Journal of Biomaterials and Nanobiotechnology
Vol.4 No.1(2013), Article ID:26874,8 pages DOI:10.4236/jbnb.2013.41007
Osteoconductivity of Superhydrophilic Anodized TiO2 Coatings on Ti Treated with Hydrothermal Processes
![]()
1Department of Materials Science & Engineering, Graduate School of Engineering, Nagoya University, Nagoya, Japan; 2EcoTopia Science Institute, Nagoya University, Nagoya, Japan; 3Hamri Co., Ltd., Tokyo, Japan.
Email: yamamoto@f2.numse.nagoya-u.ac.jp
Received October 30th, 2012; revised November 30th, 2012; accepted December 10th, 2012
Keywords: Titanium; Anodizing; Superhydrophilic; Hydrothermal Treatment; Phosphate Buffered Saline; Osteoconductivity
ABSTRACT
Surface hydrophilicity is considered to have a strong influence on the biological reactions of bone-substituting materials. However, the influence of a hydrophilic surface on osteoconductivity is not completely clear, especially for superhydrophilic surfaces. In this study, we conferred superhydrophilic properties on anodized TiO2 coatings using a hydrothermal treatment, and developed a method to maintain this surface until implantation. The osteoconductivity of these coatings was evaluated with in vivo tests. A hydrothermal treatment made the surface of as-anodized samples more hydrophilic, up to a water contact angle of 13 (deg.). Storage in both air and distilled water increased the water contact angle after several days because of the adsorption of hydrocarbon. However, storage in phosphate buffered solution led to a reduction in the water contact angle, because of the adsorption of the inorganic ions in the solution, and the sample retained its high hydrophilicity for a long time. As the water contact angle decreased, the hard tissue formation ratio increased continuously up to 58%, which was about four times higher than the hard tissue formation ratio on as-polished Ti.
1. Introduction
Titanium (Ti) is widely used in dental and orthopedic implants because it is very biocompatible and highly resistant to corrosion; however, it has poor bone-forming properties. An implant’s osteoconductivity is usually influenced by its surface characteristics, because the formation of new bone on the implant is an interfacial reaction between the implant and body fluid. Therefore, it is important to modify the surface characteristics to improve the osteoconductivity of Ti, using, for example, hydroxyapatite coatings [1-8] or TiO2 coatings [9-14].
Surface roughness and hydrophilicity are considered two of the most important characteristics of biomaterials because of their strong influence on protein adsorption and cell adhesion after implantation into the body [15- 19]. In previous studies, we assessed the influence of surface roughness and hydrophilicity on the osteoconductivity of anodized TiO2 coatings [11,14]. We found that the osteoconductivity of TiO2 coatings was improved by a fine (Ra/μm < 0.1) and hydrophilic surface. We also found that changing the anodizing condition altered the hydrophilicity of anodized TiO2 coatings. However, the water contact angle (WCA) did not decrease below 20
(deg.) when only anodizing was used. An additional surface treatment is required to achieve a more hydrophilic surface.
Processes using ultraviolet irradiation [20], plasma irradiation [21], and hydrothermal treatment [22] have been reported to confer high hydrophilicity on sol-gel or sputtered TiO2 coatings and on an anodized coating on a Ti substrate. Ultraviolet irradiation and plasma irradiation have merit in that they do not cause dynamic changes in the film properties, such as the surface morphology or crystal structure, but these processes are difficult to apply to complex-shaped substrates with complex topographies, which typify many implants, because of the line-of-sight nature of the methods. In contrast, hydrothermal treatments can be applied regardless of the shape of the implant, although these processes may influence the film properties because heat is applied. After hydrophilic surfaces are prepared, it is also important to maintain those surfaces until implantation, because surface hydrophilicity can change with time, as reported by Att et al. [23] Therefore, optimum methods must be explored both to prepare hydrophilic surface and to maintain their hydrophilicity. In this study, we attempted to confer superhydrophilic properties on anodized TiO2
coatings using a hydrothermal treatment, and developed a method to maintain the hydrophilic surface until implantation. The osteoconductivity of these coatings was then evaluated in in vivo tests.
2. Materials and Methods
2.1. Preparation of Anodized TiO2 Coatings
Commercially pure Ti (cp-Ti) disks (area = 1.13 cm2) and rods (dimensions = f2 × 5 mm) were used to evaluate the coatings and in the in vivo tests, respectively. The cp-Ti disks were covered with epoxy resin, except for the face that would be in contact with the aqueous solution. All of the substrates were polished with emery paper, and then buffed with Al2O3 particles (particle size = 0.05 μm). After they were polished, the substrates were cleaned and degreased with distilled water and ethanol, and then used for anodizing. A Ti substrate and a Pt coil were used as the anode and cathode, respectively, and no reference electrode was used. Anodizing was performed in a 0.1 M H2SO4 aqueous solution (pH = 1.0) by increasing the applied voltage from 0 V to 100 V at 0.1 Vs−1. After anodizing, each sample was sterilized at 394 K for a period of 20 min. This sample is designated the “as-anodized Ti” in the following description.
2.2. Hydrothermal Treatment
The hydrothermal treatment was only applied to as-anodized samples. Therefore, samples that were both anodized and subjected to the hydrothermal treatment are simply designated “hydrothermally treated Ti” in the following sections: The as-anodized samples were immersed in a beaker of distilled water, which was then placed in an autoclave unit. During the hydrothermal treatment, the samples were heated to 393 K or 453 K at a rate of 4 K∙min−1 in the autoclave unit and kept at that temperature for up to 180 min. After the treatment, the beaker was immediately taken out from the autoclave unit, and the samples cooled naturally to the room temperature in the beaker.
2.3. Storage of Samples
The samples were stored under one of the following four conditions (at room temperature): in air, in distilled water, in phosphate buffered saline (×1 PBS(-), pH 7.2), or in five times concentrated phosphate buffered saline (×5 PBS(-), pH 7.5). The composition of ×1 PBS(-) was (in g∙L−1) 8 NaCl, 0.2 KCl, 1.44 Na2HPO4, 0.24 KH2PO4, and <0.1 diethyl dicarbonate. The samples were stored in each of the conditions for up to 170 h.
2.4. Analysis of the Coatings
Surface morphology was observed with an SEM. The crystal structure of the coating was identified with XRD. The elements on the coated surface were identified using XPS. Surface roughness was measured with a confocal laser scanning microscope, with a measurement area of 150 ´ 112 μm2, and was expressed as the arithmetical mean of the surface roughness (Ra), because this value is not susceptible to any local scarring of the sample [18]. WCA was measured at three different points on each sample, using a 2 μL droplet of distilled water, after a specified time in each of the storage environments, and the average value was used as the WCA value.
2.5. In Vivo Test
Because the experimental procedure for our in vivo study was almost the same as that described in a previous report [6], it is described only briefly here. Before surgery, all the implants were cleaned in distilled water and immersed in a chlorhexidine gluconate solution. Ten-weekold male Sprague Dawley rats (Charles River Japan, Inc., Tokyo, Japan) were used in our experimental procedures. The samples were implanted in the tibial metaphysis of the rats. A slightly oversized hole, which did not pass through to the rear side of the bone, was created using a low-speed rotary drill. Subsequently, the implants were inserted into these holes, and then the subcutaneous tissue and skin were closed and sterilized.
The rats were sacrificed after a period of 14 d, and the implants with their surrounding tissue were retrieved. The samples were fixed in a 10% neutral buffered formalin solution, dehydrated in a graded series of ethanol, and embedded in methylmethacrylate. Following polymerization, each implant block was sectioned longitudinally into 20 μm thick slices. These sections were then stained with toluidine blue.
The sum of the linear bone contact with the implant surface was measured and was expressed as a percentage over the sum of hard tissue contact and soft tissue contact with the specimen (the bone-implant contact ratio, RB-I) in the cortical bone part [6]. Significant differences in the bone-implant contact ratios were analyzed statistically using the Tukey-Kramer method [24]. Differences were considered statistically significant at the p < 0.05 level. This animal study was conducted in the laboratory of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International.
3. Results and Discussion
3.1. Effect of Hydrothermal Treatment on Film Properties
Figure 1 shows the X-ray diffraction (XRD) patterns of the anodized samples before and after the hydrothermal treatment performed at different temperatures. Figure 2 shows typical surface scanning electron microscope

Figure 1. XRD patterns of Ti samples processed with different surface modification methods: (a) as-anodized; (b) hydrothermally treated at 393 K for 180 min in distilled water; and (c) hydrothermally treated at 453 K for 180 min in distilled water.
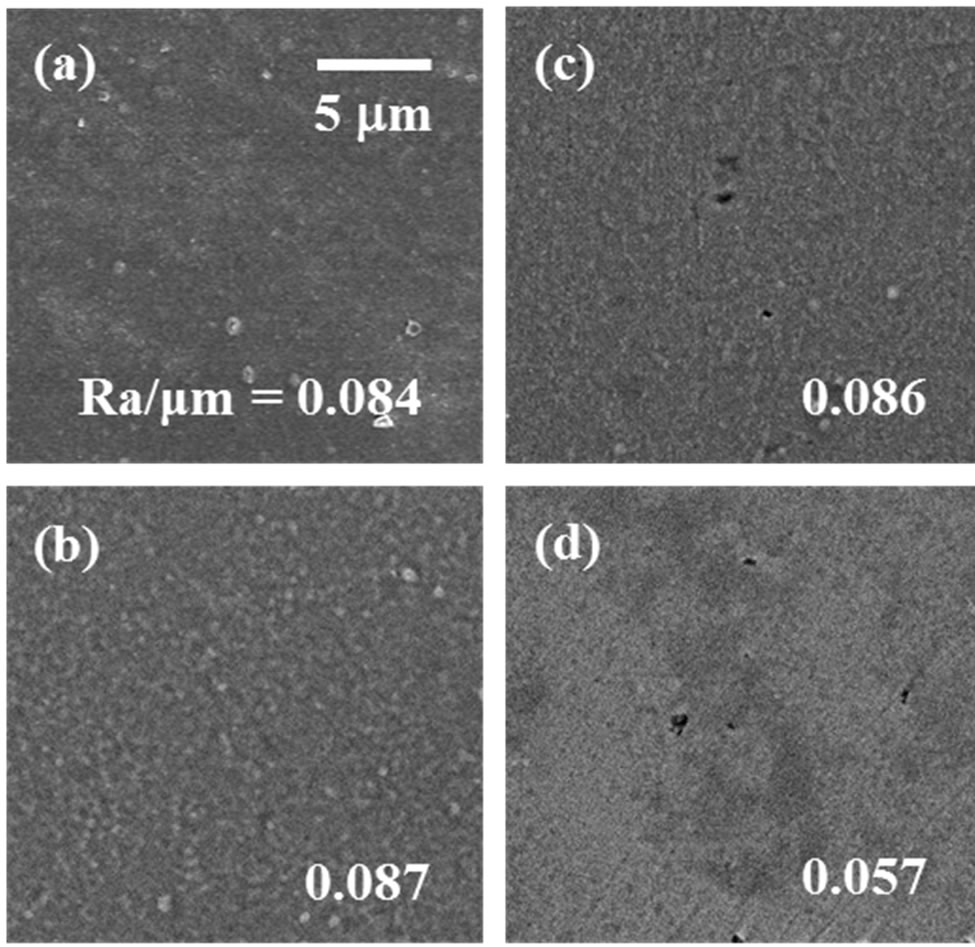
Figure 2. Surface morphology and surface roughness (Ra) of Ti samples processed with different surface modification methods: (a) as-anodized; (b) hydrothermally treated at 393 K for 180 min in distilled water; (c) hydrothermally treated at 453 K for 180 min in distilled water; and (d) as-polished.
(SEM) images of the samples. Figure 3 shows the influence of the period of hydrothermal treatment at different processing temperatures on the WCA value immediately after treatment.
The surfaces of the Ti samples were covered with oxide coatings consisting of only anatase-type TiO2 after anodizing and after the additional hydrothermal treatments at both temperatures (393 K and 453 K) (Figure 1). Neither anodizing nor the additional hydrothermal treatment made the surfaces dramatically rougher than that of the as-polished Ti; rather, the fine surfaces were retained (Ra/μm < 0.1) (Figure 2). In contrast, WCA of the as-polished Ti decreased from 71 (deg.) (shown later in Figure 4) to 28 (deg.) after anodizing, and further still to 13 (deg.) after the additional hydrothermal treatment at both processing temperatures (Figure 3). WCA reached a stable value of 13 (deg.) in a shorter period when either the hydrothermal processing temperature or the hydrothermal processing period increased (Figure 3). A similar stable value was previously reported by Takebe et al., although their results for surface roughness differed from ours [22].

Figure 3. The relationship between WCA and the processing time for the hydrothermal treatment on as-anodized Ti in distilled water at 393 K (○) and at 453 K (●).
When we used X-ray photoelectron spectroscopy (XPS) to analyze the chemical species on the surfaces of the as-polished Ti, as-anodized Ti, and hydrothermally treated Ti samples processed at 453 K, the elements C, O, and Ti were detected on all the samples. In general, WCA is strongly related to the surface chemical species, such as OH groups and adsorbed hydrocarbon, each of which has the opposite effect on WCA [23,25]. The numbers of OH groups and amounts of adsorbed hydrocarbon on the as-polished Ti, the as-anodized Ti, and the hydrothermally treated Ti are shown in Figure 4. The number of OH groups decreased after anodizing (1(d)), probably because the pH decreased locally during the anodizing process, but did not change after the additional hydrothermal treatment (1(g)) compared with that on the as-polished sample (1(b)). Therefore, the OH groups were not introduced by the additional hydrothermal treatment. In contrast, the amount of adsorbed hydrocarbon did not change after anodizing (2(d)), but decreased after the additional hydrothermal treatment (2(g)). Therefore, the TiO2 surface was cleaned by the removal of the adsorbed hydrocarbon after the additional hydrothermal treatment. Despite the smaller number of OH groups, the hydrothermally treated Ti was more hydrophilic than the as-polished Ti. This means that the reduction in adsorbed hydrocarbon more strongly influenced the surface hydrophilicity than the reduction in the OH groups, resulting in a significant reduction in WCA. Thus, the hydrothermal treatment converted the surface to a more hydrophilic state by removing the adsorbed hydrocarbon from it. The temperature for the hydrothermal treatment did not influence the final WCA achieved, as mentioned above. In the following experiment, the hydrothermal treatment was performed at a temperature of 453 K for 180 min, which was sufficient to produce a small and stable WCA value (θ < 15 (deg.)).
3.2. Storage of the Samples in Different Environments
Figure 5 shows the effects of the four different storage

Figure 4. The relationship between WCA and (1) the number of OH group and (2) the amount of adsorbed hydrocarbon on the Ti samples processed with different surface modifications and different storage methods, as listed in the table. The concentrations of [-OH], [C-H], and [TiO2] were calculated from O 1s, C 1s, and Ti 2p spectra obtained with XPS.
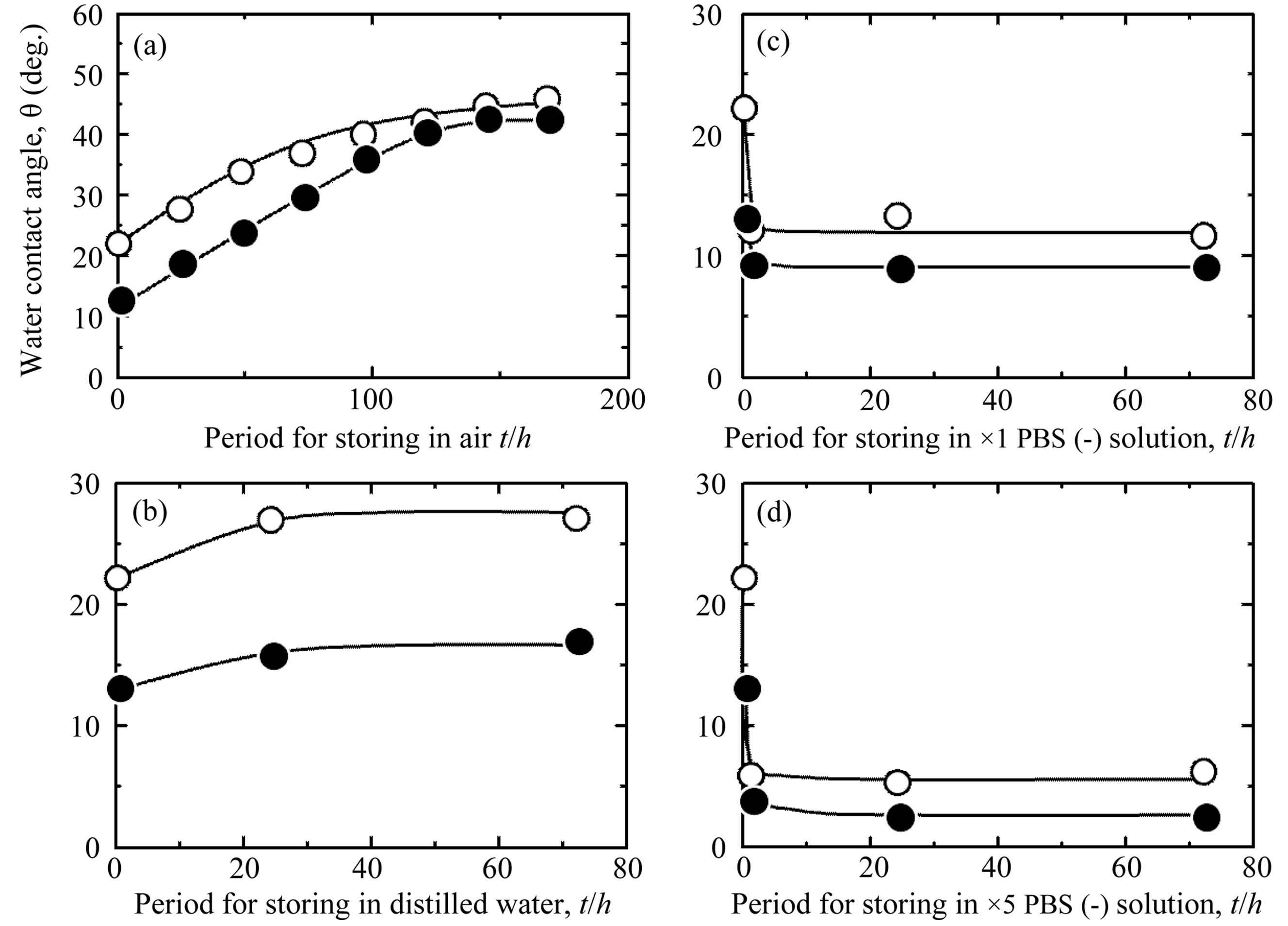
Figure 5. Temporal changes in WCA on the Ti samples processed with different surface modifications (○: as-anodized; ●: hydrothermally treated at 453 K for 180 min in distilled water) and then stored under different conditions for different periods: (a) in air; (b) in distilled water; (c) in ×1 PBS(-) solution; and (d) in ×5 PBS(-) solution.
environments on the variation of WCA of the samples with time. The WCA values of the samples varied greatly according to the storage condition and the period of storage. Regardless of whether a sample was hydrothermally treated, storing it in air caused a continuous increase in its WCA as the storage period increased, resulting in similarly high WCA values after 168 h for all samples (Figure 5(a)). Storage in distilled water also caused WCA to increase slightly (Figure 5(b)). However, storage in PBS(-) solution, which provides the same wet environment as distilled water, reduced WCA of the asanodized Ti (○ in Figures 5(c) and (d)). This tendency was enhanced when the sample was hydrothermally treated and when it was stored in a higher concentration of PBS(-) solution, ×5 PBS(-) (● in Figures 5(c) and (d)).
The variations of WCA with time in the different storage environments were influenced by chemical species on the surface. For the samples with hydrophilic surfaces immediately after their preparation, WCA values increased as the amount of adsorbed hydrocarbon increased when the samples were stored in air, although the amount of OH did not change, even after 168 h (Figures 4(1) and (2) (c)→(d) →(e), (f)→(g)→(h)). The same result has been reported previously [23,26]. For the as-polished Ti, which was relatively hydrophobic immediately after its preparation, the amount of adsorbed hydrocarbon did not change as the storage period increased. It is thought that hydrocarbon does not easily adsorb to surfaces with an originally high WCA value. On the other hand, the adsorption of hydrocarbon to the hydrothermally treated Ti was suppressed by storing the samples in distilled water (Figure 4(2)(i)). This was a reason for the lower stable WCA value of hydrothermally treated Ti samples stored in distilled water, even after 72 h, compared with that of the sample stored in air (Figure 5(b)).
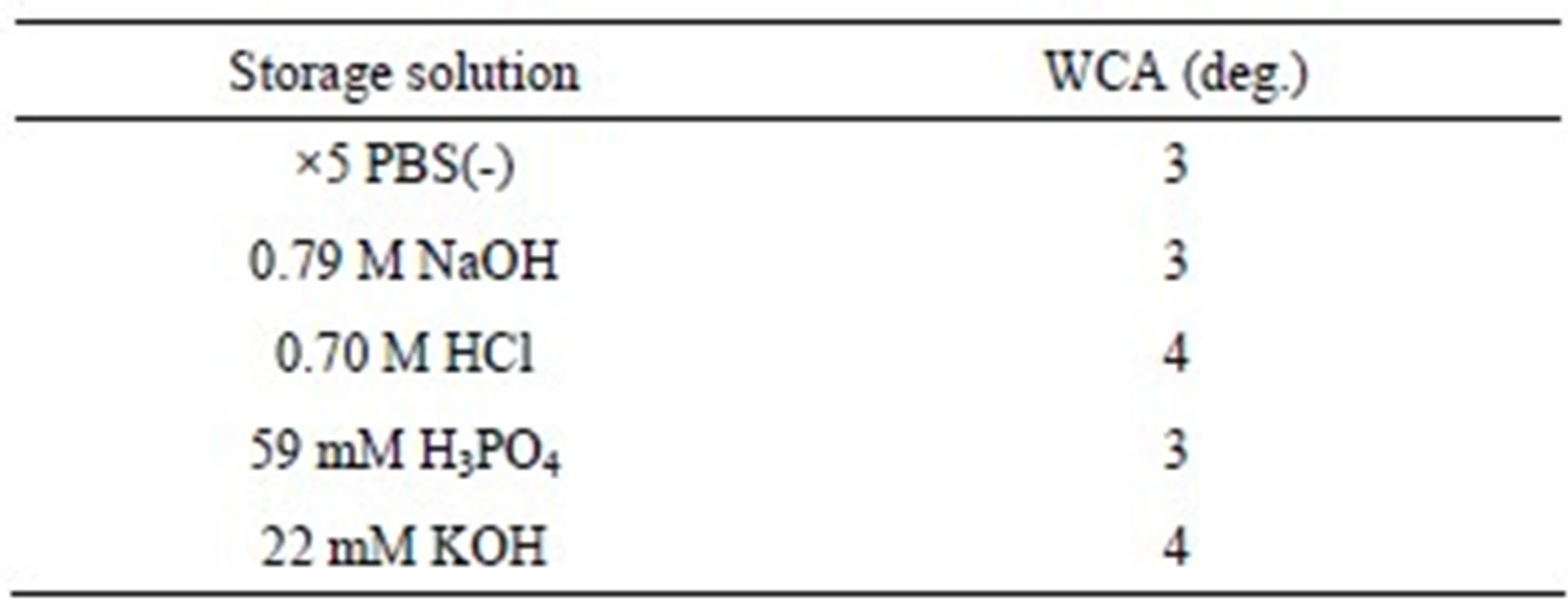
The hydrothermally treated Ti samples stored in PBS(-) solution showed lower WCA values than the hydrothermally treated Ti samples stored in distilled water, despite the slightly larger amounts of adsorbed hydrocarbon (Figure 4(2)(g)). In addition to the elements C, O, and Ti, the elements Na and Cl, which are components of PBS(-), were detected on the surfaces of the samples stored in PBS(-) solution (Figure 6). Similarly, it has been reported that an exsiccation layer of PBS salts can stabilize originally hydrophilic surfaces [26]. To understand the influence of the components of PBS(-) on the hydrophilicity of the samples, hydrothermally treated Ti samples were immersed for 24 h in an aqueous solution of 0.79 M NaOH (pH 13.6), 22 mM KOH (pH 12.2), 59 mM H3PO4 (pH 1.8), or 0.70 M HCl (pH 0.5), each of which had the same concentration of Na, K, P, or Cl as ×5 PBS(-) aqueous solution (Table 1). All of these solute ions adsorbed to the surfaces of the samples, regardless of the type of ion or the pH of the solution, consequently reducing the WCA values to the same extent, about 3 (deg.), as immersion in ×5 PBS(-) solution. There was no difference between the types of solute ions in their ca pacity to reduce WCA, but when the samples were immersed in ×5 PBS(-), the Na and Cl ions adsorbed markedly to the surfaces of the samples because their concentrations in the solution were high. Therefore, it is clear that the adsorbed solute ions from the ×5 PBS(-) influenced the surface hydrophilicity of the TiO2 coatings more strongly than the adsorbed hydrocarbon, and formed stable superhydrophilic surfaces when the samples were stored in ×5 PBS(-) solution. All things considered, storing the samples in ×5 PBS(-) solution effectively maintained the superhydrophilic surface for a long time.
3.3. In Vivo Test
To investigate the relationship between WCA < 20 (deg.) and RB-I in cortical bone, we used two types of samples in this in vivo study, which were stored in either distilled water and ×5 PBS(-) solution after hydrothermal treat-

Figure 6. XPS spectra of (a) Na 1s, (b) Cl 2p, (c) P 2p, and (d) K 2p, detected in Ti samples hydrothermally treated at 453 K for 180 min in distilled water and then stored in ×5 PBS(-) solution for a period of 170 h.
Table 1. WCA values of Ti samples hydrothermally treated at 453 K for 180 min in distilled water and then stored for a period of 24 h in different aqueous solutions.
ment at 453 K for a period of 180 min. The results are plotted in Figure 7, which shows the relationship between WCA and the RB-I value in cortical bone. To show the relationship between RB-I and WCA > 20 (deg.), our previous in vivo results for TiO2-coated samples with Ra/μm < 0.1 are also plotted in the figure [11,13,14]. Each of the hydrothermally treated samples (WCA = 16 (deg.) or 3 (deg.)) showed quite high RB-I values, up to 48% and 58%, respectively, which are about four times higher than the RB-I value for the as-polished surface, indicating a significant improvement in osteoconductivity. These results also clearly show that the RB-I values continued to increase to 58% as WCA decreased, in the range of WCA < 55 (deg.). It is unclear whether the adsorbed Na and Cl ions on the sample surface aggressively induced the formation of hard tissue, but these ions contributed to the creation of a superhydrophilic surface. The superhydrophilic surface prepared in this study remained stable for at least 720 h after the samples were immersed in ×5 PBS(-) solution. This means that the high hydrophilicity of the samples can be maintained until just before their implantation during surgery by simply immersing them in ×5 PBS(-) solution. The high hydrophilicity of the hydrothermally treated Ti was lost after storage for 168 h in air, as shown in Figure 5(a), but was recovered to the original level by another hydrothermal treatment at 453 K for 180 min in distilled water. This indicates that contamination during storage in air can be reversed with a further hydrothermal treatment, as long as the as-prepared surface is hydrophilic. In summary, the storage of an anodized sample with less adsorbed hydrocarbon in ×5 PBS(-) effectively maintained the high hydrophilicity and resulting osteoconductivity of the sample for a long time.
4. Conclusions
In this study, a hydrothermal treatment was used as an additional surface treatment for anodized TiO2 coatings to produce a more hydrophilic surface, and various storage processes were investigated to identify a treatment that would maintain the hydrophilic surface for a long time after the surface treatment. The osteoconductivity of
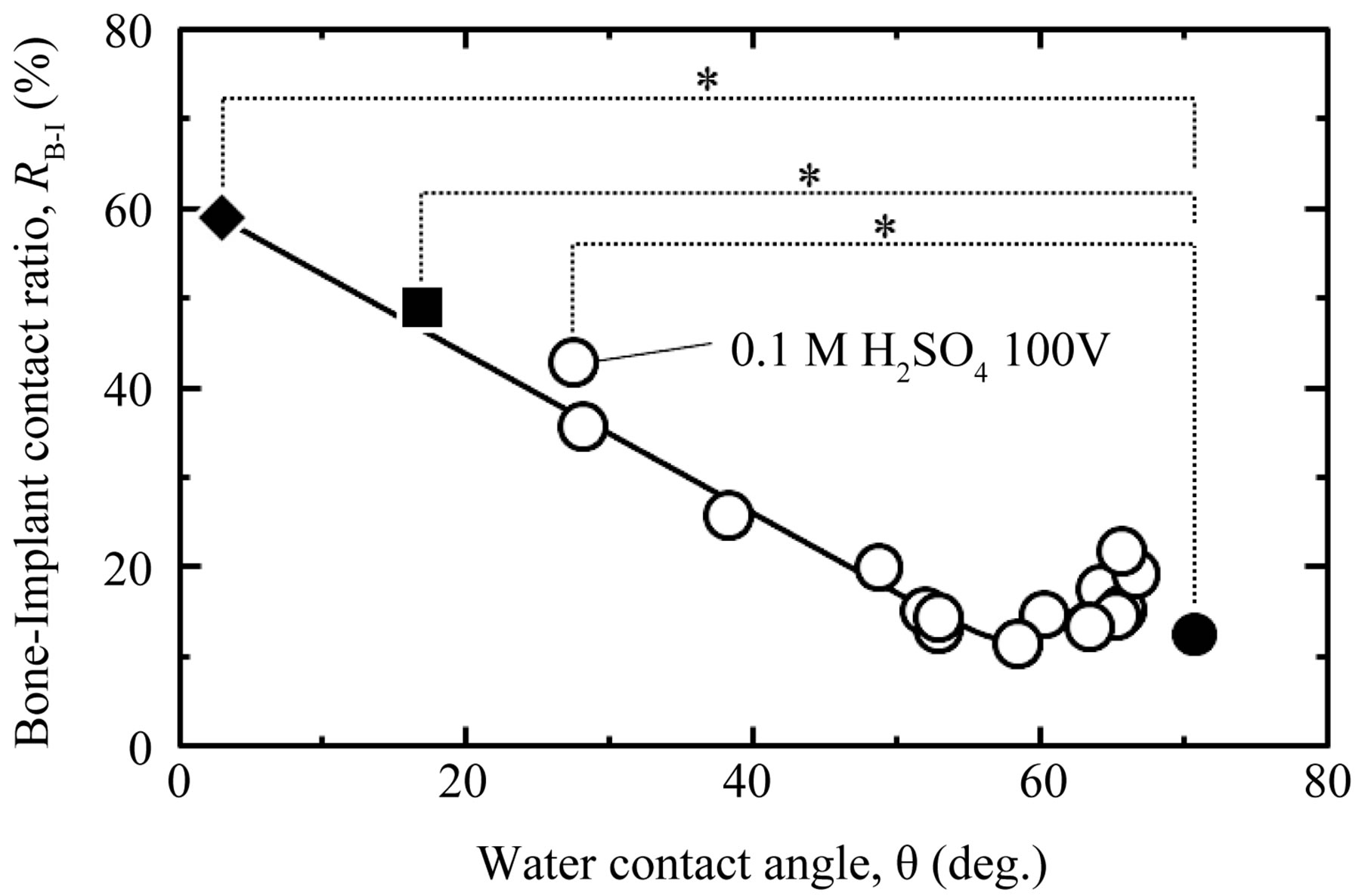
Figure 7. Relationship between WCA and RB-I in the cortical bone part of Ti samples processed with different oxidizing processes and subsequent storage methods. Symbols: ○ = anodized in various aqueous solutions (Ra/μm < 0.1) [14]; ■ = hydrothermally treated at 453 K for 180 min in distilled water and then stored in distilled water; ◆: hydrothermally treated at 453 K for 180 min. in distilled water and then stored in ×5 PBS(-) solution; and ●: as-polished. *p < 0.05.
the samples with enhanced hydrophilicity was evaluated in an in vivo test. The following results were obtained.
1) The hydrophilicity of anodized TiO2 was enhanced by the hydrothermal treatment, and WCA decreased to 13 (deg.). The hydrothermal treatment temperature influenced the processing time required to reduce WCA value to 13 (deg.), but it did not affect the final WCA value.
2) Storing in air or in distilled water increased WCA after several days because of the adsorption of hydrocarbon. However, storage in phosphate buffered saline reduced WCA, converting the surface to a superhydrophilic surface, because of the adsorption of inorganic solute ions to the surface. This form of storage thus maintained the high hydrophilicity of the surface for a long time.
3) As WCA decreased, the hard tissue formation ratio increased continuously up to 58%, which was about four times higher than the hard tissue formation ratio on as-polished Ti.
5. Acknowledgements
This work was partially supported by a Grant-in-Aid for JSPS fellows (No. 2310401), and the Global COE program (COE for Education and Research of Micro-Nano Mechatronics) from the Japan Society for the Promotion of Science (JSPS), and a Grant-in-Aid for Scientific Research (C) (No.21560719).
REFERENCES
- J. Park and R. S. Lakes, “Biomaterials,” 3rd Edition, Springer, New York, 2007.
- K. Kuroda, R. Ichino, M. Okido and O. Takai, “Hydroxyapatite Coating on Titanium by Thermal Substrate Method in Aqueous Solution,” Journal of Biomedical Materials Research Part A, Vol. 59, No. 2, 2002, pp. 390-397. doi:10.1002/jbm.10002
- K. Kuroda, R. Ichino, M. Okido and O. Takai, “Effects of Ion Concentration and pH on Hydroxyapatite Deposition from Aqueous Solution onto Titanium by the Thermal Substrate Method,” Journal of Biomedical Materials Research Part A, Vol. 61, No. 3, 2002, pp. 354-359. doi:10.1002/jbm.10197
- K. Kuroda, Y. Miyashita, R. Ichino, M. Okido and O. Takai, “Preparation of Calcium Phosphate Coatings on Titanium Using the Thermal Substrate Method and Their in Vitro Evaluation,” Materials Transactions, Vol. 43, No. 12, 2002, pp. 3015-3019. doi:10.2320/matertrans.43.3015
- K. Kuroda, S. Nakamoto, R. Ichino, M. Okido and R. M. Pilliar, “Hydroxyapatite Coatings on a 3D Porous Surface Using Thermal Substrate Method,” Materials Transactions, Vol. 46, No. 7, 2005, pp. 1633-1635. doi:10.2320/matertrans.46.1633
- K. Kuroda, S. Nakamoto, Y. Miyashita, R. Ichino and M. Okido, “Osteoinductivity of HAp Films with Different Surface Morphologies Coated by the Thermal Substrate Method in Aqueous Solutions,” Materials Transactions, Vol. 47, No. 5, 2006, pp. 1391-1394. doi:10.2320/matertrans.47.1391
- K. Kuroda, M. Moriyama, R. Ichino, M. Okido and A. Seki, “Formation and in Vivo Evaluation of Carbonate Apatite and Carbonate Apatite/CaCO3 Composite Films Using the Thermal Substrate Method in Aqueous Solution,” Materials Transactions, Vol. 49, No. 6, 2008, pp. 1434-1440. doi:10.2320/matertrans.MRA2007330
- K. Kuroda, M. Moriyama, R. Ichino, M. Okido and A. Seki, “Formation and Osteoconductivity of Hydroxyapatite/Collagen Composite Films Using a Thermal Substrate Method in Aqueous Solutions,” Materials Transactions, Vol. 50, No. 5, 2009, pp. 1190-1195. doi:10.2320/matertrans.MRA2008459
- H.-J. Song, S.-H. Park, S.-H. Jeong and Y.-J. Park, “Surface Characteristics and Bioactivity of Oxide Films Formed by Anodic Spark Oxidation on Titanium in Different Electrolytes,” Journal of Materials Processing Technology, Vol. 209, No. 2, 2009, pp. 864-870. doi:10.1016/j.jmatprotec.2008.02.055
- X. Cui, H.-M. Kim, M. Kawashita, L. Wang, T. Xiong, T. Kokubo and T. Nakamura, “Preparation of Bioactive Titania Films on Titanium Metal via Anodic Oxidation,” Dental Materials, Vol. 25, No. 1, 2009, pp. 80-86. doi:10.1016/j.dental.2008.04.012
- D. Yamamoto, I. Kawai, K. Kuroda, R. Ichino, M. Okido and A. Seki, “Osteoconductivity of Anodized Titanium with Controlled Micron-Level Surface Roughness,” Materials Transactions, Vol. 52, No. 8, 2011, pp. 1650-1654. doi:10.2320/matertrans.M2011049
- D. Yamamoto, T. Iida, K. Kuroda, R. Ichino, M. Okido and A. Seki, “Formation of Amorphous TiO2 Film on Ti Using Anodizingin Concentrated H3PO4 Aqueous Solution and Its Osteoconductivity,” Materials Transactions, Vol. 53, No. 3, 2012, pp. 508-512. doi:10.2320/matertrans.M2011234
- D. Yamamoto, I. Kawai, K. Kuroda, R. Ichino M. Okido and A. Seki, “Osteoconductivity and Hydrophilicity of TiO2 Coatings on Ti Substrates Prepared by Different Oxidizing Processes,” Bioinorganic Chemistry and Applications, in Press.
- D. Yamamoto, T. Iida, K. Arii, K. Kuroda, R. Ichino M. Okido and A. Seki, “Surface Hydrophilicity and Osteoconductivity of Anodized Ti in Aqueous Solutions with Various Solute Ions,” Materials Transactions, Vol. 53, No. 11, 2012, pp. 1956-1961. doi:10.2320/matertrans.M2012082
- K. L. Kilpadi, P. L. Chang, and S. L. Bellis, “Hydroxylapatite Binds More Serum Proteins, Purified Integrins, and Osteoblast Precursor Cells than Titanium or Steel,” Journal of Biomedical Materials Research Part A, Vol. 57, No. 2, 2001, pp. 258-267. doi: 10.1002/1097-4636(200111)
- F. Rupp, L. Scheideler, N. Olshanska, M. de Wild, M. Wieland and J. Geis-Gerstorfer, “Enhancing Surface Free Energy and Hydrophilicity through Chemical Modification of Microstructured Titanium Implant Surfaces,” Journal of Biomedical Materials Research Part A, Vol. 76A, No. 2, 2006, pp. 323-334. doi: 10.1002/jbm.a.30518
- K. Das, S. Bose and A. Bandyopadhyay, “Surface Modifications and Cell-Materials Interactions with Anodized Ti,” Acta Biomaterialia, Vol. 3, No. 4, 2007, pp. 573-585. doi:10.1016/j.actbio.2006.12.003
- M. Bigerelle, K. Anselme, B. Noel, I. Ruderman, P. Hardouin and A. Iost, “Improvement in the Morphology of Ti-Based Surfaces: A New Process to Increase in Vitro Human Osteoblast Response,” Biomaterials, Vol. 23, No. 7, 2002, pp. 1563-1577. doi:10.1016/S0142-9612(01)00271-X
- Y. Arima and H. Iwata, ”Effect of Wettability and Surface Functional Groups on Protein Adsorption and Cell Adhesion Using Well-Defined Mixed Self-assembled Monolayers,” Biomaterials, Vol. 28, No. 20, 2007, pp. 3074- 3082. doi:10.1016/j.biomaterials.2007.03.013
- M. E. Simonsen, Z. Li and E. G. Sogaard, “Influence of the OH Groups on the Photocatalytic Activity and Photoinduced Hydrophilicity of Microwave Assisted Sol-Gel TiO2 Film,” Applied Surface Science, Vol. 255, No. 18, 2009, pp. 8054-8062. doi:10.1016/j.apsusc.2009.05.013
- K.-X. Zhang, W. Wang, J.-L. Hou, J.-H. Zhao, Y. Zhang and Y.-C. Fang, “Oxygen Plasma Induced Hydrophilicity of TiO2 Thin Films,” Vacuum, Vol. 85, No. 11, 2011, pp. 990-993. doi:10.1016/j.vacuum.2011.02.006
- J. Takebe, S. Ito, S. Miura, K. Miyata and K. Ishibashi, “Physicochemical State of the Nanotopographic Surface of Commercially Pure Titanium Following AnodizationHydrothermal Treatment Reveals Significantly Improved Hydrophilicity and Surface Energy Profiles,” Materials Science and Engineering: C, Vol. 32, No. 1, 2012, pp. 55- 60. doi:10.1016/j.msec.2011.09.011
- W. Att, N. Hori, M. Takeuchi, J. Ouyang, Y. Yang, M. Anpo and T. Ogawa, “Time-Dependent Degradation of Titanium Osteoconductivity: An Implication of Biological Aging of Implant Materials,” Biomaterials, Vol. 30, No. 29, 2009, pp. 5352-5363. doi:10.1016/j.biomaterials.2009.06.040
- C. Y. Kramer, “Extension of Multiple Range Tests to Group Means with Unequal Numbers of Replications,” Biometrics, Vol. 12, No. 3, 1956, pp. 307-310. doi:10.2307/3001469
- S. Takeda and M. Fukawa, “Surface OH Groups Governing Surface Chemical Properties of SiO2 Thin Films Deposited by RF Magnetron Sputtering,” Thin Solid Films, Vol. 444, No. 1-2, 2003, pp. 153-157. doi:10.1016/S0040-6090(03)01094-0
- H. P. Jennissen, “Stabilizing Ultra-Hydrophilic Surfaces by an Exsiccation Layer of Salts and Implications of the Hofmeistereffect,” Materialwissenschaft und Werkstofftechnik, Vol. 41, No. 12, 2010, pp. 1035-1039. doi: 10.1002/mawe.201000705

