Journal of Environmental Protection
Vol.06 No.10(2015), Article ID:60609,8 pages
10.4236/jep.2015.610102
Impact of Improving Water Quality at the Tala Drain on the Rosetta Branch Water Quality
Mohamed K. Mostafa
Department of Civil, Construction, and Environmental Engineering, University of Alabama at Birmingham, Birmingham, USA
Email: mkhaled@uab.edu
Copyright © 2015 by author and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 21 September 2015; accepted 24 October 2015; published 27 October 2015
ABSTRACT
The Tala drain is the second major source of pollution along the Rosetta branch. The Tala drain receives discharge from dairy industry and agricultural drainage, as well as untreated domestic wastewater. This research involved attempting to improve water quality at the Rosetta branch by improving water quality at the Tala drain. Water quality at the Tala drain will be improved through improving effluent water quality from the dairy industry using aluminum chloride (AlCl3) as a coagulant, with injections of carbon dioxide (CO2), and constructing a new WWTP. Results indicated that the optimum aluminum chloride dosage was 225 mg/L at a pH value of 6.15. The estimated treatment cost of 1.0 m3 of dairy wastewater is $0.0425 per day. The river pollutant (RP) modeling was also used to study the effect of improving water quality at the Tala drain in the Rosetta branch water quality. The RP modeling showed that applying the proposed solutions will significantly improve water quality at the Tala drain and at the Rosetta branch.
Keywords:
Aluminum Chloride, Carbon Dioxide, RP Modeling, Tala Drain, Rosetta Branch

1. Introduction
The Nile River in Egypt divides at Cairo into two branches, Damietta and Rosetta, which form the Nile delta. The Tala drain is the second major source of pollution along the Rosetta branch [1] . The Tala drain is located north of Cairo in the El-Menufia governorate. This drain is polluted mainly by agricultural drainage, domestic wastewater, as well as by industrial wastewater from dairy industry located along its path. The analysis of the water samples revealed relatively high levels of BOD and COD at the Tala drain [1] . Twenty samples were collected from the Tala drain before discharging to the Rosetta branch and the average COD, BOD, TSS, TDS, TOC, Cl− and DO concentrations were recorded to be 181.77, 89.60, 94.87, 896.40, 10, 265 and 2.23 mg/L, respectively [1] . The average value for pH in the Tala drain was recorded to be 7.8 [1] . The average flow at the Tala drain is about 450,000 m3/day (118,877,400 gal/day) [1] . Four villages discharge more than 200,000 m3 of raw sewage daily to the Tala drain. The dairy industry generates about 5500 m3 (1,452,946 gallons) of wastewater every year. The Tala drain receives also about 250,000 m3 (66,043,000 gal) of agricultural drainage water every day.
Dairy products include fresh milk, icecream, yogurts, processed milk, and cheeses. Milk and whey, the main components of waste streams, contain a high concentration of BOD (reaching up to 100,000 mg/L). Thus, any loss likely leads to a significant increase in the concentration of BOD in wastewater [2] . Several studies were conducted to improve effluent quality from dairy industries. Parmar et al. [3] studied the use of ferrous sulfate and aluminum sulfate (alum) in the treatment of dairy wastewater and found optimum dosages for ferrous sulfate and alum of 225 and 100 mg/L, respectively. The results showed COD removal efficiencies using ferrous sulfate and alum of 53% and 60%, respectively. The removal efficiency for turbidity reaches 55% using alum and 60% using ferrous sulfate. In their study, Harush et al. [4] investigated the treatment of dairy wastewater using coagulation and aerobic biodegradation. The removal efficiency of COD and odor increased with increasing aeration rate to an optimum rate of aeration of 320 mL/min. The removal efficiency for COD and odor reached 87.05% and 80%, respectively. Dabhi [5] conducted a comparison study involving the use of ferrous sulfate and ferric chloride in the treatment of dairy wastewater. The average COD concentration in the collected samples ranged from 1500 to 2900 mg/L, whereas turbidity ranged 15 to 30 NTU. The optimum dosages for ferrous sulfate and ferric chloride were found to be 260 and 220 mg/L, respectively. The results showed that the removal efficiencies for COD using ferrous sulfate and ferric chloride were 57 and 83 mg/L, respectively, and that those rates for turbidity reached 73.7% using ferrous sulfate and 76.8% using ferric chloride [5] .
Wastewater samples were collected from the effluent of the dairy industry and analyzed for different parameters. The parameters include pH, COD, TSS, TDS, and turbidity. The concentration of contaminants must not exceed the limits specified in Egyptian Law 44/2000. The analysis of wastewater samples thus far has indicated that the concentrations of TDS, COD, and TSS exceeded the limits specified in Egyptian Law 44/2000, as shown in Table 1. Therefore, this type of waste must be treated to meet waste specific standards before being discharged to various waterways.
This research involved attempting to improve water quality at the Rosetta branch by improving water quality at the Tala drain. Water quality at the Tala drain will be improved through improving effluent water quality from the dairy industry using aluminum chloride (AlCl3) as a coagulant, with injections of carbon dioxide (CO2), and constructing a new WWTP.
2. Materials and Methods
2.1. The Dairy Industry
Dairy wastewater samples were collected in 10.0 liter plastic containers from the effluent of the plant. Waste
Table 1. Physicochemical characterization of raw effluent from a dairy industry [1] .
COD: chemical oxygen demand, BOD: biochemical oxygen demand, TOC: total organic carbon, TSS: total suspended solids, TDS: total dissolved solids, Cl−: chlorides.
water samples were placed in an ice box for transfer to the laboratory. A series of jar tests were used to evaluate the effectiveness of AlCl3 in dairy wastewater treatment. pH, is an important component for proper coagulation performance, it can affect the surface charge of floc particles and the coagulant solubility. The optimal pH values for the elimination of the turbidity, BOD, COD, and TSS ranged from 6.1 to 6.2 for the AlCl3 [6] . The optimum settling time was determined by treating the wastewater in the beakers with the same coagulant dosage at pH 6.15. The contents were rapidly stirred at a speed of 150 rpm for 1 minute, followed by slow mixing for 10 minutes at 30 rpm. Jar tests were also used to determine the optimum dosage of AlCl3. The samples were then analyzed for BOD, pH, COD, TOC, TSS, Cl−, TDS, and turbidity according to the standard methods for wastewater analysis [7] . Standard quality control procedures were used for the analysis of the samples [7] . The TDS, pH, and DO were measured in the field, while the other parameters were measured in Egyptian Housing Building Research Center (HBRC) laboratory, located in Cairo city. The HM digital TDS meter enabled measurement of TDS level in the wastewater samples. The WTW multi 340i meter was used to measure DO and pH levels in the samples. The 5-day BOD test 5210B and the test method 2540D was used in the determination of the BOD and TSS concentrations in wastewater samples, respectively. The ion chromatography method 4110B and the closed reflux, titrimetric method 5220C enabled determination of the chlorides (Cl−) and COD concentration in the samples, respectively. A Shimadzu TOC-4200 analyzer enabled measurement of TOC level. Then, the removal efficiency (%) for each parameter was calculated by subtracting the parameter concentration after coagulation treatment from the parameter concentration at the blank sample, and then dividing the result by the parameter concentration at the blank sample. Mass balances were used to estimate the concentrations of different parameters at the Tala drain after effluent water quality was improved at the dairy industry and installing new WWTP.
2.2. River Pollutant (RP) Modeling
The river pollutant (RP) modeling was then used to study the effect of improving water quality at the Tala drain in the Rosetta branch water quality [8] . The RP modeling is a river water quality model used to calculate water quality parameters such as DO, TOC, Cl−, BOD, COD, TDS, TSS, pH, and temperature along the Nile River delta [8] . Data required to run the RP modeling include the cross-sectional area and the water velocity upstream of the Tala drain, the parameters concentration upstream the Tala drain, the parameters concentration and the flow rate at the Tala drain, and the river water velocity downstream of the Tala drain [1] . The RP modeling uses the mass balance and the exponential equations which enabled seasonal estimations of the values of different parameters downstream the Tala drain. The mass balance helps to mathematically estimate the mass loading at any point along the study area. Consequently, the RP modeling aided in the identification of the mixing condition and in the determination of mass balance along the study area.
3. Results and Discussion
3.1. The Dairy Industry
The proposed scenario is to improve water quality at the Tala drain through improving effluent water quality from the dairy industry and constructing a WWTP with a capacity of more than 200,000 m3/day (52,834,410.5 gal/day). Equal amounts of the wastewater sample were poured into a series of plastic beakers, and then each beaker was treated with 180 mg/L AlCl3. The contents were rapidly stirred at a speed of 150 rpm for 1 minute, followed by slow mixing for 10 minutes at 30 rpm. The optimum time for settling was found to be about 65 min, as shown in Figure 1.
Jar tests were also used to determine the optimum dosage of AlCl3. According to the above results, the following doses were selected: 120, 150, 180, 225, 250, and 300 mg/L. Results indicated that the optimum aluminum chloride dosage was 225 mg/L at a pH value of 6.15, as shown in Figure 2. The results showed that aluminum chloride at a dose of 225 mg/L results in removal efficiencies reaching 86.60%, 84.10%, 87.40%, 42.30%, 50.10%, 91.80%, and 42.0% for BOD, COD, TSS, TDS, TOC, turbidity, and chlorides, respectively.
Water samples for analysis were collected from point sources discharging to the Tala drain. Mass balances were used to estimate the concentrations of different parameters at the Tala drain after applying the proposed solution, as shown in the equations below. Water flow and characterization at the Tala drain for the current and proposed solution, are presented in Table 2.
Figure 1. Removal efficiencies of BOD, COD, TSS, and turbidity at different treatment times.
Figure 2. Removal efficiencies of BOD, COD, TSS, TOC, TDS, chlorides, and turbidity at different AlCl3 doses.
Table 2. Water flow and characterization at the Tala drain.
COD: chemical oxygen demand, BOD: biochemical oxygen demand, TOC: total organic carbon, TSS: total suspended solids, TDS: total dissolved solids, DO: dissolved oxygen.
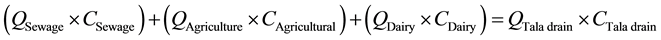 (1)
(1)
For COD:
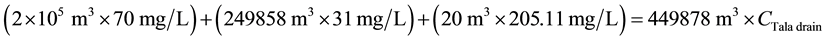
\ 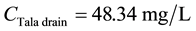
For BOD:
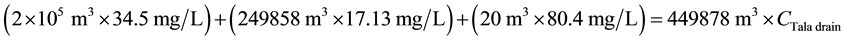
\ 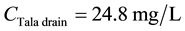
For TSS:
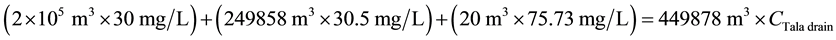
\ 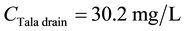
For DO:
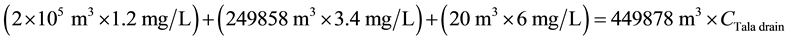
\ 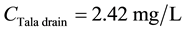
For TDS:
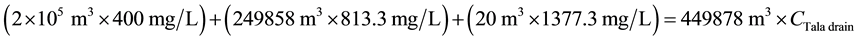
\ 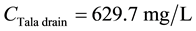
For TOC:
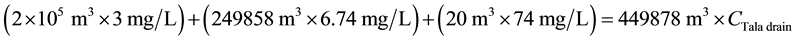
\
For Chloride:
\
For pH:

\
3.2. Economical Study
Cost is one of the most important parameter in wastewater treatment. A sedimentation tank and a chemical mixer tank are needed for treating wastewater by using coagulants. The treatment cost of dairy wastewater with AlCl3 and CO2 is calculated using the below equation. The cost of aluminum chloride to treat one cubic meter of dairy wastewater at pH equal 6.14 is 0.2936 EGP. The total cost to treat 20 m3 of dairy wastewater is 6.0 EGP or $0.85.

3.3. RP Modeling Results
The river pollutant (RP) modeling was used to predict improvement in the Rosetta branch water quality after improving water quality at the Tala drain by constructing a WWTP with a capacity of more than 200,000 m3/day, and after improving effluent water quality from the dairy industry with the application of AlCl3 at a lower pH value. The simulations of the current situation and the proposed solution are shown in Figures 3-10. The water quality standard specified in Egyptian law 48/1982 and EPA standards for BOD is 6.0 mg/L [9] [10] . In the current situation, the BOD concentrations downstream of the Tala drain was about 14.02 mg/L. These values clearly exceeded the water quality standards, as shown in Figure 3. After water quality at the Tala drain is improved, the BOD concentration downstream of the Tala drain will decrease to 12.85 mg/L. In comparison with findings for the current situation, those resulting from applying the proposed solution will reduce the BOD concentration downstream of the Tala drain by about 8.4%. Consequently, if the proposed solution is applied, the BOD concentration downstream of the Tala drain will decrease, and thus, improve the water quality at the Rosetta branch. The chlorides concentration downstream of the Tala drain did not exceed the 250 mg/L maximum value specified in Egyptian law 48/1982 and EPA standards [9] [10] . After water quality at the Tala drain is improved, the chlorides concentration along the study area will decrease by about 2.34% (see Figure 4).
The water quality standard specified in Egyptian law 48/1982 and EPA standards for COD is 10.0 mg/L [9] [10] . For the current situation, the COD concentration downstream of the Tala drain is about 27.59 mg/L. This value clearly exceeded the water quality standards, as shown in Figure 5. After water quality at the Tala drain is improved, the COD concentration downstream of the Tala drain will decrease to 25.18 mg/L. In comparison with findings for the current situation, those resulting from applying the proposed solution will reduce the COD
Figure 3. BOD concentration along the Rosetta branch for current situation and proposed solution.
Figure 4. Chlorides concentration along the Rosetta branch for current situation and proposed solution.
Figure 5. COD concentration along the Rosetta branch for current situation and proposed solution.
concentration downstream of the Tala drain by about 8.77%. Applying the proposed solution will decrease the negative affect of the Tala drain in the Rosetta branch water quality. The water quality standard specified in Egyptian law 48/1982 and EPA standards for TDS is ≤500 mg/L [9] [10] . For the current situation, the TDS concentration along the branch did not exceed the 500 mg/L maximum value specified in Egyptian and EPA standards; thus, the TDS concentration in the Rosetta branch is not negatively affected by receiving discharge from the Tala drain (see Figure 6). After water quality at the Tala drain is improved, the TDS concentration along the study area will decrease by about 2.15%. The pH value specified in Egyptian law 48/1982 and EPA standards ranges from 7.0 to 8.5 [9] [10] . The pH value upstream of the Tala drain was about 8.15, which agrees with water quality standards (see Figure 7). The pH value downstream of the Tala drain is expected to increase from 8.098 to 8.101 after improving water quality at the Tala drain. For the current situation and the proposed
Figure 6. TDS concentration along the Rosetta branch for current situation and proposed solution.
Figure 7. pH value along the Rosetta branch for current situation and proposed solution.
Figure 8. DO concentration along the Rosetta branch for current situation and proposed solution.
solution, the pH value along the Rosetta branch is in agreement with the water quality standards specified in Egyptian and EPA standards. Consequently, the pH value in the Rosetta branch is not negatively affected by using carbon dioxide in dairy wastewater treatment.
The DO concentration specified in Egyptian law 48/1982 and EPA standards is ≥4.0 mg/L [9] [10] . In the two cases, the DO concentration downstream of the Tala drain was lower than limits (see Figure 8). In comparison with findings for the current situation, those resulting from applying the proposed solution will slightly increase DO concentration along the Rosetta branch. The TOC concentration upstream of the Tala drain was approximately 1.25 mg/L, which clearly is within the 3.0 mg/L maximum value specified in Egyptian law 48/1982 [9] . In the two cases, the TOC concentration along the study area was also within the permissible limits. After water quality at the Tala drain is improved, the TOC concentration will decrease by about 6.20% downstream the Tala drain (see Figure 9). The TSS concentration upstream of the Tala drain was about 46 mg/L, which clearly ex-
Figure 9. TOC concentration along the Rosetta branch for current situation and proposed solution.
Figure 10. TSS concentration along the Rosetta branch for current situation and proposed solution.
ceeded the 20 mg/L maximum value specified in Egyptian law 48/1982 and EPA standards [9] [10] . After water quality at the Tala drain is improved, the TSS concentration downstream of the Tala drain is expected to decrease from 47.03 to 45.95 mg/L, with a percentage decrease of about 2.30% (see Figure 10). These values clearly exceeded the water quality standards, so the TSS concentration in the Rosetta branch is negatively affected by receiving discharge from the Tala drain.
4. Conclusions
Environmental laws have been enacted to protect public health and the environment. The company may be exposed to the risk of criminal and civil liability as a result of noncompliance with environmental regulations. Treating the dairy wastewater is very important for preventing high loading pollutants from entering and polluting the environment. The optimum time for settling was found to be 65 min. The application of 225 mg/L of AlCl3 at pH value of 6.15 caused a reduction in COD, BOD, TSS, TDS, TOC, chlorides, and turbidity of up to 84.10%, 86.60%, 87.40%, 42.30%, 50.10%, 42.0%, and 91.80%, respectively. Aluminum chloride is very effective in treating dairy wastewater, especially at pH between 6.1 and 6.2; the estimated treatment cost of treating the daily effluent (20 m3) is approximately $0.85.
The RP modeling showed that the concentrations of COD, TDS, BOD, TSS, and TOC along the Rosetta branch downstream of the Tala drain are expected to decrease after improving water quality at the Tala drain. The modeling results also showed that the proposed scenario, improving water quality at the Tala drain, will have a minor effect in reducing the chloride concentration and increasing the DO concentration. The pH is also expected to increase slightly downstream of the Tala drain after the proposed scenario is applied. In the two cases, the BOD, TSS, and DO concentrations along the study area exceeded the limit specified in the Egyptian and EPA standards; in contrast the TDS, pH, TOC, and chlorides values along the study area are within the limits specified by Egyptian and EPA standards.
Acknowledgements
This research was supported by the Department of Civil, Construction, and Environmental Engineering at the University of Alabama at Birmingham. The authors also thank the Egyptian Housing Building Research Center for their help in collecting samples and performing the chemical analyses.
Cite this paper
Mohamed K.Mostafa, (2015) Impact of Improving Water Quality at the Tala Drain on the Rosetta Branch Water Quality. Journal of Environmental Protection,06,1149-1157. doi: 10.4236/jep.2015.610102
References
- 1. Mostafa, M. (2014) Modeling of Pollutant Transport in the Nile Delta Egypt. Ph.D. Dissertation, Department of Civil, Construction, and Environmental Engineering, University of Alabama at Birmingham, Birmingham.
- 2. Cleaner Production Opportunities (1999) SEAM Project, Food Processing Sector, Ministry of State for Environmental Affairs, Egyptian Environmental Affairs Agency, Technical Cooperation Office for the Environment and Entec UK Limited.
http://www.ripecap.net/Uploads/466.pdf - 3. Parmar, K.A., Sarju, P., Rinku, P. and Yogesh, D. (2011) Effective Use of Ferrous Sulfate and Alum as a Coagulant in Treatment of Dairy Industry Wastewater. ARPN Journal of Engineering and Applied Sciences, 6, 42-45.
- 4. Harush, D.P., Hampannavar, U.S. and Mallikarjunaswami, M.E. (2011) Treatment of Dairy Wastewater Using Aerobic Biodegradation and Coagulation. International Journal of Environmental Sciences and Research, 1, 23-26.
- 5. Dabhi, Y.M. (2013) Physicochemical Treatment of Dairy Plant Wastewater Using Ferrous Sulfate and Ferric Chloride Coagulants. International Journal of Basic and Applied Chemical Sciences, 3, 9-14.
- 6. Mostafa, M.K. (2015) Improve Effluent Water Quality at Abu-Rawash WWTP Using Aluminum Chloride and Carbon Dioxide. Journal of Water Resource and Protection, 7, 1049-1057.
http://dx.doi.org/10.4236/jwarp.2015.713086 - 7. Andrew, D.E., Lenore, S.C., Eugene, W.R. and Arnold, E.G. (2005) Standard Methods for the Examination of Water and Wastewater. 21st Edition, American Public Health Association, Washington DC.
- 8. Mostafa, M.K. and Peters, R.W. (2015) Use River Pollutant Modeling to Simulate and Predict the Change in the Damietta Branch Water Quality before and after Construction of the Ethiopian Dam. Journal of Environmental Protection, 6, 935-945.
http://dx.doi.org/10.4236/jep.2015.69083 - 9. National Water Research Center (NWRC) (1995) River Nile Protection and Development Project Phase II. Environmental Pollution and Legislative Regulations (Law 48. 1982 & Decree 8, 1993). Ministry of Public Works and Water Resources, Cairo.
- 10. Environmental Protection Agency (EPA) (2007) Drinking Water Standards and Health Advisories Table. San Francisco.


















