World Journal of Vaccines
Vol.2 No.1(2012), Article ID:17227,5 pages DOI:10.4236/wjv.2012.21007
Clinical Outcome of Children with Post Bacillus Calmette and Guerin Vaccination Complications: A Single Center Experience*
![]()
Department of Pediatrics, Cairo University, Cairo, Egypt.
Email: Nermeengalal@gmail.com
Received August 23rd, 2011; revised October 21st, 2011; accepted November 4th, 2011
Keywords: BCG Related Complications; Primary Immunodeficiency
ABSTRACT
Background: Bacillus Calmette ET Guerin (BCG) vaccine, compulsory in endemic areas, remains the only available vaccine for prevention of Tuberculosis (TB) despite its modest protective value. Complications may arise in healthy/ immunocompromized hosts. Methods: Children presenting with BCG vaccine related complications in the form of local/distant complications were enrolled from 2007-2010 at Cairo University Pediatric hospital. Objectives: Assess outcome of BCG related complications in a group of children with post vaccination incidents, identify risk factors for complications among vaccinated children and identify cases of underlying Primary Immunodeficiency (PID) among presenting cases. Results: Fifty one eligible patients were included, forty three were proved immunocompetent, and eight had underlying primary immunodeficiency disorders. Presentations included localized axillary lymphadenopathy, cervical sinuses, granulomatous lesions and disseminated forms Faulty injection sites were strongly associated with complications (p value < 0.001). Patients without underlying PID had larger scar size and younger age at presentations (p values: 0.02, 0.0001 respectively).Resolution of lesions was observed in 97% (95% CI 97% ± 3%) of cases without underlying PID versus fatal outcome in all cases with underlying immune defects. Conclusion: Local BCG related complications do not necessarily indicate underlying PID, disseminated complications are more serious and warrant further investigations. If PID is suspected, vaccination should be deferred to avoid its potentially fatal outcome.
1. Introduction
Bacillus Calmette Guerin (BCG) vaccine, compulsory in endemic areas, remains the only available vaccine for prevention of Tuberculosis (TB) despite attempts to produce newer generations [1]. The overall protective effect of BCG in preventing TB was described in a Meta analysis as 51% with a value of 78% against pulmonary and disseminated TB, 64% against tuberculous meningitis and 71% against death [2] though it does not prevent primary infection and, more importantly, does not prevent reactivation of latent pulmonary infection.
Egypt has achieved better treatment success and case detection rates according to the World Health Organization (WHO) bringing the prevalence rate from 40 to 23 cases per 100 000 per year [3].
The vaccination dose is 0.05 ml and is administered intradermally. Usually, two weeks after vaccination, a small papule develops which, over a period of 6 weeksenlarges and ulcerates. It heals with a scar. Nonspecific complications such as keloid, eczema, maculopapular rash, erythema nodosum, infections, poor healing, ulceration, and abscess formation can occur after the vaccination. The specific complications include development of lupus vulgaris, verrucous tuberculosis, tuberculous chancre, lymphadenitis, and Koch phenomenon [4].
The clinical response to BCG is primarily mediated by cellular immunity and therefore BCG is contraindicated in T cell or combined defects as it may cause disseminated infections in the form of infiltrating and ulcerating lesions at the impact of the vaccination and in the regional lymph nodes, papular cutaneous lesions, osteolytic lesions and or organ impairment of liver, spleen, lymph-node and lung [5].
The study aims at assessing the outcome of BCG related complications in a group of children with post vaccination incidents, identify risk factors for complications among vaccinated children and identify cases of primary immunodeficiency among presenting cases.
2. Material and Methods
Setting: The study was conducted at Cairo University Specialized Pediatric Hospital which serves as a tertiary referral hospital serving greater Cairo and neighboring governates.
Subjects: participants were enrolled after informed consents were obtained from parents from 2007 through 2010. Approval was obtained from Cairo University Pediatric research committee.
Inclusion criteria:
Children presenting with BCG vaccine related complications in the form of:
1) Localized (Injection site-related).
Ipsilateral axillary lymphadenopathy following BCG vaccination;
Cervical sinuses related to tuberculous lymph nodes;
Granulomatous Tuberculous lesions (evidenced by fine needle aspiration/excision biopsy/Polymerase chain reactions).
2) Disseminated-Distant or Spreading Tuberculous infections following vaccination found in distant lymph nodes, lung, bone, gastrointestinal tract involvement and or other sites.
Confirmation of TB infection was done by cultures, biopsy and Polymerase Chain Reaction (PCR) testing whenever possible.
Data collection: Comprehensive history and thorough examination were done with emphasis on vaccination information (through history and vaccination cards yet information could not be verified).
Immune functions were assessed by complete blood counts, nitro blue tetrazolium reduction test, T, B and NK cell subsets by flow cytometry (using FACSE Pics, coulter) CD3, CD4, CD8, CD56, CD19, immunoglobulins (Radio immunoassay), and human immunodeficiency virus serology (HIV) status when indicated.
Treatment was received by patients with disseminated disease in the form of triple therapy (Isoniazide, Rifampicin, and Streptomycin); a decision to start treatment for local complications was taken on a case by case basis especially with large lesion size and or sinus complications.
Monthly follow up was started with emphasis on the following outcomes:
Resolution of BCG related complications/Mortality/ Residual disease.
Cases were categorized into Group A: Immunocompetent with BCG complications, Group B: Immunocompetent without BCG complications (uneventful vaccination), Group C: Immunodeficient with BCG complications and Group D: Immunodeficient without BCG complications (vaccinated prior to diagnosis).
Primary immunodeficiency disorders were diagnosed according to International Union of Immunological Societies (IUIS) criteria [6].
3. Results
Between 2007-2010 fifty one eligible cases presenting with BCG related complications were enrolled, forty three were proved immune competent (Group A). A comparable age and sex matched immune competent group, who received BCG vaccine uneventfully, were assessed for comparison (Group B). Eight cases within the study group were proved to have underlying immune defects (Group C). A group of patients (30 patients) with underlying T cell immune deficiency who received BCG vaccine and developed no complications (relevant to BCG) were also evaluated (Group D).
Age at presentation with BCG complications ranged between 2 and 12 months with a mean value of 2.2 months (Standard Deviation 1.7). There were 28 males and 22 females in the study population. Age at vaccination had a median value of 30 days (range day 3 to 2 months).There was no significant difference between the different groups regarding age/sex/age at vaccination. Anthropometric measurements were observed to be significantly lower for age in Groups C and D with a p value of 0.003 and 0.005 respectively. Vaccination was received at the immunization health offices in 98% of cases versus 2% in private clinics.
Scar of vaccination was observed to be higher than deltoid insertion (close to the shoulder tip) in group A when compared to other groups with a p value of 0.001. Presentations of BCG related complications included localized (Injection site-related) ipsilateral axillary Lymphadenopathy (n = 34 with size ranging from 5 to 30 mm mean 12mm SD 5.1.), cervical sinuses related to lymph nodes (n = 9) and granulomatous lesions (n = 26). Patient characteristics with BCG complications with and without underlying PID are represented in Table 1.
Disseminated tuberculous infections following vaccination were observed in all cases with PID and two patients without PID who developed generalized lymphadenopathy which responded to treatment. Patient profiles with underlying immune defects are presented in Table 2.
Outcome Measures
In the healthy group outcome was resolution in 97% (95% CI 97% ± 3%), whereas the remaining three percent still had lymphadenopathy, no deaths were recorded. As for children with PID mortality was reported in all cases however death could not be solely attributed to BCG related complications.
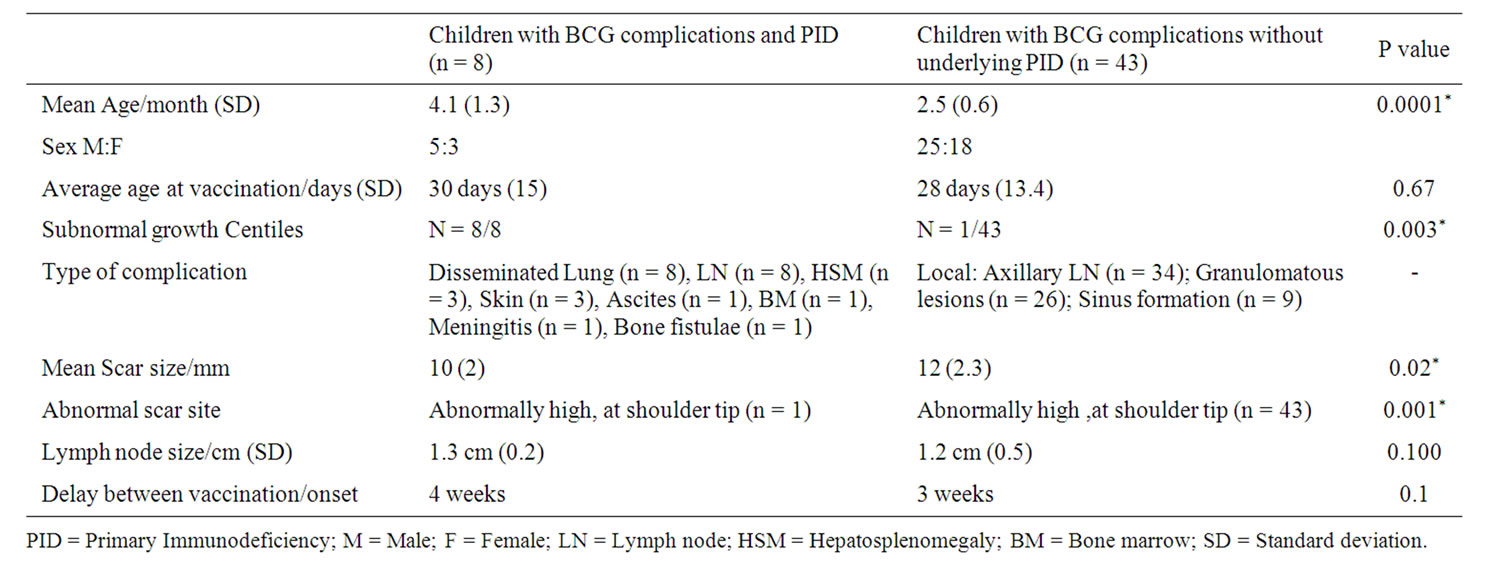
Table 1. Characteristics of patients in the groups with and without underlying primary immunodeficiency disorders (PID).
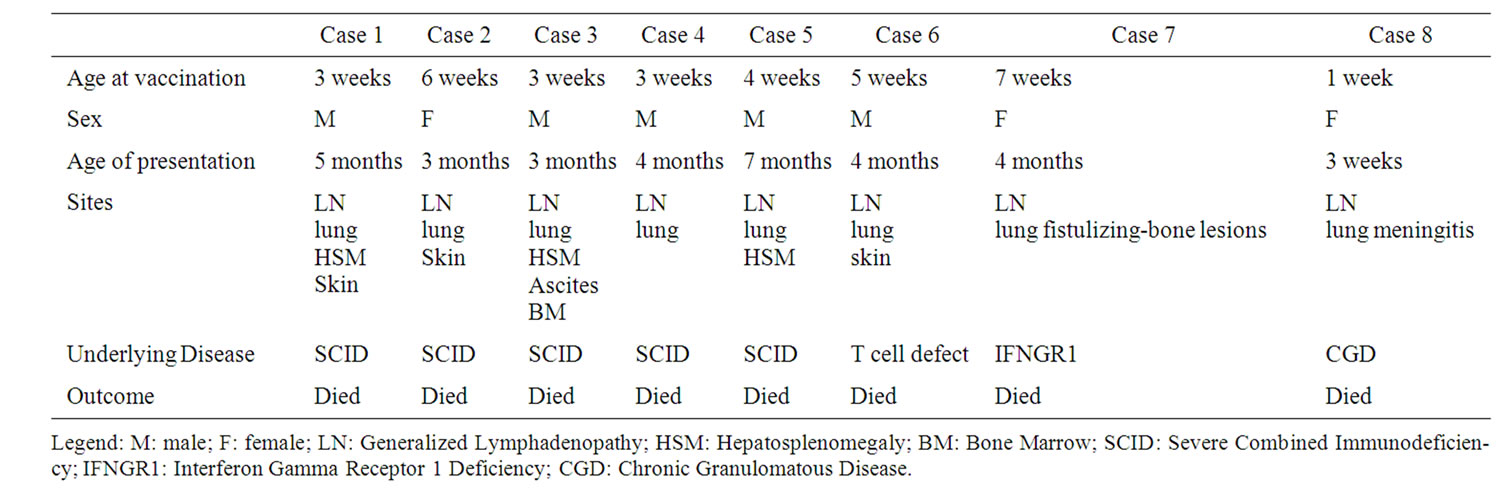
Table 2. Patients’ profiles with disseminated BCG-related complications (Group C).
The average duration till resolution of symptoms was equal to 3 months in local complications.
The likelihood ratio of having underlying PID in BCGosis affected cases equals 0.11.
4. Discussion
There are growing numbers of children presenting with local BCG related complications presenting to paediatricians. The annual rates of BCG complications in vaccinated infants was reported as (19.1/1000) at the United Nations Relief in Gaza strip [7] however no recent data was found for Egypt or neighboring countries describing complications rate. There was no relation between the age at administration of vaccine and complications as cases in all groups were vaccinated around third or fourth week of life, other studies reported a statistically significant difference where BCG was administered during the first week of life [8]. The average interval between vaccination and complications equaled 3 weeks in group A (without PID) as opposed to 4 weeks in group C (underlying PID). A statistically significant difference was noted in the age of presentation where cases without underlying defects had an earlier presentation probably because of the speculated high doses/faulty administration sites.
The site of vaccine administration as evidenced by the scar was significantly higher than that indicated by guidelines at the deltoid insertion in affected cases. Other Factors that may influence the development of adverse reactions to BCG include the potency and dose of the vaccine strain, the route of delivery, the age and immune status of the host, and the skill levels of the operator administering the vaccine [9,10].
The mean size of skin lesions was 12 mm; sizes up to 210 mm have been reported [11]. Sinus formation was related to attempts at surgical drainage for lesions. Confirmation of vaccine doses, strains and technique of administration could not be assessed in all cases and is recognized as a study limitation.
Regarding outcome, resolution was noted in 97% of the group with local complications (3% with residual lymphadenopathy) and zero mortality in contrast to 100% mortality in the disseminated group with underlying immune defects. Disseminated BCG related complications were suggested as an alert for underlying immune defects whether Human Immunodeficiency virus or primary immunodeficiencies [12].
In our study immune defects included Severe combined immunodeficiency (SCID), Chronic Granulomatous disease (CGD) and isolated T cell lymphopenia with a fatal outcome for all cases however death could be attributed solely to TB in the CGD case who died of tuberculous meningitis and IFNGR1 patient who had no other infections. SCID cases suffered other several infections which might have caused death. A study conducted in Tehran testing immunological function for children presenting with BCG complications reported death in all cases with underlying PID .[13]
The course of complications might extend into several years even in the absence of underlying immune defects as reported by Suthermann and colleagues [4]. In our series the case with IFNGR1 deficiency was vaccinated at 7 weeks of age, manifested at 4 months and suffered extensive spreading lesions over the course of several years.
Choice of BCG vaccination policy should depend upon tuberculosis rates in the locality as well as changing recommendations and policies for treatment according to infection type [14]. Shift from universal to targeted screening might be associated with decreased coverage in high risk children [15].
Though BCG remains compulsory, cases with family history suggestive of PID should be screened prior to vaccination especially with the expected existence of autosomal recessive forms favored by high consanguinity and the fatal outcome observed. Another aspect that needs to be addressed is proper training of personnel in charge of vaccination to standardize method of administration and dose.
5. Conclusion
BCG related complications exist in two forms; local and disseminated. Local forms have a better outcome and may be minimized by proper training of personnel. Disseminated forms may indicate underlying immunodeficiency and vaccination should be deferred in suspected children for its potentially fatal outcome.
REFERENCES
- K. B. Walker, M. J. Brennan, M. M. Ho, J. Eskola, G. Thiry, J. Sadoff, R. Dobbelaer, L. Grode, M. A. Liu, U. Fruth and P. H. Lambert, “The Second Geneva Consensus: Recommendations for Novel Live TB Vaccines,” Vaccine, Vol. 28, No. 11, 2010, pp. 2259-2270. doi:10.1016/j.vaccine.2009.12.083
- G. A. Colditz, C. S. Berkey, F. Mosteller, T. F. Brewer, M. E. Wilson, E. Burdick and H. V. Fineberg, “The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-Analyses of the published literature,” Pediatrics, Vol. 96, No. 1, 1995, pp. 29-35.
- World Health Organization (WHO), “The Work of WHO in the Eastern Mediterranean Region: Annual Report of the Regional Director,” 2009.
- G. Sethuraman, V. Ramesh, M. Ramam and V. Sharma, “Skin Tuberculosis in Children: Learning from India,” Dermatologic Clinics, Vol. 26, No. 2, 2008, pp. 285-294. doi:10.1016/j.det.2007.11.006
- N. Rezaei, A. Aghamohammadi and L. Notarangelo, “Primary Immunodeficiency Diseases: Definition, Diagnosis, and Management,” Springer, Berlin, 2008.
- R. S. Geha, L. D. Notarangelo, J. L. Casanova, H. Chapel, M. E. Conley, A. Fischer, L. Hammarström, S. Nonoyama, H. D. Ochs, J. M. Puck, C. Roifman, R. Seger and J. Wedgwood, “Primary immunodeficiency diseases: an update from the International Union of Immunological Societies Primary Immunodeficiency Diseases Classification Committee,” The Journal of Allergy and Clinical Immunology, Vol. 120, No. 4, 2007, pp. 776-794. doi:10.1016/j.jaci.2007.08.053
- R. Awad, “BCG vaccine and post-BCG complications among infants in Gaza Strip, 1999,” Eastern Mediterranean Health Journal, Vol. 7, No. 1-2, 2001, pp. 211-220.
- WHO, “Regional Epidemiological Data on Tuberculosis,” Eastern Mediterranean Health Journal, Vol. 2, 1996, pp. 164-166.
- N. Ritz, “Too much of a good thing: management of BCG vaccine overdose,” Vaccine, Vol. 27, No. 41, 2009, pp. 5562-5564. doi:10.1016/j.vaccine.2009.07.043
- D. Murphy, “Adverse reactions to Mycobacterium bovis bacille Calmette-Guérin (BCG) vaccination against tuberculosis in humans, veterinary animals and wildlife species,” Tuberculosis, Vol. 88, No. 4, 2008, pp. 344- 357. doi:10.1016/j.tube.2007.11.010
- K. Farsinejad, “Lupus vulgaris at the site of BCG vaccination: report of three cases,” Clinical and Experimental Dermatology, Vol. 34, No. 5, 2009, pp. e167-e169. doi:10.1111/j.1365-2230.2008.03041.x
- A. Santos, “Severe axillary lymphadenitis after BCG vaccination: alert for primary immunodeficiencies,” Journal of Microbiology, Immunology and Infection, Vol. 43, No. 6, 2010, pp. 530-537. doi:10.1016/S1684-1182(10)60082-5
- M. Sadeghi-Shabestari and N. Rezaei, “Disseminated bacille Calmette-Guérin in Iranian children with severe combined immunodeficiency,” International Journal of Infectious Diseases ,Vol. 13, No. 6, 2009, pp. e420-e423. doi:10.1016/j.ijid.2009.02.008
- D Shingadia and H. Baumer, “Tuberculosis, diagnosis, prevention and management,” Archives of Disease in Childhood Education & Practice, Vol. 92, No. 1, 2007, pp. 27-29. doi:10.1136/adc.2006.110577
- J. P. Guthmann, D. Antoine, L. Fonteneau, D. Che and D. Lévy-Bruhl, “Assessing BCG vaccination coverage and incidence of paediatric tuberculosis following two major changes in BCG vaccination policy in France,” Eurosurveillance, Vol. 16, No. 12, 2011.
NOTES
*Conflict of interest: The author declares no conflict of interest. Funding: No funding was received during the study.

