Rao, G.S., Pandya, K.P. and Shanker, R. (1992) Trichloroethylene toxicity in mice: A biochemical, hematological and pathological assessment. Indian Journal of Experimental Biology, 30, 402-406.
- Khan, S., Priyamvada, S., Khan, S.A., Khan, W., Farooq, N., Khan, F., et al. (2009) Effect of trichloroethylene (TCE) toxicity on the enzymes of carbohydrate metabolism, brush border membrane and oxidative stress in kidney and other rat tissues. Food and Chemical Toxicology, 47, 1562-1568. doi:10.1016/j.fct.2009.04.002
- Mostafa, S.A. (1998) Effect of allyl as glutathione depleting agent on carbohydrate metabolism in rats. Journal of Egyptian German Society of Zoology, 26, 13-34.
- Fletcher, L.M., Roberts, F.D., Irving, M.G., Powell, L.W. and Halliday, J.W. (1989) Effects of iron loading on free radical scavenging enzymes and lipid peroxidation in rat liver. Gastroenterology, 97, 1011-1018.
- Sadani, G. and Nadkarni, G.D. (1994) Role of antioxidant enzyme defense in sparing rat hepatocytes from toxicity of ricin at low dose. Indian Journal of Experimental Biology, 32, 354-355.
- Kumar, P., Prasad, A.K., Maji, B.K., Mani, U. and Dutta, K.K. (2001) Hepato-toxic alterations induced by inhalation of trichloroethylene (TCE) in rats. Biomedical and Environmental Sciences, 14, 325-332.
- Hultberg, M. and Hultberg, B. (2005) Glutathione turnover in human cell lines in the presence of agents with glutathione influencing potential with and without acivicin inhibition of gamma-glutamyl transferase peptidase. Biochimica Biophysica Acta, 1726, 42-47. doi:10.1016/j.bbagen.2005.08.007
- Dashti, H.M., Al-Sayer, H., Behbehani, A., Madda, J. and Christenson, J.T. (1992) Liver cirrhosis induced by carbon tetrachloride and the effect of superoxide dismutase and xanthine oxidase inhibitor treatment. Journal of the Royal College of Surgeons of Edinburgh, 37, 23-28.
- Keen, C.L., Ensunsa, J.L., Watson, M.H., Baly, D.L., Donovan, S.M., et al. (1999) Nutritional aspects of manganese for experimental studies. Neurotoxicology, 20, 213-223.
- Pathak, C.M., Avti, P.K., Kumar, S., Khanduja, K.L. and Sharma, S.C. (2007) Whole body exposure to low dose gamma radiation promotes kidney antioxidant status in Balble/c-mice. Journal of Radiation Research, 48, 113- 120. doi:10.1269/jrr.06063
NOTES
*Corresponding author.

