World Journal of Nano Science and Engineering
Vol.3 No.3(2013), Article ID:36678,6 pages DOI:10.4236/wjnse.2013.33012
Comparison of Acidic and Polymeric Agents in Synthesis of TiO2 Nanoparticles via a Modified Sol-Gel Method
Department of Physics, Vali-E-Asr University of Rafsanjan, Rafsanjan, Iran
Email: masoud.karimipour@gmail.com
Copyright © 2013 Masoud Karimipour. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received July 11, 2013; revised August 15, 2013; accepted August 21, 2013
Keywords: Sol-Gel Method; Photo Catalyst; Rutile; Anatase; Complex Agent
ABSTRACT
TiO2 nano particles were synthesized in Rutile and Anatase phases by sol-gel method using two kind of complex agents, acidic (Citric Acid) and organic complex agent (acetyl acetone) at 400˚C, 500˚C, 650˚C sintering temperatures. The structural analysis by XRD diffraction confirmed phase formation of titanium oxide. Particles sizes were determined by using Scherrer formula. TEM was employed to confirm nano particles formation. The size of nano particles as well as Phase formation can be controlled by the type of complex agent and sintering temperature. Acetyl Acetone causes a more crystalline structure and more uniformity of size distribution in 400˚C sintering temperatures. Moreover, it results in obtaining single phase TiO2 nanoparticles at 400˚C and 650˚C sintering temperature. On the other hand, at high sintering temperature, the particles obtained from polymeric agent tend to agglomerate larger in size than the acidic product.
1. Introduction
Titanium dioxide which is known as Titania occurs in four forms: Rutile (Eg = 3.0 eV), Anatase (Eg = 3.2 eV), Brookite (Eg = 2.96 eV) and TiO2-β. Brookite and Anatase phases are rare and have more photocatalytic activity than Rutile phase [1]. TiO2 is desirable due to its inertness, stability, and low cost [2]. It is also self regenerating and recyclable. Its redox potential of the H2O/*OH couple (−2.8 eV) lies within the band gap. However, its large band gap (Eg = 3.2 eV) only allows the UV absorption of the solar spectrum. Titania nano particles are highly considered for the technological and industrial applications because of its photo catalytic activities [3], such as self-cleaning [4], anti bacterial [5], fog proofing [6]; and also, as a new source of energy saving in dye sensitized solar cells [7-9]. Titania nano particles are prepared by many techniques such as sol-gel [10], hydrothermal [11] and co-precipitation processes [12]. But, there are still many ongoing researches [13] to develop new features and simplification of nanoparticles synthesis within a desired size distribution. In this study, we used a simple Pechini sol-gel process [14,15] with two different complex agents in order to study the role of polymeric and non-polymeric agents in phase purity and size formation.
2. Experimental Details
The titanium oxide nano particles have been prepared by Sol-gel process in two steps. Titanium isopropoxide (TTIP) was used as precursor. Acetyl acetone (AcAc: CH3COCH2COCH3) and acetic acid (HOC(COOH)(CH2COOH)2) were used as both complex and polymer agent and finally absolute ethanol as solvent. In first step, starting solution was prepared with diluting 0.1 M TTIP by 40 cc absolute ethanol with the same volume ratio and then mixed with 0.1 M AcAc (Citric Acid) and stirred with magnet for 1 hr in 40˚C to establish initial sol. This sol was red (yellow)-color and pH = 6.5 (3.0). For gaining transparent sol witch has nano particles of Titania as complex compounds, the sol was refluxed in oil bath at 130˚C for 4 h. After this step, sol was orange(yellow-green)-color and pH = 8.4(6.0). In the next step, we used oil bath to heat the sol, indirectly at T = 80˚C for 36 hrs until forming gel. The gel firstly was dried up by direct heating on hot plate at 120˚C for 2hrs and then for final drying, the gel was heated at 150˚C for 30 minutes. The titanium oxide nano particles cannot be obtained unless the dried gel calcinated at 400, 500, 650˚C for 1 hr. the weight of powders measured before and after calcinations that the results have come in Tables 1 and 2.
The X-ray diffractometer (D8 Advance Bruker), with
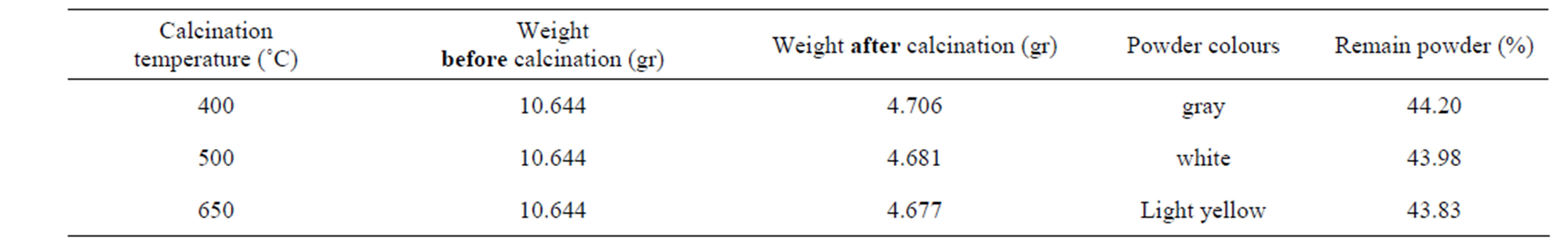
Table 1. Powder prepared by sol-gel process using AcAc as complex and polymer agent.
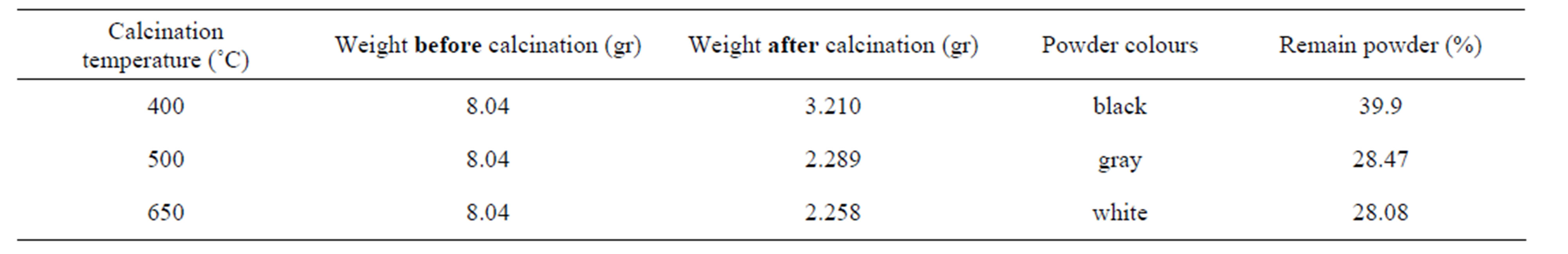
Table 2. Powder prepared by sol-gel process using citric acid as complex and polymer agent.
Cu-Kα radiation of wavelength 1.5405 Å (operated at 25 kV, 20 mA) was employed for structural studies. TEM was employed for nano particles size analysis. The UvVis spectra were prepared by spectrophotometer (Agilent 8453) for determining band gap of nano particles.
3. Results and Discussion
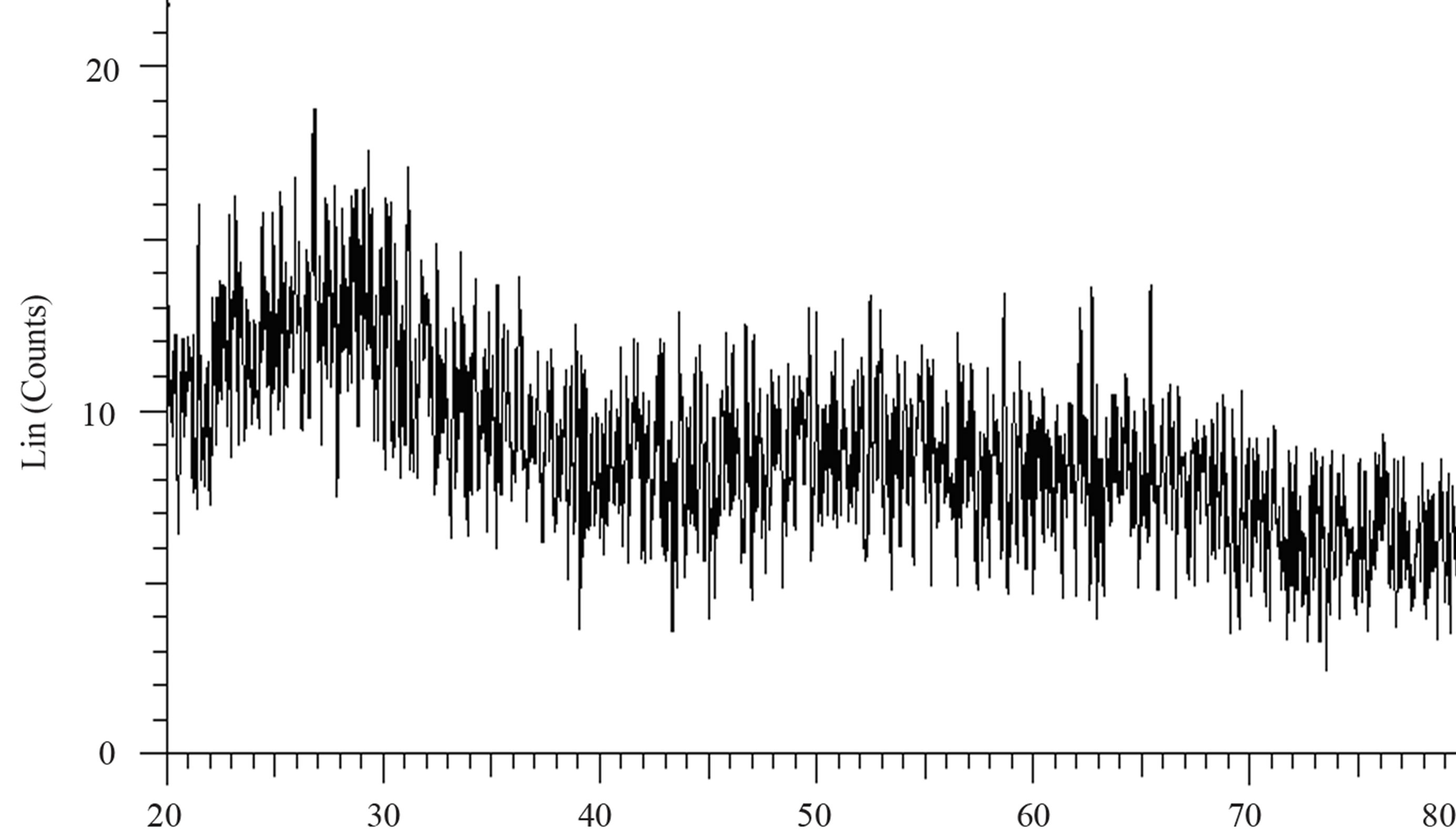
Structural characterization: the XRD patterns of titanium oxide nano particles (Figures 1 and 2) show Rutile and Anatase phases formation. The (101)-plane for Anatase and (110)-plane for Rutile are the main diffraction planes seen in the figures. the mean crystalline size of nanoparticles is estimated using scherrer formula, and Anatase/Rutile phase ratio also is determined using Equation (1) [11] in which, xA is the Anatase phase contribution in the powder, IR and IA are the intensity of the highest peaks of Rutile and Anatase in XRD patterns, respectively.
 (1)
(1)
Figure 1(a) show phase formation of Anatase and small crystalline size (Table 3) in lowest sintering temperature, which may due to using organo-metalic precursor and organic complex agent, it allows Ti-O bonds formation and postpones the condensation process rather than hydrolysis [12]. Comparison of Tables 3 and 4 shows that in the sintering temperature at which both Anatase and Rutile phases coexist, AcAc case results in a uniform crystalline growth process other than Citric Acid one. The observation of mixed Anatase and Rutile phases at 500˚C (Figure 1(b)) and Rutile phase at 650˚C (Figure 1(c)) indicates phase transition of Anatase to Rutile. Moreover, application of AcAc as complex agent results in obtaining single phase TiO2 nanoparticles in 400˚C and 600˚C sintering temperatures. Actually powders did not show any crystalline phase below 400˚C sintering tem-
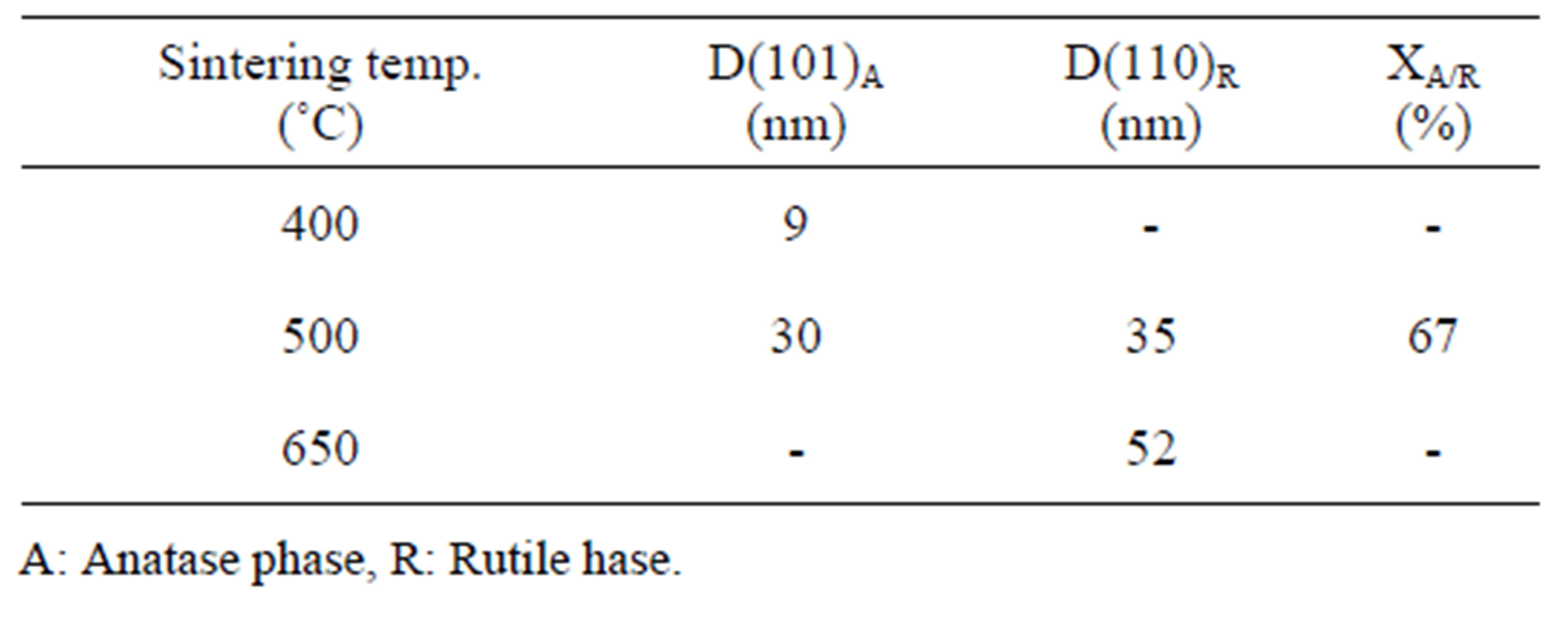
Table 3. Nano crystalline size of calcined powders prepared by AcAc as complex agent.
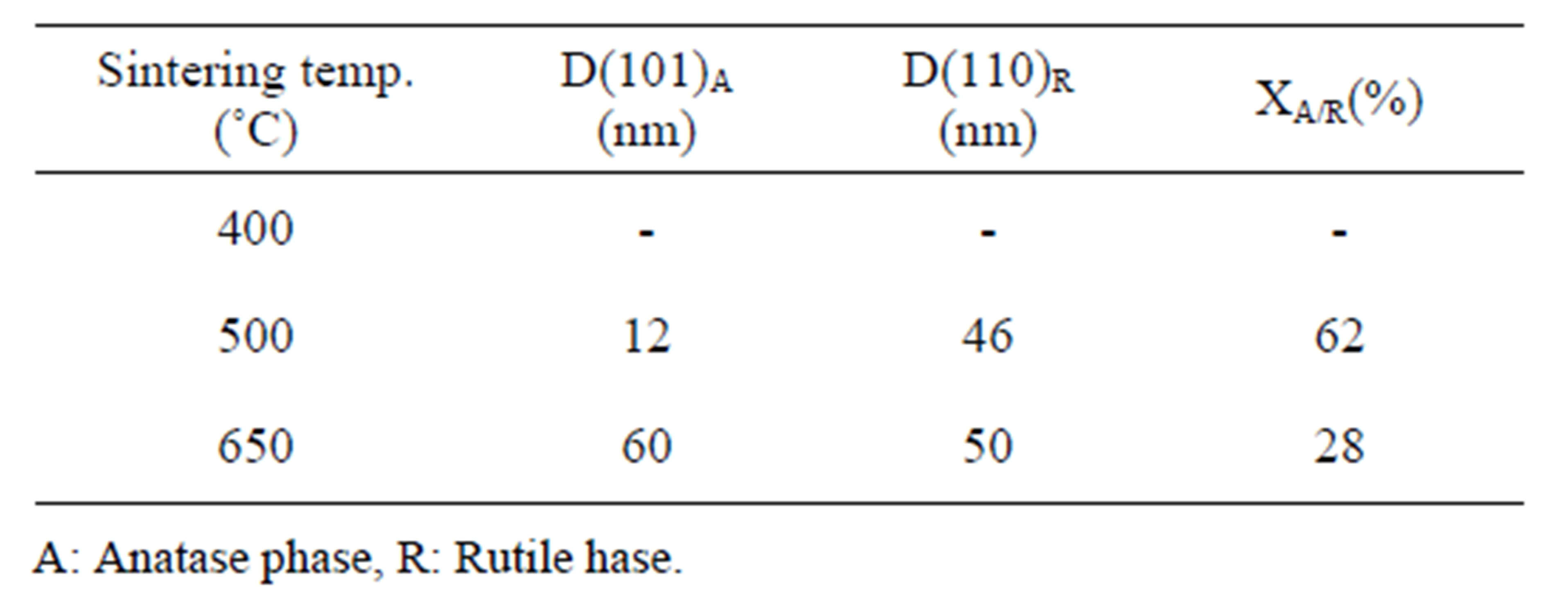
Table 4. Nano crystalline size of calcined powders prepared by citric acid as complex agent.
perature. thus, comparing citric acid and AcAc chemical formula one can be said that the lower the amount of carbon existing in the dried powders, the lower the sintering temperature at which phase formation occurs.
TEM images (Figures 3 and 4) show that with increasing of sintering temperature, agglomeration of nano particles has been occurred. Acetyl Acetone causes a more crystalline structure and more uniformity of size distribution in 400˚C sintering temperatures. On the other hand, in highest sintering temperature, the particles obtained from polymeric agent tends to agglomerate larger in size than the acidic one.
Optical characterization: Using Uv-Vis spectra and Tauc formula, The optical absorption edge was analyzed by the following relationship [16], αhν = A (hν − Eg)m where A is a constant, m value is respectively 1/2 and 2
 (a)
(a) (b)
(b)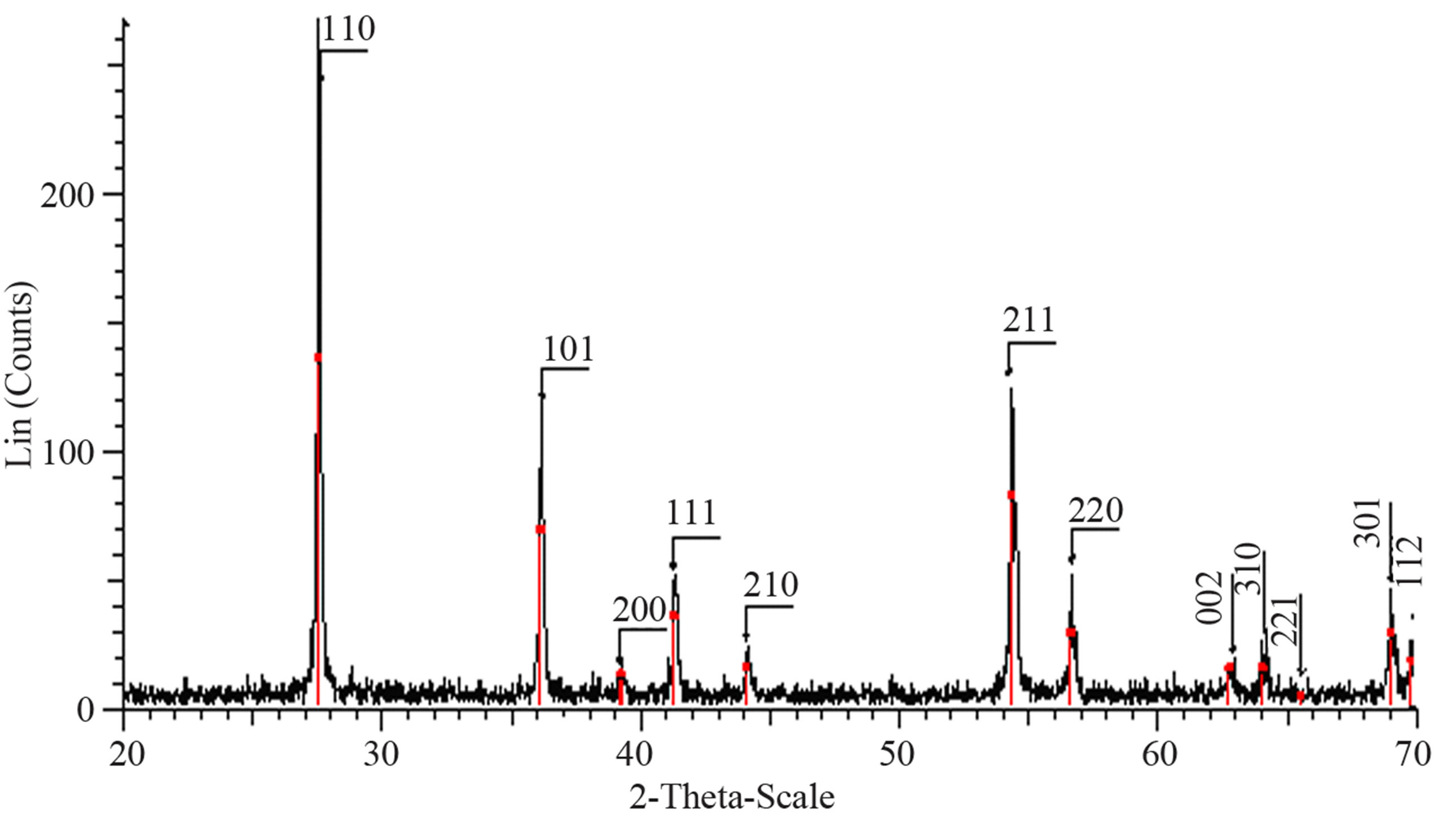 (c)
(c)
Figure 1. XRD pattern of nano powder prepared by AcAc as complex agent, calcined at (a) T = 400˚C, (b) T = 500˚C, (101) red-peak and (110) blue-peak implies to Anatase and Rutile phases, respectively, and (c) T = 650˚C.
 (a)
(a) (b)
(b)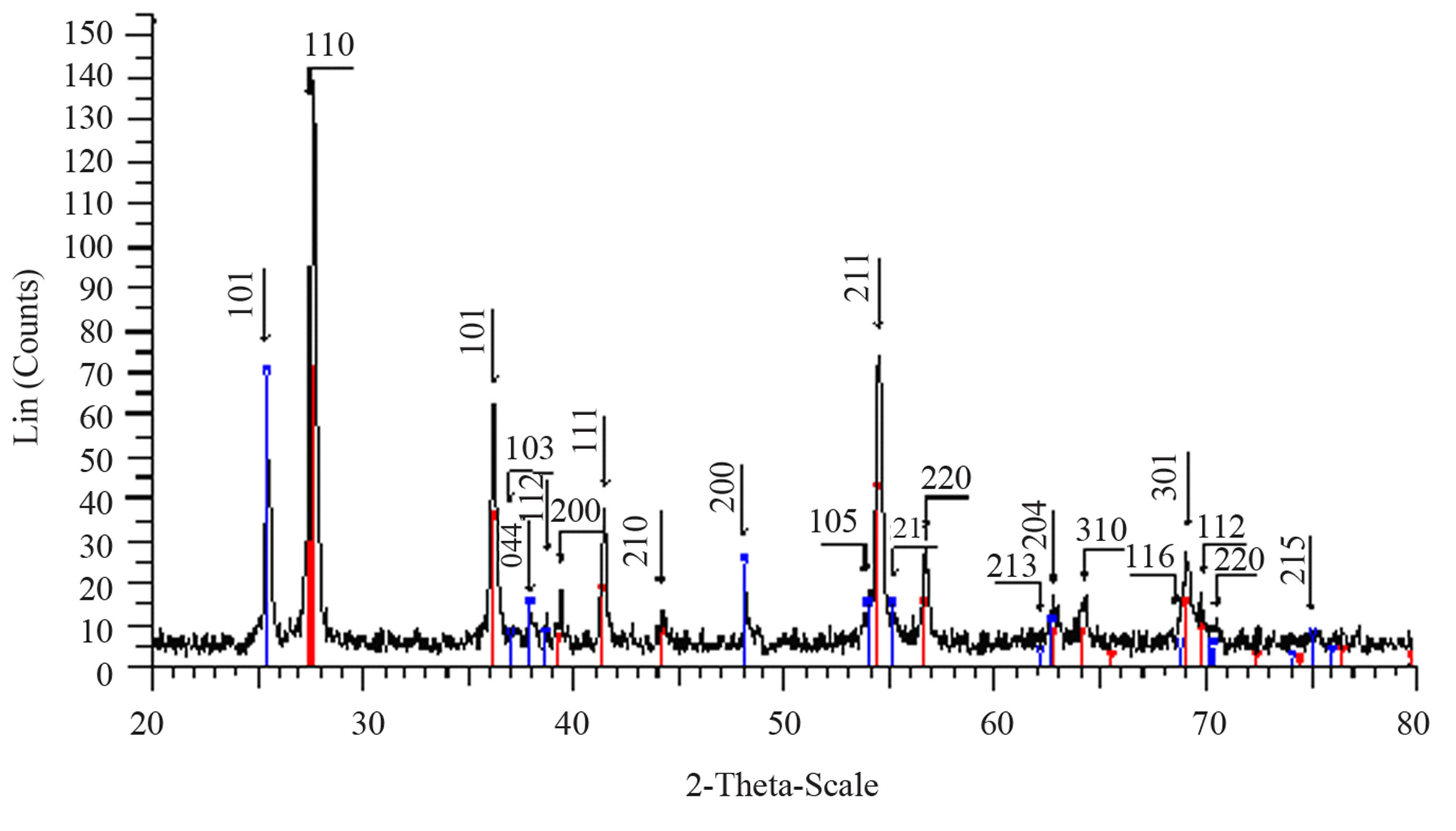 (c)
(c)
Figure 2. XRD pattern of nano powder prepared by citric acid as complex agent, calcined at (a) T = 400˚C, (b) T = 500˚C, (101) red-peak and (110) blue-peak implies to Anatase and Rutile phases, respectively, and (c) T = 650˚C.
 (a)
(a)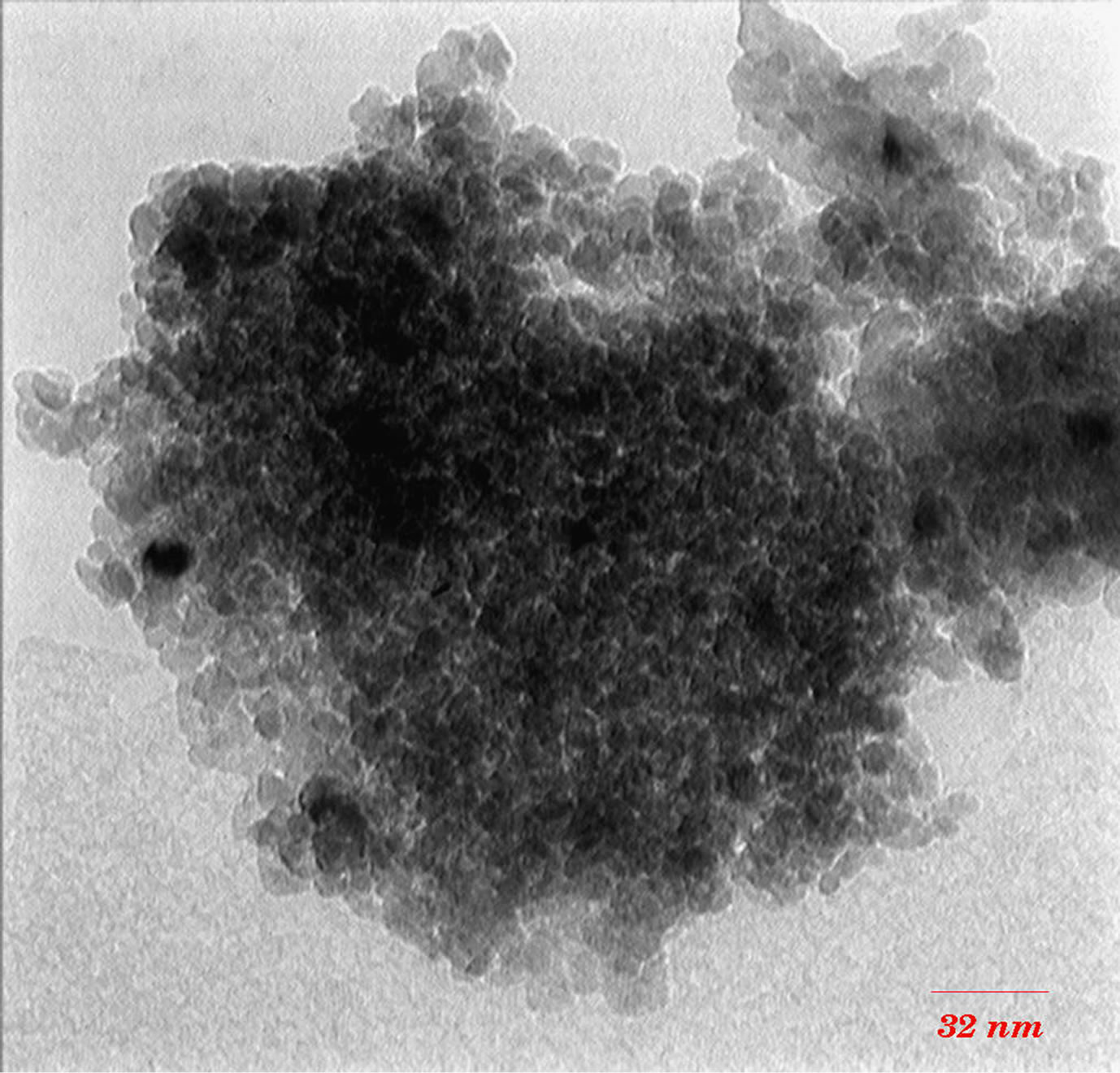 (b)
(b)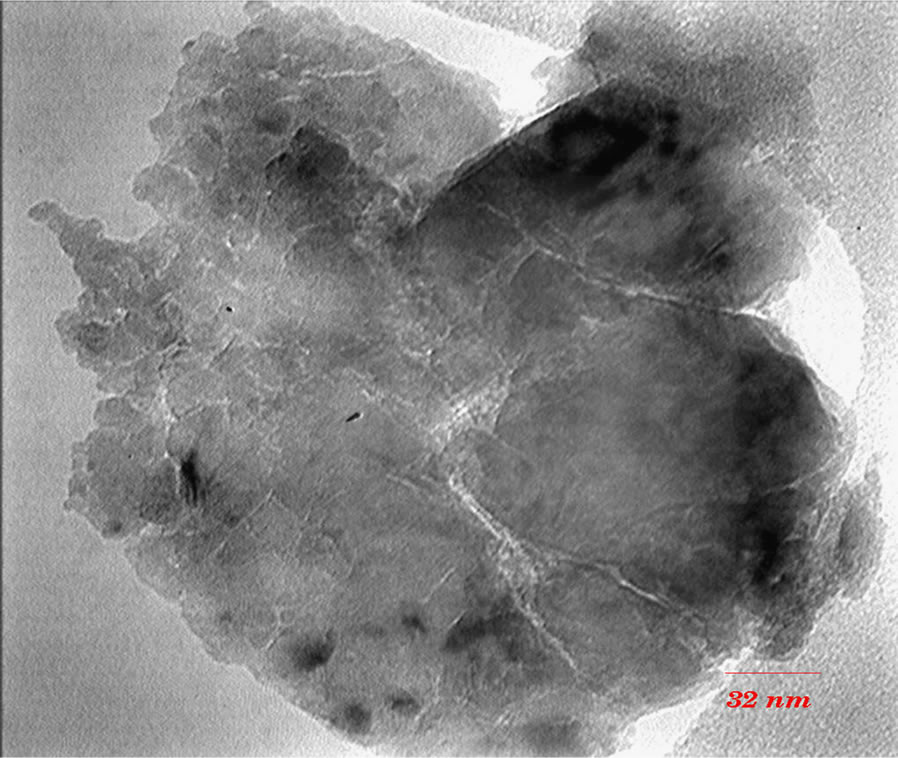 (c)
(c)
Figure 3. TEM images of nano powder prepared by AcAc as complex agent, calcined at (a) T = 400˚C, (b) 500˚C, (c) 650˚C.
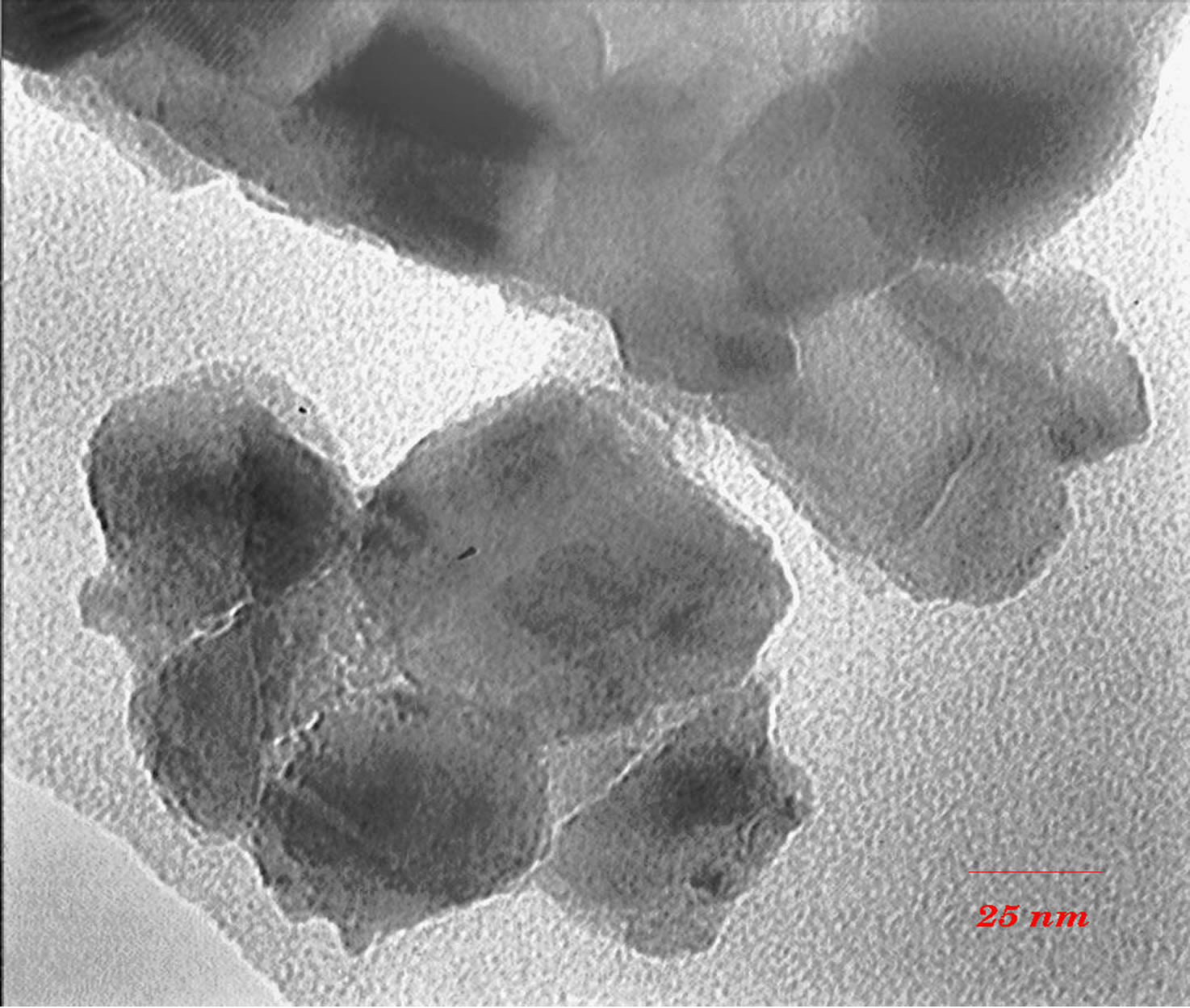 (a)
(a)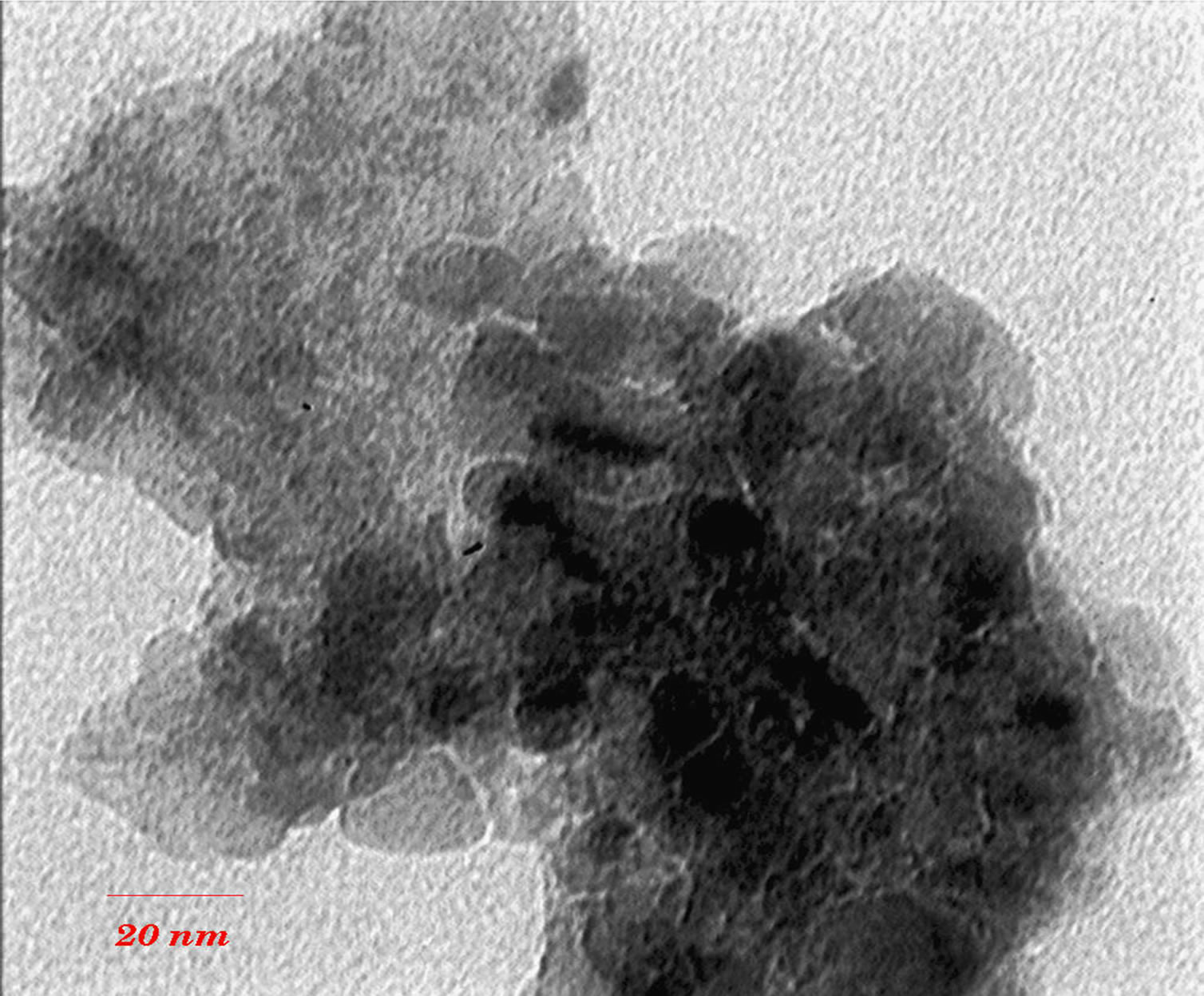 (b)
(b)
Figure 4. TEM images of nano powder prepared by citric acid as complex agent, calcined at (a) T = 500˚C, (b) 650˚C.
for direct and indirect transitions and Eg is the optical band gap. We estimated the direct energy band gap of nano particles. The optical results show that nano particles with smaller size which sintered at 500˚C have smaller band gap rather than nano particles which sintered at 650˚C (Figure 5). This may be due to existing of traps that lies within the band gap. Nano particles that have been sintered at 500˚C which contain two phases (Anatase and Rutile) have more traps rather than those who sintered at 650˚C.
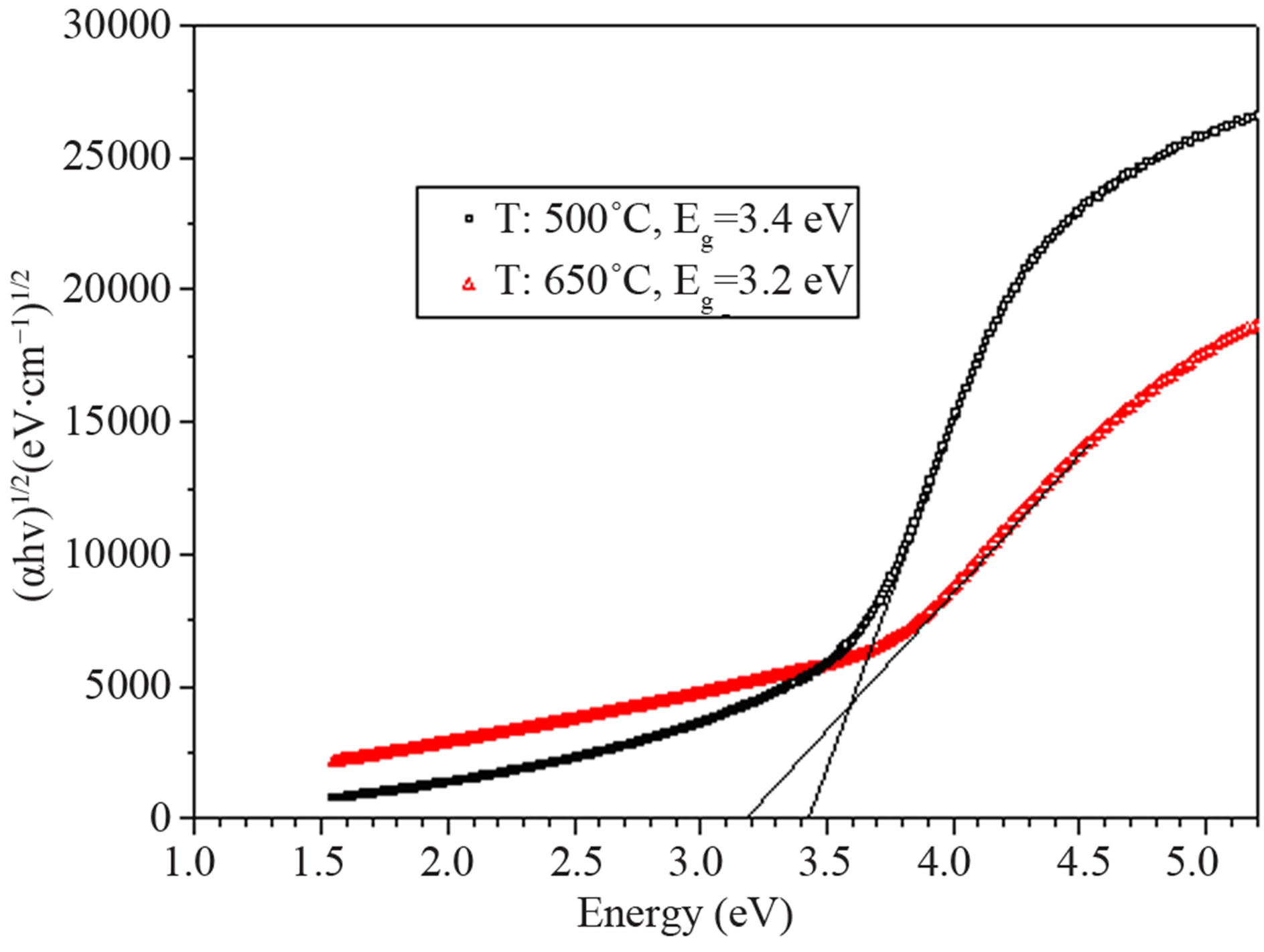
Figure 5. energy gap of nano particles prepared with solgel process and using AcAc complex agent (T: sintering temperature).
4. Conclusion
TiO2 nanoparticles have been synthesized simply via complexing-polymerizing sol-gel method. The effect of polymer agent on size distribution of particles shows that AcAc is a proper candidate to synthesize particles in pure Anatase phase below 10 nm. Also, sintering at 650˚C shows pure Rutile phase with narrow size distribution about 100 nm. Moreover, application of Citric acid gives rise to a less agglomeration of particles after annealing at 650˚C which does not have pure phase.
REFERENCES
- K. Mori, KONA, “Photo-Functionalized Materials Using Nanoparticles: Photocatalysis,” KONA, Vol. 3, No. 23, 2005.
- D. M. Blake, P. C. Maness, Z. Huang, E. J. Wolfrum and J. Huang, “Application of the Chemistry of Titanium Oxide to Disinfection and the Killing of Cancer Cells,” Separation and Purification Methods, Vol. 28, 1999, pp. 1-50.
- M. O. Abou-Helal and W. T. Seeber, “Preparation of TiO2 Thin Films by Spray Pyrolysis to Be Used as a PhotoCatalyst,” Applied Surface Science, Vol. 195, No. 1-4, 2002, pp. 53-62. doi:10.1016/S0169-4332(02)00533-0
- A. Zaleska “Doped-TiO2: A Review,” Recent Patents on Engineering, Vol. 2, No. 3, 2008, pp. 157-164. doi:10.2174/187221208786306289
- Y. L. Jeyachandran, S. K. Narayandass, D. Mangalaraj, C. Y. Bao and P. J. Martin, “The Effect of Surface Composition of Titanium Films on Bacterial Adhesion,” Biomedical Materials, Vol. 1, No. 1, 2006. doi:10.1088/1748-6041/1/1/L01
- H. Irie, Y. Watanabe and K. J. Hashimoto, “NitrogenConcentration Dependence on Photocatalytic Activity of TiO2-xNx Powders,” The Journal of Physical Chemistry B, Vol. 107, No. 23, 2003, pp. 5483-5486. doi:10.1021/jp030133h
- M. Okuya, K. Nakade, D. Osa, T. Nakano, G. R. A. Kumara and Sh. Kaneko, “Fabrication of Dye-Sensitized Solar Cells by Spray Pyrolysis Deposition (SPD) Technique,” Journal of Photochemistry and Photobiology A: Chemistry, Vol. 164, No. 1-3, 2004, pp. 167-172.
- P. Cameron and L. Peter, “Compact TiO2 Blocking Layers in Dye Sensitized Nanocrystalline Solar Cells,” The Journal of Physical Chemistry B, Vol. 104, No. 5, 2000, pp. 949-958. doi:10.1021/jp993220b
- A. Heft, T. To. lke, A. Pfuch and C. Erbe, “Photocatalytically Active Thin Films on Float Glass with Enhanced Hydrophilicity and Transmission for Photovoltaic Applications,” Solar Energy Materials & Solar Cells, Vol. 90, No. 17, 2006, pp. 2846-2854. doi:10.1016/j.solmat.2006.04.008
- W. Chen, X. D. Sun and D. Weng, “Morphology Control of Titanium Oxides by Tetramethylammonium Cations in Hydrothermal Conditions,” Materials Letters, Vol. 60, No. 29-30, 2006, pp. 3477-3480. doi:10.1016/j.matlet.2006.03.035
- Ch. Wang and J. Y. Ying, “Sol-Gel Synthesis and Hydrothermal Processing of Anatase and Rutile Titania Nano Crystals,” Chemistry of Materials, Vol. 11, No. 11, 1999, pp. 3113-3120.
- Z. Tang, J. Zhang, Zh. Cheng and Zh. Zhang, “Synthesis of Nanosized Rutile TiO2 Powder at Low Temperature,” Materials Chemistry and Physics, Vol. 77, No. 2, 2002, pp. 314-317. doi:10.1016/S0254-0584(02)00003-2
- X. B. Chen and S. S. Mao, “Titanium Dioxide Nanomaterials: Synthesis,Properties, Modifications, and Applications,” Chemistry of Materials, Vol. 107, No. 7, 2007, pp. 2891-2959. doi:10.1021/cr0500535
- M. Kakihana, “Invited Review ‘Sol-Gel’ Preparation of High Temperature Superconducting Oxides,” Journal of Sol-Gel Science and Technology, Vol. 6, No. 1, 1996, pp. 7-55. doi:10.1007/BF00402588
- M. P. Pechini, “Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor,” US Patent No3330697, 1967.
- J. Tauc, “Optical Properties of Solids,” Academic, New York, 1966.

