World Journal of Vaccines
Vol.3 No.2(2013), Article ID:31520,9 pages DOI:10.4236/wjv.2013.32011
Clinical and Immunological Effects in Patients with Advanced Non-Small Cell Lung-Cancer after Vaccination with Dendritic Cells Exposed to an Allogeneic Tumor Cell Lysate*
![]()
1Department of Oncology, Herlev University Hospital, Herlev, Denmark; 2DanDrit Biotech A/S, Copenhagen, Denmark; 3Department of International Health, Immunology and Microbiology, The Panum Institute, University of Copenhagen, Copenhagen, Denmark.
Email: anders.mellemgaard@regionh.dk
Copyright © 2013 Lotte Engell-Noerregaard et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 25th, 2013; revised February 27th, 2013; accepted March 10th, 2013
Keywords: NSCLC; Dendritic Cells; Vaccination; Tumor Lysate
ABSTRACT
Background: We evaluated the clinical and immunological effects of dendritic cell (DC) vaccination of patients with NSCLC. Autologous DCs were pulsed with a MAGE containing allogeneic melanoma cell lysate (MelCancerVac®, Dandrit Biotech, Copenhagen, Denmark). Imiquimod cream, proleukin and celecoxib were used as adjuvants to the vaccines. The objective of the study was to evaluate specific T cell response in vitro by IFNg EliSpot. Secondary objectives were overall survival, response and quality of life (QoL). Results: Twenty-two patients initiated the vaccination program consisting of ten vaccinations. Seven patients remained in stable disease (SD) three months after the first vaccination. After ten vaccinations (six months), four patients still showed SD and continued vaccinations on a monthly basis. These four patients received a total of 12, 16, 26 and 35 vaccinations, respectively. Five patients showed an unexpectedly prolonged survival. The treatment was well tolerated and only minor adverse events were reported. Quality of life did not change during the study period. In four of the seven patients with SD, vaccine-specific T cells were detected by IFNγ EliSpot assays, whereas only one patient with progressive disease (PD) showed vaccine-specific responses. Conclusion: This DC-based vaccine trial has indicated a correlation between vaccine-specific immunity and sustained SD. Furthermore, we observed an unexpectedly prolonged survival in some patients, which may indicate delayed effect of DC vaccination after completion of the treatment. A prospective randomized phase-IIb or -III is needed to further evaluate the use of MelCancerVac® vaccine treatment in patients with progressive NSCLC.
1. Introduction
Lung cancer is one of the most common causes of cancer related deaths in the Western world [1]. Non-small cell lung cancer (NSCLC) accounts for 80% of all lung cancers and the majority of patients are diagnosed in an advanced stage.
In spite of an increasing number of treatment options, including various targeted agents, the outcome for patients with advanced disease remains poor with median survival of less than 1 year [2,3]. Thus, there is a need for new treatment modalities of which immunotherapy remains a possibility. Progress is being made in numerous cancers with immunotherapy ranging from non-specific Immune stimulants as interferons and interleukins, to antigen-specific vaccines [4]. In prostate cancer, a cellular cancer vaccine (Provenge®) has achieved FDA approval [5], and for advanced melanoma, Ipilimumab (BMS) was approved by FDA last year [6]. In lung cancer, phase III trials are presently conducted to examine the role of vaccines combined with chemo-radiotherapy to locally advanced NSCLC and as adjuvant therapy after radical surgery [7-9].
In the present trial, we tested a vaccine (MelCancerVac®, Dandrit Biotech, Copenhagen, Denmark) consisting of autologous DCs pulsed with an allogeneic tumor lysate known to express several tumor antigens including the major cancer testis antigens (CTA) MAGE-A, MAGE-B, GAGE, BAGE, SAGE, CSAG, IL13Ra2, CAGE and TPTE [10]. mRNA encoding many of these CTA’s are expressed by tumors from NSCLC cancer patients [11]. Thus, MAGE-A is expressed by NSCLC tumor cells in more than 50% of the biopsies from bone metastasis [12] and in more than 35% of biopsies from lymph node metastasis [13]. MAGE-A was also found to be expressed in more than 40% of NSCLC cell lines [14].
Previously, MelCancerVac® has been tested in a phase II trial in MAGE-A tumor-positive patients with metastatic colorectal carcinoma. In that study, a 40% clinical beneficial response (SD and partial response) was observed [15]. The aim of the present clinical trial was to examine clinical and immunological responses in NSCLC patients treated with MelCancerVac®.
2. Materials and Methods
2.1. Patients
Patients with a histological or cytological confirmed diagnosis of NSCLC in loco-advanced or metastatic stage were eligible, irrespective of histological subtype. All patients were to have received anticancer treatment, but no further treatments were to be planned. Further inclusion criteria were age > 18 years, performance status < 2 (Eastern Cooperative Oncology Group), expected survival of at least 12 weeks, more than 4 weeks from last chemoor radiation-therapy, more than 1 week after treatment with immunosuppressive drugs, adequate hepatic and renal function, normal blood cell count, and at least one measurable lesion according to RECIST. Women of childbearing age were requested to use safe contraception. The exclusion criteria were any other malignancy within the last 5 years, brain metastasis, severe systemic diseases e.g. infection (including acute/chronic infection of HIV, hepatitis or tuberculosis), uncontrolled hypertension, severe asthma, unstable angina and other cardiac, hepatic, renal and metabolic diseases. Also patients who of any reason had impaired oral uptake, present or previous gastric ulcer within three months, serious allergy or previous anaphylactic reactions, autoimmune diseases, pregnant or lactating women and mental diseases which could affect the patients’ compliance to the study were excluded from the study. Furthermore, we excluded patients with known hypersensitivity to the substances used as adjuvants (celecoxib, imiquimod cream, proleukin (IL-2)).
This trial was approved by the Danish Medicines Agency and the local ethics committee, and was registered in ClinicalTrials.gov with identifier NCT00442754. The trial opened at December 5th 2006 and closed at December 9th 2008.
2.2. Objectives
The primary objective was to evaluate the MelCancerVac®-specific T cell response in vitro by IFNg EliSpot. The secondary objectives were overall survival, response according to RECIST and quality of life (QoL). The patients were followed up for survival until December 31st 2011.
2.3. Treatment
The treatment (Figure 1) included 5 vaccinations administered at weekly intervals with a 6th booster vaccination 3 weeks after the 5th vaccine. Subsequent to the initial 6 vaccinations, the patients were evaluated by CT scans. If the patient showed stable disease (SD), partial response or complete response according to RECIST, treatment proceeded with four additional vaccinations given every 4 weeks. After the 10th vaccination, patients were again evaluated by CT scans. Patients who did not progress after the second CT scan at 6 months after entry into the trial, were allowed to continue off-study with additional vaccinations every 4 weeks with CT evaluation every 3rd month.
To enhance the immune response, patients received subcutaneously (SC) proleukin (2 MIU/day), day 1-5 after each vaccination, a cox-2 inhibitor (celecoxib) 200 mg/day throughout the study, and imiquimod cream, a TLR7 agonist (Aldara®), applied to the site of vaccine injection for 8 hours, days −2, −1 and +1 to each vaccination. MelCancerVac® was administered intradermally in the femoral triangle. Adverse events were evaluated at each visit and scored according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 2006.
2.4. Quality of Life (QoL)
QoL was measured by self-administered questionnaire using European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire (QLQ)-C30 version 3 and QLQ-LC13 [16]. The questionnaire was filled at baseline, and by the time of the 5th, 6th, 7th, 8th, 9th and 10th vaccinations.
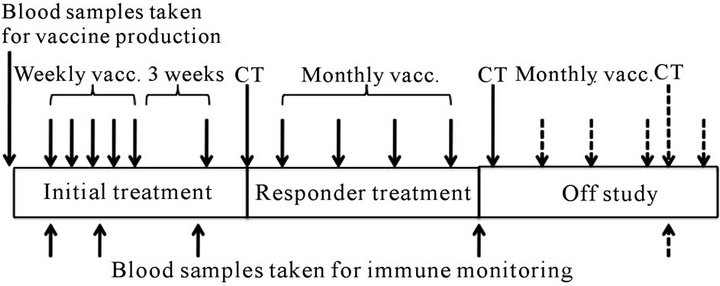
Figure 1. Schematic overview of the vaccination protocol.
2.5. Preparation of Vaccine (MelCancerVac®)
Autologous peripheral blood mononuclear cells (PBMCs) were obtained either from a peripheral whole blood sample of 200 ml or by a leukapheresis. PBMCs were iso lated by Ficoll gradient centrifugation (Nycomed, OsloNorway) and cryopreserved in 10% DMSO and autologous plasma. Monocytes were isolated by plastic adherence and monocyte-derived DCs were generated as previously described by Romani et al [17]. Briefly, the monocytes were cultured for 5 days in AIM-V (Thermo Fisher Scientific,US) supplemented with 5% autologous plasma and GM-CSF and IL-4 (1400 U/ml and 700 U/ml respectively, Gentaur, Brussels, Belgium). At day 5 the immature DCs were pulsed with allogeneic cell lysate prepared from the melanoma cell line DDM-1. DDM-1 express high levels of a wide range of CTA’s as reported in [10]. To induce maturation of DCs, IL-1 (10 ng/ml, Gentaur), TNF (10 ng/ml, Gentaur), IL-6 (10 ng/ml, Gentaur) and PGE2 (1 mM, Sigma Aldrich, US) were added at day 6 of culture and after one additional day the DCs were harvested. The vaccine was initially frozen in boxes containing isopropanol at −80°C and subsequently moved to a nitrogen cont ainer after 24 h and stored until used. Vaccinations were performed as described previously [18].
2.6. IFNγ Enzyme-Linked Immunospot (EliSpot) Assay
Blood samples of 50 ml were collected at baseline and after the 3rd, 6th and 10th vaccinations for immune monitoring.
In vitro stimulation was conducted prior to the EliSpot assay. MelCancerVac® was co-cultured with monocytedepleted PBMC at a ratio of 1:20 - 1:30 for eight days in the presence of human IL-2 (20 U/ml) (Gentaur). As culture medium we used AIM-V (Thermo Fisher Scientific, Waltham, MA, USA) with 2% L-glutamine and 5% autologous plasma. EliSpot analyses were performed as previously described [19]. Briefly, 96 well plates with nitrocellulose membrane bottoms (Multiscreen HTS system, Milipore, Roskilde, Denmark) were coated with antiIFNγ mAb (1-D1k, Mabtech, Nacka Strand, Sweden) overnight. Lymphocytes were added at 105, 5 × 104 and 104 cells per 200 µl together with 104 DCs, either unloaded (unspecific IFNγ release) or loaded with tumor cell lysate (MelCancerVac®). The assays were conducted in triplicates when the available amounts vaccine and/ or cells were present. Plates were incubated at 37°C/5% CO2 for 20 - 22 hours. The next day plates were developed with biotinylated anti-IFNγ mAb (7-B6-biotin, Mabtech), streptavidin (Mabtech) and BCIP/NBT enzyme substrate (Mabtech). The spots were counted by a digitalized EliSpot counter (ImmunoSpot, CTL Inc. USA). Monocyte-depleted PBMCs cultured with PHA (Sigma Aldrich St. Louis, MO, US) were set up as a positive control, and the negative control consisted of monocyte-depleted PBMCs cultured with media alone. EliSpot assays were performed only after patients had completed the treatment and all samples could be analyzed in parallel to avoid interexperimental variability.
2.7. Statistical Analysis
EliSpot results: student’s t tests (two-tailed, unpaired) were performed to compare background values and experimental values, and between preand post-vaccination time points for patients that displayed vaccine-specific IFNγ release. Results were considered to be statistically significant when p ≤ 0.05. To assess any correlation between SD and vaccine specific IFNγ release, a two-tailed Fisher’s Exact Test was used. Graph pad prism was used to conduct the statistical analyses.
3. Results
3.1. Clinical Results
Twenty-eight NSCLC patients were included in this trial. Six patients did not receive any treatment: One patient withdrew her consent prior to the first vaccine, 2 patients developed brain metastasis, 1 patient did not have enough monocytes to generate vaccines, 1 patient decreased in performance status to below 2 and 1 patient was found to have a synchronous cancer in the esophagus at pre-treatment evaluation. Thus, 22 patients initiated treatment and received a total of 199 vaccines. Six patients were excluded from the study prior to the 1st evaluation because of decline in performance status and/ or rapid disease progression. The remaining 16 patients received at minimum 6 vaccines and were evaluated by CT scans. Of those, 9 patients showed progression on the 1st evaluation CT scan 3 months after initiation of treatment, and seven patients had stable disease (SD) and continued with monthly vaccinations. At the time of the 2nd evaluation (6 months after initiation of treatment), 3 patients were excluded from the study due to progression whereas the remaining 4 patients were allowed to continue off-study with additional vaccinations every 4th week and CT evaluation every 3rd month until progression. These 4 patients received 12, 16, 26 and 35 vaccinations, respectively.
3.2. Patient Characteristics
Treatments prior to DC vaccinations, tumor histology, smoking status, number of vaccinations, age and gender are summarized in Table 1. Histology subtypes for the 4
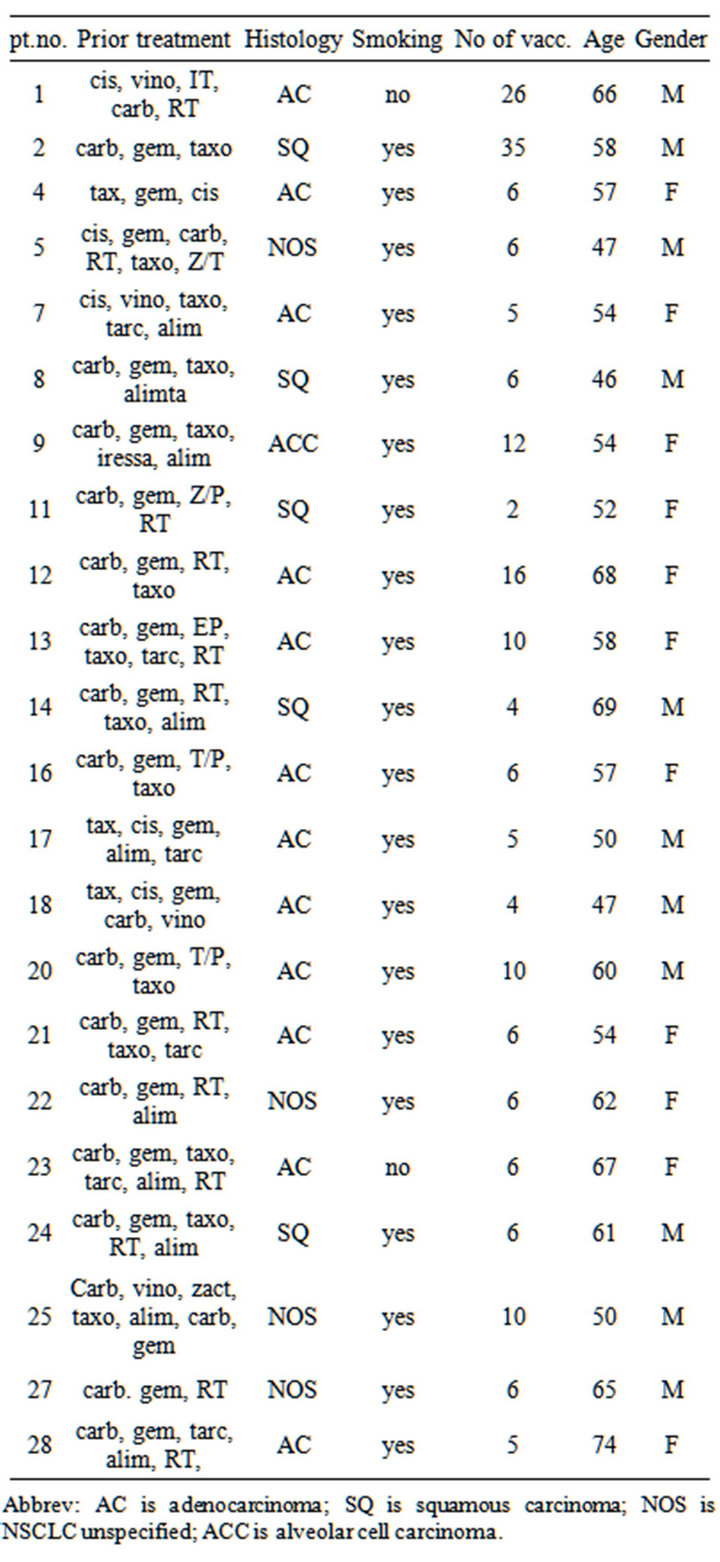
Table 1. Characteristics of patients included in phase II trial of vaccination with dendritic cells exposed to an allogeneic tumor cell lysate. Cis: cisplatin; Carbo: carboplatin; Vino: vinorelbine; Vande: Vandetanib; Doce: Docetaxel; Erlo: Erlotinib; Gem: gemcitabine; Pem: pemetrexed; Gefi: gefitinib; XD: experimental drug; RT: Radiotherapy; NOS: Not otherwise specified; Ca: carcinoma; M: male; F: female; pt no.: patient number; NSCLC: non-small cell lung cancer.
Abbrev: AC is adenocarcinoma; SQ is squamous carcinoma; NOS is NSCLC unspecified; ACC is alveolar cell carcinoma.
patients who remained in stable disease after more than 10 vaccinations were: broncho-alveolar carcinoma (n = 1), squamous cell carcinoma (n = 1) and adenocarcinoma (n = 2). The median age was 58.5 years (46 - 74 years). All patients received systemic anti-cancer treatment prior to inclusion.
At the time of inclusion, 15 were in performance status (PS) 0 and 7 patients were in PS 1. 25 months after termination of the trial, 3 patients (patient number 1, 2 and 12) were still alive. Kaplan Meier curves for progression free survival (PFS) and overall survival (OS) are shown in Figures 2 and 3 respectively. At the first evaluation 3 months after initiation, 15 patients either showed progression or had experienced a decrease in performance necessitating withdrawal from the trial within the first months. However, 7 patients remained in SD for a variable period of time (Figure 2). The overall survival curve (Figure 3) shows a plateau after 2 years.
3.3. Adverse Events
Several minor adverse events in vaccinated patients were noted (Table 2).
One patient developed a deep vein thrombosis in the left leg following leukapheresis and was treated with low molecular heparin where-after the symptoms resolved over weeks. One patient developed renal-failure at the time of the 5th vaccine and was excluded from the trial.
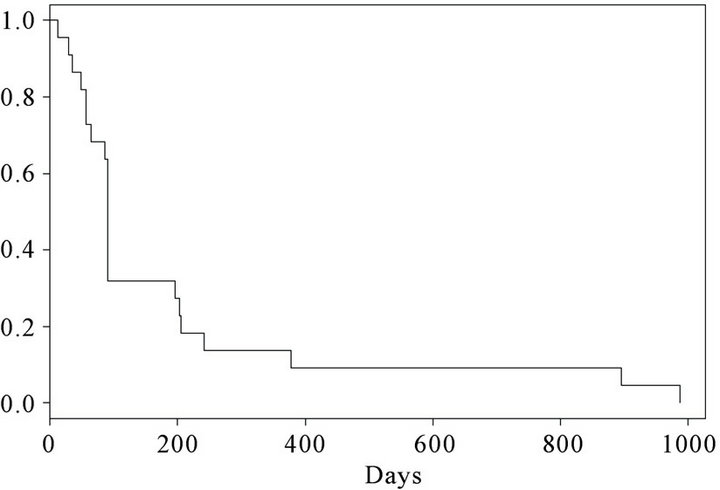
Figure 2. Kaplan-Meier curve. Progression free survival (PFS) in 22 vaccinated patients.
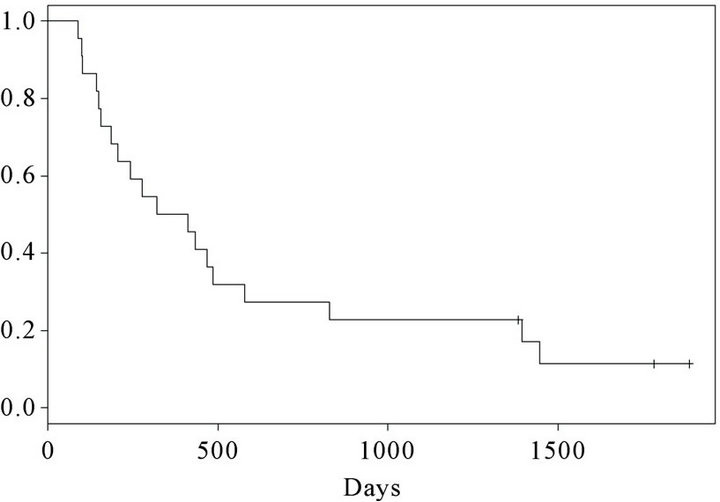
Figure 3. Kaplan-Meier curve. Overall survival of 22 vaccinated patients.
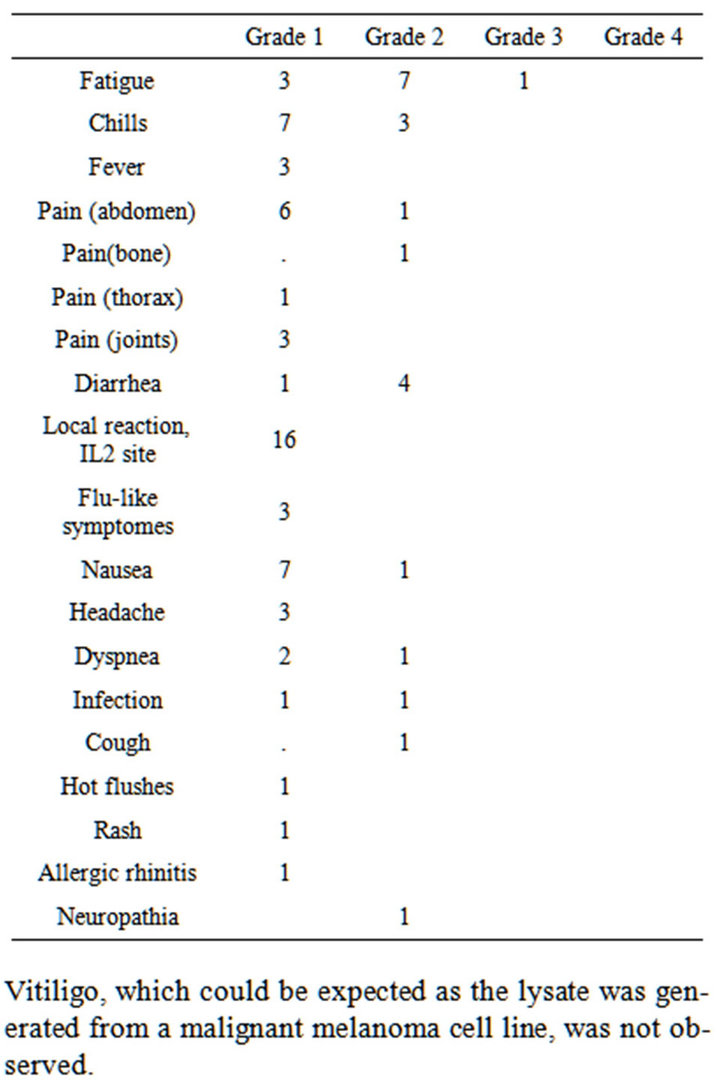
Table 2. Adverse events observed in patients with advanced non-small cell lung-cancer after vaccination with dendritic cells exposed to an allogeneic tumor cell lysate.
Vitiligo, which could be expected as the lysate was generated from a malignant melanoma cell line, was not observed.
3.4. Quality of Life
The data from the QoL questionnaires were collected and coded according to EORTC [18]. Due to patients excluded during the course of the study, the numbers of completed QOL questionnaires were limited. An overall evaluation of general QoL-score for the global question of “How do you rate your overall quality of life during the past week” remained stable throughout the study period. More specific factors such as anxiety and lung specific symptoms also remained unchanged during the studyperiod (data not shown).
3.5. IFNγ EliSpot
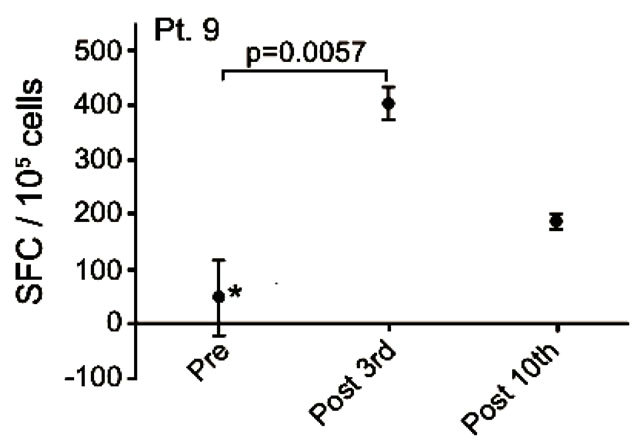
Blood-samples from 20 patients were screened for MelCancerVac®-specific IFNγ release. Responses were observed only in 5 patients of whom 4 remained in SD at the first evaluation 12 weeks after inclusion. Vaccinespecific IFNγ release was detected in 4 out of 7 patients with SD and 1 out of 13 the patients with PD. The correlation between vaccine-specific immune-responses and clinical response to treatment was found to be significant (p = 0.03, Fisher’s Exact test). EliSpot data for the 5 patients with vaccine-specific responses are shown in Figure 4 and these patients are described below.
Patient no. 9 was a 54 year old woman with an alveolar cell carcinoma in the lung, mediastinum, liver and bone. She was diagnosed in 2005 and by the time of inclusion the disease was in progression after treatment with carboplatin/gemcitabine, docetaxel, gefitinib and pemetrexed. She remained in SD after a total of 10 vaccinations, but died shortly after the 12th vaccination 249 days after inclusion. After the 3rd vaccination a significant (p < 0.006) vaccine-induced release of IFNγ was detected (Figure 4(a)). The vaccine-specific IFNγ response was decreased after the 10th vaccination.
Patient no. 13 was a 58 years old woman with an adenocarcinoma in the lung and mediastinum. She had SD at the time of inclusion and remained in SD at the first evaluation, but progressed at the second evaluation after 10 vaccinations. She was diagnosed in 2004 and prior to inclusion she received carboplatin/gemcitabine, epo906 (experimental treatment), docetaxel, erlotinib and radiation therapy. After termination of vaccines (due to disease progression), she received further treatment with docetaxel, pemetrexed and carboplatin/vinorelbine. She died 1446 days after the first vaccination. During the course of treatment we detected an increased number of IFNγ-secreting cells in response to the vaccine and the value obtained at the 10th vaccine was significantly increased compared to baseline (pre-vaccination) (p = 0.0132) (Figure 4(b)).
Patient no. 20 was a 60 year old man with an adenoid cystic carcinoma in the lung and liver. He was diagnosed in 2007 and prior to inclusion he received carboplatin/ gemcitabine, docetaxel and was included in a randomized blinded trial of erlotinib plus/minus experimental drug. He had SD at the time of inclusion and remained in SD after 6 vaccines but progressed after 10 vaccines. After exclusion from this trial he was treated with pemetrexed, erlotinib and experimental treatment. He died 828 days after the first vaccination. Vaccine specific IFNγ response was detected at baseline but this response decreased after 3 and 6 vaccinations. Interestingly, after 10 vaccinations an increase in vaccine-specific IFNγ release was detected which was significantly higher compared to the value obtained after 6 vaccinations (p < 0.004) (Figure 4(c)).
Patient no. 2 was a 58 years old man, diagnosed in 2006 with squamous cell carcinoma in the lung and mediastinum. He had SD at the time of inclusion and remained in SD for 29 months. He received a total of 35 vaccinations. Prior to inclusion in this trial he received treatment with carboplatin/gemcitabine and docetaxel.
After termination of vaccinations he was treated with erlotinib and radiation therapy and he was still alive at last follow up (December 31st 2011), 1783 days after the first vaccination. The EliSpot assay showed high levels of non-specific IFNγ spot formation in pre-treatment samples. The detected increase in vaccine-specific spot formation from baseline to the 10th vaccination was statistically significant (p < 0.025) as depicted in Figure 4(d).
Patient no. 17 was a 50 year old man with an adenocarcinoma in the lung and bones. He was diagnosed in 2006 and prior to inclusion he received cisplatin/gemcitabine, docetaxel, pemetrexed and erlotinib. After the 5th vaccination, the patient progressed rapidly and died 1 month after exclusion from the trial, 100 days after the first vaccination. A pre-existing vaccine specific IFNγ response to MelCancerVac® was detected. However, this response decreased after 3 vaccinations (Figure 4(e)).
4. Discussion
In the present phase II trial, 22 patients were treated with DC vaccinations and 16 patients were evaluable by CT scans, QoL questionnaires and EliSpot. We found that 7 patients had SD and we observed a few long-term survivors. Self-evaluated QoL remained unchanged throughout the treatment period. Vaccine-specific immune-responses were detected in 4 of 7 patients with SD and only in 1 of 13 patients with PD. In addition, we found that the treatment was feasible and the logistics manageable in this group of patients.
 (a)
(a)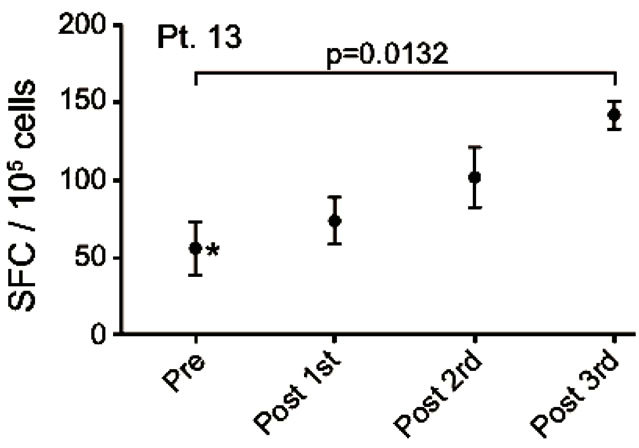 (b)
(b)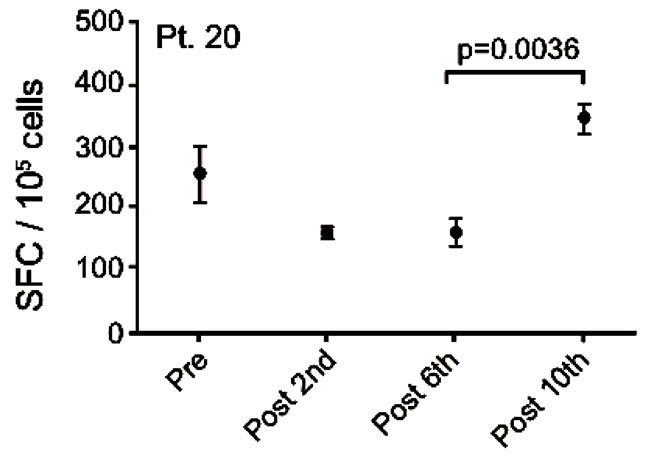 (c)
(c)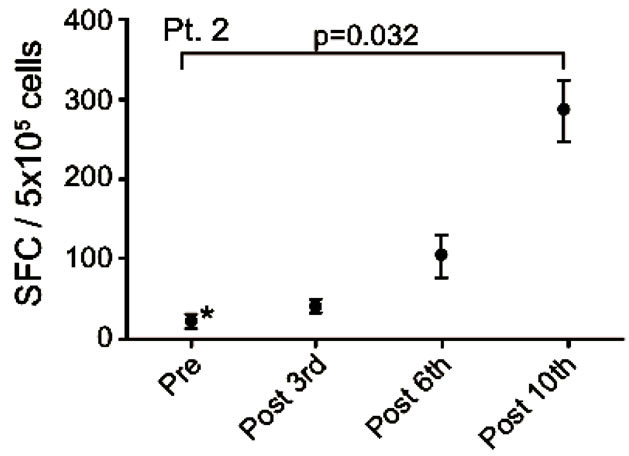 (d)
(d)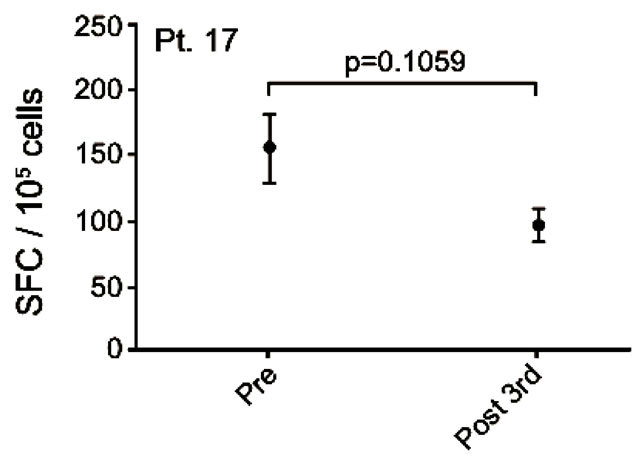 (e)
(e)
Figure 4. IFNg EliSpot results from 5 patients (Pt. 2, 9, 13, 17, 20). All results except patient no.2 (see below) were from EliSpot assays conducted subsequent to in vitro stimulation. Effector cells were plated in three concentrations, 105, 5 × 104, 104 cells per 200 µl together with 104 DCs in triplicates. The values shown in the figures are from wells with the highest concentration of effector cells to stimulator cells i.e. 10:1 (backgrounds are subtracted). Asterisks indicate where the experimental values were not significantly higher than background. Data are presented as mean numbers of IFNγ spot-forming cells (SFC). Bars represent two SEM values. For patient no.2 the Elispot was conducted directly from PBMC without in vitro stimulation.
We observed that a few patients were still alive years after termination of the trial and after additional lines of treatment. This finding was surprising taking the usual course of NSCLC into account. We are unable to evaluate whether the long survival was due to a long-lasting effect of vaccines or whether the prolonged survival reflects a selection of patients. Supporting the first theory, Fay et al observed a small number of patients with a long survival and slow progression of the disease after DC vaccinations of patients with malignant melanoma [20]. It has been shown that patients can benefit from the treatment with immunotherapeutic agents in terms of prolonged survival but without affecting progression free survival (PFS) as described by Kantoff et al. [5] and discussed by Hoos et al. [21].
We evaluated vaccine-specific IFNγ release in EliSpots in 20 patients and specific responses were detected in 4/7 patients with SD, and only in 1/13 patients with PD—this difference was statistically significant (p = 0.03). In line with these data, correlations between immune responses and clinical responses have been demonstrated previously in tumor peptide-based DC vaccination and peptide vaccination trials [20-22]. Also Fay et al found a correlation between clinical outcome and tumor peptide-specific induced T cell responses in peripheral blood after vaccinations with tumor peptide-loaded DCs [20]. Lonchay et al showed that long term survival was associated with the occurrence of tumor peptide specific immune responses [22]. However, it remains to be elucidated if the presences of vaccine-induced immune-responses merely is reflecting immune competence of the patients in general or if these responses are clinical relevant. The finding of a significant correlation between prolonged disease stabilization and vaccine-specific cellular responses may support the latter notion and support the hypothesis that immune responses may play a role in disease control even long time after the actual treatment. This is in contrast to the rapid effect of other anti-cancer treatments such as chemotherapy and radiotherapy.
The DCs generated for vaccine production in the present study expressed levels of differentiation molecules necessary for their functional activity in vivo (data not shown), similar to that found in a previous study using the same set-up [18]. The DCs were mature autologous monocyte-derived cells, pulsed with a lysate from an allogeneic melanoma cell line. This cell line was selected based on its high expression of the CTA MAGE-A [10]. A relatively high frequency of NSCLC tumors have been reported to express CTA’s, including MAGE [11-14]. Thus, the treatment of patients with advanced NSCLC with autologous DCs pulsed with allogeneic lysate expressing high levels of MAGE is relevant, as it might trigger a beneficial immune response against CTA’s expressed by the tumor. In light of these considerations, screening for MAGE positive tumors in the NSCLC patients would have been scientifically justified but was abandoned due to ethical considerations.
Consistent with other studies utilizing tumor cell lysate [23-29], the present study showed that administration of autologous DCs loaded with allogeneic tumor cell lysate rich in CTA can induce or enhance vaccine-specific release of IFNγ in EliSpot cultures in some patients.
The first—and so far only—FDA approved vaccine for cancer treatment is the DC-like vaccine Sipuleucel-T (Provenge®, Dendreon, USA). This vaccine is based on autologous dendritic-like cells exposed to a fusion protein: prostatic acid phosphatase-GM-CSF. Three vaccinations increased median survival of metastatic prostate cancer patients by 4 months, interestingly without affecting PFS and thus illustrating the proposed delayed onset of antitumor responses after vaccine immunotherapy [5]. The patients seem to benefit from the treatment without this being reflected by the standard evaluation criteria. Thus, changing the evaluation strategy might provide better understanding of the effects of the treatment in question as already suggested by Hoss et al [21].
In conclusion, 7 out of 22 NSCLC patients vaccinated with autologous DC pulsed with an allogeneic CTA containing tumor cell lysate had prolonged disease stabilization. In the course of DC vaccination vaccine-specific IFNg responses were detected in peripheral blood from 4 of the patients with SD and one patient with progressive disease. However, from the present study, it is not possible to conclude whether the vaccine treatment and the subsequent IFNg responses are involved in the clinical cause of these patients. To elucidate the full effect of MelCancerVac® vaccinations of patients with NSCLC, a randomized trial should be conducted.
5. Acknowledgements
This work was supported by DanDrit Biotech, Copenh gen, Denmark.
REFERENCES
- A. Jemal, F. Bray, M. M. Center, J. Ferlay, et al., “Global Cancer Statistics,” Cancer Journal for Clinicians, Vol. 61, No. 2, 2011, pp. 69-90. doi:10.3322/caac.20107
- J. Ferlay, D. M. Parkin and E. Steliarova-Foucher, “Estimates of Cancer Incidence and Mortality in Europe in 2008,” European Journal of Cancer, Vol. 46, No. 4, 2010, pp. 765-781. doi:10.1016/j.ejca.2009.12.014
- L. S. Gloeckler Ries, M. E. Reichman, D. Riedel Lewis, et al., “Cancer Survival and Incidence from the Surveillance, Epidemiology, and End Results (SEER) Program,” The Oncologist, Vol. 8, No. 6, 2003, pp. 541-552. doi:10.1634/theoncologist.8-6-541
- B. A. Quinn, N. Kasahara, F. Farzaneh, et al., “Recent Advances and Current Challenges in Tumor Immunology and Immunotherapy,” Molecular Therapy, Vol. 15, No. 6, 2007, pp. 1065-1071.
- P. W. Kantoff, C. S. Higano, N. D. Shore, et al., “Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer,” The New England Journal of Medicine, Vol. 363, 2010, pp. 411-422. doi:10.1056/NEJMoa1001294
- A. Pollack, “Approval for Drug That Treats Melanoma,” The New York Times, 25 March 2011.
- C. Butts, R. N. Murray, C. J. Smith, et al., “A Multicenter Open-Label Study to Assess the Safety of a New Formulation of BLP25 Liposome Vaccine in Patients with Unresectable Stage III Non-Small-Cell Lung Cancer,” Clinical Lung Cancer, Vol. 11, No. 6, 2010, pp. 391-395. doi:10.3816/CLC.2010.n.101
- J. Vansteenkiste, M. Zielinski, A. Linder, et al., “Final Results of a Multi-Center, Double-Blind, Randomized, Placebo-Controlled Phase II Study to Assess the Efficacy of MAGE-A3 Immunotherapeutic as Adjuvant Therapy in Stage IB/II Non-Small Cell Lung Cancer (NSCLC),” Journal of Clinical Oncology, Vol. 25, No. 18, 2007, p. 7554.
- P. Tyagi and B. Mirakhur, “MAGRIT: The Largest-Ever Phase III Lung Cancer Trial Aims to Establish a Novel Tumor-Specific Approach to Therapy,” Clinical Lung Cancer, Vol. 10, No. 5, 2009, pp. 371-374. doi:10.3816/CLC.2009.n.052
- B. T. Weinert, K. K. Krishnadath, F. Milano, et al., “RealTime PCR Analysis of Genes Encoding Tumor Antigens in Esophageal Tumors and a Cancer Vaccine,” Cancer Immunity, Vol. 9, No. 1, 2009, p. 9.
- M. J. Scanlan, A. J. Simpson and L. J. Old, “The Cancer/ Testis Genes: Review, Standardization, and Commentary,” Cancer Immunity, Vol. 4, No. 1, 2004, pp. 1-15.
- W. Sienel, I. Mecklenburg, S. Dango, et al., “Detection of MAGE-A Transcripts in Bone Marrow Is an Independent Prognostic Factor in Operable Non-Small-Cell Lung Cancer,” Clinical Cancer Research, Vol. 13, No. 13, 2007, pp. 3840-3847. doi:10.1158/1078-0432.CCR-06-2507
- S. Dango, X. T. Wang, M. Gold, et al., “Expression of Melanoma-Antigen-A (MAGE-A) in Disseminated Tumor Cells in Regional Lymph Nodes of Patients with Operable Non-Small Cell Lung Cancer,” Lung Cancer, Vol. 67, No. 3, 2010, pp. 290-295. doi:10.1016/j.lungcan.2009.04.012
- M. Sugita, M. Geraci, B. Gao, et al., “Combined Use of Oligonucleotide and Tissue Microarrays Identifies Cancer/Testis Antigens as Biomarkers in Lung Carcinoma,” Cancer Research, Vol. 62, No. 14, 2002, pp. 3971-3979.
- H. C. Toh, W. W. Wang, W. K. Chia, et al., “Clinical Benefit of Allogeneic Melanoma Cell Lysate-Pulsed Autologous Dendritic Cell Vaccine inMAGE-Positive Colorectal Cancer Patients,” Clinical Cancer Research, Vol. 15, 2009, pp. 7726-7736. doi:10.1158/1078-0432.CCR-09-1537
- P. Fayers, N. K. Aaronson, K. Bjordal, et al., “On Behalf of the EORTC Quality of Life Study Group. EORTC QLQ-C30 Scoring Manual (3rd Edition),” Brussels, EORTC Quality of Life Group 2001.
- N. Romani, S. Gruner, D. Brang, et al., “Proliferating Dendritic Cell Progenitors in Human Blood,” The Journal of Experimental Medicine, Vol. 180, No. 1, 1994, pp. 83- 93. doi:10.1084/jem.180.1.83
- S. K. Burgdorf, A. Fischer, M. H. Claesson, et al., “Vaccination with Melanoma Lysate-Pulsed Dendritic Cells, of Patients with Advanced Colorectal Carcinoma: Report from a Phase I Study,” Journal of Experimental & Clinical Cancer Research, Vol. 25, No. 2, 2006, pp. 201-206.
- P. Kvistborg, M. Boegh, A. W. Pedersen, et al., “Fast Generation of Dendritic Cells,” Cellular Immunology, Vol. 260, No. 1, 2009, pp. 56-62. doi:10.1016/j.cellimm.2009.09.003
- J. W. Fay, A. K. Palucka, S. Paczesny, et al., “Long-Term Outcomes in Patients with Metastatic Melanoma Vaccinated with Melanoma Peptide-Pulsed CD34(+) Progenitor-Derived Dendritic Cells,” Cancer Immunology, Immunotherapy, Vol. 55, No. 10, 2006, pp. 1209-1218. doi:10.1007/s00262-005-0106-6
- A. Hoos, G. Parmiani, K. Hege, et al., “Cancer Vaccine Clinical Trial Working Group. A Clinical Development Paradigm for Cancer Vaccines and Related Biologics,” Journal of Immunotherapy, Vol. 30, No. 1, 2007, pp. 1- 15.
- C. van der Lonchay, B. P. Connerotte, T. Hanagiri, et al., “Correlation between Tumor Regression and T Cell Responses in Melanoma Patients Vaccinated with a Mage Antigen,” Proceedings of the National Academy of Sciences, Vol. 101, No. 2, 2004, pp. 14631-1438. doi:10.1073/pnas.0405743101
- N. Bercovici, N. Haicheur, S. Massicard, et al., “Analysis and Characterization of Antitumor T-Cell Response after Administration of Dendritic Cells Loaded with Allogeneic Tumor Lysate to Metastatic Melanoma Patients,” Journal of Immunotherapy, Vol. 31, No. 1, 2008, pp. 101- 112. doi:10.1097/CJI.0b013e318159f5ba
- I. de Vries, W. J. Lesterhuis, N. M. Scharenborg, et al., “Maturation of Dendritic Cells Is a Prerequisite for Inducing Immune Responses in Advanced Melanoma Patients,” Clinical Cancer Research, Vol. 9, No. 14, 2003, pp. 5091-5100.
- I. de Vries, M. R. Bernsen, W. J. Lesterhuis, et al., “Immunomonitoring Tumor-Specific T Cells in DelayedType Hypersensitivity Skin Biopsies after Dendritic Cell Vaccination Correlates with Clinical Outcome,” Journal of Clinical Oncology, Vol. 23, No. 24, 2005, pp. 5779- 5787. doi:10.1200/JCO.2005.06.478
- F. O. Nestle, S. Alijagic, M. Gilliet, et al., “Vaccination of Melanoma Patients with Peptideor Tumor LysatePulsed Dendritic Cells,” Nature Medicine, Vol. 4, No. 3, 1998, pp. 328-332. doi:10.1038/nm0398-328
- J. D. Geiger, R. J. Hutchinson, L. F. Hohenkirk, et al., “Vaccination of Pediatric Solid Tumor Patients with Tumor Lysate-Pulsed Dendritic Cells Can Expand Specific T Cells and Mediate Tumor Regression,” Cancer Research, Vol. 61, No. 23, 2001, pp. 8513-8519.
- M. S. Mitchell, W. Harel, R. A. Kempf, et al., “ActiveSpecific Immunotherapy for Melanoma,” Journal of Clinical Oncology, Vol. 8, No. 5, 1990, pp. 856-869.
- M. Griffioen, M. Borghi, P. I. Schrier, et al., “Analysis of T-Cell Responses in Metastatic Melanoma Patients Vaccinated with Dendritic Cells Pulsed with Tumor Lysates,” Cancer Immunology, Immunotherapy, Vol. 53, No. 8, 2004, pp. 715-722. doi:10.1007/s00262-004-0514-z
NOTES
*Conflict of interests: Mai-Britt Zocca and Mogens H. Claesson are minor shareholders in DanDrit Biotech.

