International Journal of Geosciences
Vol.06 No.07(2015), Article ID:58272,8 pages
10.4236/ijg.2015.67058
Carbon and Hydrogen Isotopes as Tracers of Methane Dynamic in Wetlands
Romina Sanci*, Héctor O. Panarello
Instituto de Geocronología y Geología Isotópica (INGEIS, UBA-CONICET), Argentina
Email: *rominasanci@hotmail.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 16 June 2015; accepted 19 July 2015; published 24 July 2015
ABSTRACT
This work presents a review of the main processes leading to the production and sinking of methane in wetlands and how they affect the stable isotope composition of carbon and hydrogen. Isotope fractionation factors associated to diffusion, ebullition, bacterial oxidation, etc., have been gathered from relevant literature in an intent of providing researchers in this thematic with practical procedures and tools for the interpretation of experimental data. Also it is presented guidelines of the most reliable field and laboratory methods used at present for the correct sampling and analyzing methane in different stages of occurrence, as well the most common tools used in their interpretation. Each statement is fully referenced to a long updated list of publications.
Keywords:
C and H Stable Isotopes, CH4 and CO2 Emissions, Wetlands

1. Introduction
CH4 is a chemically and radioactively important atmospheric trace gas and is the second most important greenhouse gas after CO2. CH4 concentration increased from ~800 ppb in the 1850s to ~1700 ppb in the late 2000s [1] , suggesting an imbalance between estimated global emissions and removals. Decadal budgets for CH4 sources and sinks between 1980 and 2010 reflect average imbalance of 6 Tg CH4 a?1 in the 2000s, 17 Tg CH4 a?1 in the 1990s and 34 Tg CH4 a?1 in the 1980s [2] . The total amount of global CH4 emissions is currently estimated as 500 - 600 Tg a?1 [3] and this CH4 is emitted from a range of natural and anthropogenic sources as a result of the anaerobic decomposition of organic matter, land use changes and fossil fuel related emissions. Roughly, natural CH4 sources (wetlands, termite activity, other wild ruminants, geological, fresh water systems, permafrost, hydrates, wild fires) are considered responsible for 40% of total emissions while 60% of emissions are related to human activities (waste decomposition, fossil fuels, rice cultivation, agriculture, livestock and the burning of biomass) [4] . Recently, living vegetation has also been suggested as an important natural source of CH4 [5] . On the other hand, CH4 is removed from the atmosphere by a variety of chemical and biological processes, which occur in different regions of the atmosphere. These include tropospheric and stratospheric oxidation and uptake by soils.
Although most sources and sinks of CH4 have been identified, their relative contributions to atmospheric CH4 levels are still highly uncertain. The quantification of individual contributions to global emissions has proved to be difficult, despite they are crucial to predict climate change and managing Earth’s carbon reservoirs. The net rate of CH4 emissions is generally estimated from three approaches: 1) extrapolation from direct flux measurements and observations; 2) process-based modelling (bottom-up approach) and 3) inverse modelling that relies on spatially distributed, temporally continuous observations of concentration, and in some cases isotopic composition in the atmosphere (top-down approach) [6] -[9] . Observations of the 13C/12C ratio of atmospheric CH4 was used in isotope mass balance models to define the magnitudes of different sources and sinks and to constrain the present-day CH4 budget using optimal estimation. Three CH4 emission scenarios are estimated: 64% - 76% biogenic, 19% - 30% fossil, and 4% - 6% pyrogenic sources [10] . Biogenic sources contain CH4-generating microbes (methanogens) and comprise anaerobic environments such as natural wetlands and rice paddies, oxygen-poor freshwater systems (lakes, rivers, streams, reservoirs such as dams), digestive systems of ruminants and termites, and organic waste deposits (such as manure, sewage and landfills). Thermogenic CH4, formed over millions of years through geological processes, is a fossil fuel. It is vented from the subsurface into the atmosphere through natural features (such as terrestrial seeps, marine seeps and mud volcanoes), and through the exploitation of fossil fuels, that is, through the exploitation of coal, oil and natural gas. Pyrogenic CH4 is produced by the incomplete combustion of biomass and soil carbon during wildfires, and of biofuels and fossil fuels. Table 1 gives a data set of the main isotopic values of carbon that involved CH4 sources.
Wetlands contribute as much as 70% of the total natural emissions [16] [17] . Their emissions from most types (marshes, swamps, bogs, and fens according to reference [18] ) can vary by a few orders of magnitude over just a small number of meters, because they are influenced by a wide range of environmental parameters. These include soil characteristics such as the availability of organic carbon and nutrients, plant physiology, community composition and cover and water table depth and soil temperature. Seasonal and inter-annual variations of CH4 wetland emissions are reported by focusing on three determinants keys: temperature, substrate availability and water table’s level [19] -[21] . Besides these factors, the actual amount of CH4 emitted to the atmosphere or rates of CH4 emission depends on the balance between CH4 production and consumption and the mode of methane transport. Wetlands transfer CH4 to the atmosphere by diffusion, ebullition, and by transport through arenchymous vascular plants but only part of the CH4 produced is emitted to the atmosphere [22] . Considerable CH4 amounts are consumed or oxidized by methane-trophic bacteria in the rhizosphere and in surficial oxic layers during diffusive transport to the soil surface [23] [24] .
The accuracy of predictions of CH4 and ultimately the extent of climate change that can be expected in the earth system depend on a better understanding of CH4 dynamics from wetlands for each region. Most studied, considering all processes, were done in wetlands from Northern Hemisphere [25] . However, until quite recently, ebullition was considered to be only locally important, and most attention was focused on matrix diffusion of dissolved CH4 and plant-mediated transport. For instance, reference [26] used measurements to argue that ebullition has been temporally and spatially very variable and it could exceed diffusive fluxes by two orders of magnitude, stating that heterogeneity in ebullition (bubbling) is a major contribution to variability of emissions. So, it is necessary to study these systems at different regions as well as to know the suitable tools that would meet this purpose. In this review we present literature data that show how stable carbon isotopic signatures, in combination with other parameters, can be used to quantify the relative contribution of the major methanogenic
Table 1. Isotopic composition range from different sources of CH4.
pathways, CH4 oxidation and transport mechanisms.
2. Why to Apply the Stable Isotopes?
Wetlands, where conditions are more favourable to the accumulation of organic matter than to its decay, are characterized by a subsurface, anaerobic zone of CH4 production, and organic soils (as peat) often submerged or water-saturated. The generation of CH4 by bacteria requires a fully saturated environments that excludes atmospheric O2 in the absence of other free-energy electron-acceptors such as 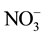 and
and 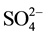 and low redox (Eh < ?200 mV), as can be observed in wetlands [27] . Methane-genesis is the final step in anaerobic decomposition and represents a complex process that proceed via two major metabolic pathways: acetate fermentation (1) and CO2 reduction (2)
and low redox (Eh < ?200 mV), as can be observed in wetlands [27] . Methane-genesis is the final step in anaerobic decomposition and represents a complex process that proceed via two major metabolic pathways: acetate fermentation (1) and CO2 reduction (2)
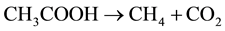
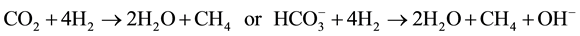
The measurement of stable isotopes of carbon on CH4 and CO2 is an effective way to identify the different phases of biodegradation of organic matter [28] [29] . According to these authors, CO2 is isotopically light during the initial aerobic and anaerobic oxidation phases of biodegradation with δ13C values that varies from −35‰ to −10‰, thus covering the range of most terrestrial plants [27] . The initial input of isotopically light CO2 associated with earlier biodegradation phases is soon overcome during the methanogen phase by the constant input of isotopically heavy CO2 associated with acetate fermentation and microbial CO2 reduction (the two primary metabolic pathways by which microbial CH4 is produced) (Table 2). During methanogenesis, CH4 is enriched in the lighter carbon isotope (12C) and the CO2 associated with microbial CH4 production is enriched in the heavier isotope (13C). Thus, in a semiclosed environment such as a wetland, the δ13C of CO2 is strongly affected by methanogen reactions with reported values between −10‰ and +20‰. Values of δ13C-CH4 due to fermentation of acetate and CO2 reduction are −65‰ to −50‰ and −110‰ to −60‰, respectively [11] [30] .
CH4 production is regulated mainly by O2 concentration as mentioned above, but also of pH, temperature, salinity, organic substrates and nutrient availability [31] . Some authors think that once anaerobic conditions are attained, the quality and supply of substrates is the major factor in CH4 production: substantial amounts of CH4 are only produced when labile carbon substrates are largely available and old (recalcitrant) wetland plays only a subordinate role as a substrate for CH4 production [32] . Reach conclusions about the relative importance of methanogen pathways for CH4 production are difficult because δ13C-CH4 values of the upper organic horizon (above 1 m) are substantiality affected by the processes of CH4 transport and oxidation [33] . So, based on differences in δ13C-CH4among organic layer and isotopic fractionation factors associated with gas transport and oxidation, these authors assessed the potential quotas of transported/oxidized CH4 Also, reference [34] used a simple mixing model to estimate the relative proportion of the acetate fermentation and CO2 reduction pathway.
CH4 is produced at depth and lost to the atmosphere from wetlands via three mechanisms: 1) diffusion through pore water to the water table and hence through the zone above the water table (if one exists) to the wetland surface; 2) diffusion or active transport through vascular plants; and 3) ebullition, that refers to methane released to the atmosphere in form of bubbles moving to the wetland surface. CH4 bubbles commonly occur in water saturated organic layers where they remain trapped and grow in size. CH4 is only sparingly soluble in
Table 2. Enrichment factors (e) to different processes for carbon.
water [35] and Henry’s law predicts that their solubility in a solvent is directly proportional to the partial pressure. Other gases, particularly nitrogen, could contribute with their partial pressure and exceed the total hydrostatic force. If this pressure is exceeded, gas bubbles, consisting of a mixture of CH4, CO2 and N2, could travel upward toward groundwater surface, perhaps along fractures [36] . This process of ebullition by which bubbles transport CH4 upward, occurs when the buoyancy of bubbles overcomes the forces that keep them in place (in particular, surface tension) when a certain threshold pressure is reached. Often this release is associated with changes in water level [37] -[39] , barometric pressure [40] -[42] and temperature [43] , fracture of the confining layer as well as mechanical disturbance [44] . The rapid transfer of CH4 bubbles through the aerobic near surface layer means there will be little or no consumption by methanotrophs. Some ebullition conceptual models suggest that bubbles form more readily in shallow layers-within 1 m-[45] , while others as sure that they form at greater depth and accumulate within the wetland profile according physical properties of subsoil [26] or presence of zones of overpressure due to transient confining layers [46] [47] . Also, these last authors affirm the occurrence of CH4 deep ebullition because they found large concentrations of dissolved CH4 and inorganic carbon (DIC) in the deeper pore water related to CO2 produced by fermentative and methanogen bacteria. Others suggest that bubbles are not trapped in subsoil but are lost directly to the water table, after which the gas moves slowly via diffusion through the zone above the water table where CH4 is oxidized [48] .
Molecular diffusion occurs through the water or air filled soil pores and the atmosphere and depends on the
vertical CH4 concentration gradient following Fick’s Law: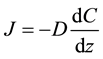 , where J is diffusive flux, D is the dif-
, where J is diffusive flux, D is the dif-
fusion coefficient, C the solute concentration and Z the depth. Fractionation (a) during diffusion is caused by differences the diffusion coefficients of the two isotope species. The stable CH4 isotopes (12CH4, 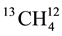 and
and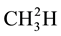 ) differ in molecular weight and their diffusion coefficient, faster diffusive transport of the lighter isotope on hence in an enrichment of the heavier isotope in the remaining gas phase. Reference [49] demonstrated that diffusion is an important transport mechanism for CH4 cover soils. They found that the fractionation factor due to transport, a transport, can be as high as 1.0178, due to the difference in molecular diffusion coefficients of CH4 isotopes (ɛ = 18‰). Diffusive transport of CH4 across the air-water interface was associated with anisotopic fractionation to 2‰ - 3‰ or ɛ = 2‰ - 3‰ [50] .
) differ in molecular weight and their diffusion coefficient, faster diffusive transport of the lighter isotope on hence in an enrichment of the heavier isotope in the remaining gas phase. Reference [49] demonstrated that diffusion is an important transport mechanism for CH4 cover soils. They found that the fractionation factor due to transport, a transport, can be as high as 1.0178, due to the difference in molecular diffusion coefficients of CH4 isotopes (ɛ = 18‰). Diffusive transport of CH4 across the air-water interface was associated with anisotopic fractionation to 2‰ - 3‰ or ɛ = 2‰ - 3‰ [50] .
Diffusion affects isotopic composition of CH4 differently than ebullition and CH4 transport from anoxic sediments and flooded soils by emergent plants. δ13C-CH4 value of released bubbles is not different from the reservoir of bubble gas held within the sediment because ebullition bypasses oxidation in the aerobic zone [33] . Many wetland plants possess aerenchymous tissue that allows for transport of oxygen into the root zone as an adaptation to rooting in waterlogged soils. Whereas this oxygen allows for oxidation of CH4 in the root zone [51] , at the same time, CH4 is transported through the aerenchyma out into the atmosphere. It was influenced by the type species and functional plant groups that affect these processes differently [52] . In vegetated wetlands, plant-mediated transport involves several steps that may further fractionate isotopes, i.e. diffusion to the roots, partial oxidation by methanotrophic bacteria in rhizosphere, diffusion into the roots, transport by convective bulk flow and emission [53] . At the moment, only the isotope effects of CH4 oxidation and convective bulk flow have been well studied. The last one, results in the little isotopic fractionation [53] whereas CH4 oxidation in the rhizosphere and in surficial oxic layers during diffusive transport to the soil surface (0 - 1 m) is notorious. Previous reports indicate that almost 60% of CH4 production is oxidized to CO2 [48] . Bacteria oxidize the 12C- isotopeslightly faster than the 13C-isotope, then, the result is an increase of the 13C/12C ratio of the remaining CH4. The enrichment factors can be seen at Table 2. This increase can be used to estimate CH4 oxidation. By the other way, variations on δ13C-DIC of water can reveal the importance of different biochemical pathways of methanogenesis and CH4 oxidation [27] . It was remarked that acetate fermentation that occur chiefly in fresh water environment is responsible for the great fractionation of 13C between CO2 and CH4. CO2 with much enriched signature incorporates to the DIC, but this signal is often masked by the simultaneous oxidation processes releasing isotope depleted CO2 to surrounding water.
3. Measurements and Analytical Methods
3.1. CH4 and CO2 Fluxes and Isotopes
Closed chamber methods are used to measure surface emissions. These methods are independent of the physical properties of soil and allow measuring the CH4 and CO2 released to the atmosphere. The closed chamber methods are applied during fieldwork, after calibration in laboratory [58] [59] . The accumulation chamber is placed on a collar that had been previously installed on the ground. Surface emissions are estimated as the increase of CH4 and CO2 concentrations over time. Flux rates are calculated fitting by linear regression the variation of concentration (C) over time and adjusting for chamber volume and covered area, according to the following eq-
uation: 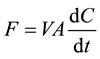 where F is the surface emission rate (mg∙m−2∙day−1), V is the chamber volume and A is the
where F is the surface emission rate (mg∙m−2∙day−1), V is the chamber volume and A is the
soil area under the chamber and dC/dt is the variation of C with t within the chamber. In the closed static chamber method, soil gases are extracted with plastic syringes and analyzed by gas chromatography (GC). In flooded soil the fluxes are estimated using floating static chambers. Sometimes chambers are covered with reflective material to minimize the increase of inside temperature during deployments. Gas samples are then stored by needle injection in pre-evacuated glass flasks sealed with butyl rubber stoppers for later concentration determination.
3.2. Methane Concentration in Sediment Gas Bubbles and Water
Bubbles are sampled by stirring up the sediments. Arising bubbles are collected with an entirely submerged 25-cm diameter inverted plastic funnel at sites devoid of vascular vegetation. The open narrow end of the funnel was sealed with a rubber stopper of 3-cm diameter. With a needle, bubble gas samples are pumped into a plastic syringe adapted with a luer-lock valve and thereafter injected in pre-evacuated glass flasks sealed with butyl rubber stoppers for later GC analysis (CH4, N2, CO2 concentration). Also, bubbles samples from natural ebullition are collected into floating traps without disturbing sediments [34] . Bubbles traps (~1 diameter) placed permanently under the surface of water bodies. Bubble N2 content may be indicative of rates of ebullition from sediments [53] . As increasing volumes of CH4 bubble from the sediments, they strip out N2, a gas that is not produced within the sediments. Thus, bubbles with low N2 concentration indicate high rates of bubble stripping or high ebullition. Continuous bubble fluxes of CH4 (mg CH4 m−2∙d−1) at shallow depths (20, 40, 60 cm below the surface and water table) are measured by gas trap funnels [45] . Other method to measure CH4 entrapped can be seen in reference [60] .
If ebullition is episodic it is very difficult to quantify its importance. The role of large ebullition events needs further research and efforts to quantify this mode of emission. Methods that can quantify the distribution (and temporal variability) of free phase gas in the peat profile are needed. CH4 bubbles accumulated at depth, episodically, can break through the confining layer to the surface causing very large rates of CH4 loss to atmosphere (in g∙m−2 as direct emission, no diffusive). Nondestructive geophysical methods that have been used in peatlands [41] such as ground penetrating radar and electrical resistivity could also be used to examine the sediment structure and gas contents within sediments. The determination of dissolved CH4 in soil solution (and DIC) is an alternative non-destructive method, which simplifies the sampling procedure and allows associating the CH4 and DIC dissolved with episodic ebullition (less DIC after ebullition).Reference [26] calculated large degassing events associating surface deformations to largest de-pressuring cycles.
Porewater CH4 can be sampled in situ using modified diffusion cambers called “peepers” [33] . Gas samples collected for dissolved CH4 analysis using a headspace equilibrium technique presented by reference [61] , a 60- ml plastic syringe adapted with a luer-lock valve containing 30 ml of water sample and 30 ml of pure nitrogen was vigorously shaken during 2 min, transferring the dissolved phase to the nitrogen headspace. The headspace was then injected with a needle in pre-evacuated glass flasks sealed with butyl rubber stoppers for later FID-GC analysis.
3.3. Isotope Analysis
After the GC analysis, the remaining gas contained in the glass flasks acquired from the emitted CH4 and CO2 (chambers), CH4 in the water column and CH4y/o CO2 in the sediment bubbles are employed for δ13C-CH4 and analysis in line with a mass spectrometer (GC-IRMS). Isotope analyses are expressed by δ13C (see Notation). Water samples are analyzed for the 13C/12C ratios of their DIC component by precipitating DIC with BaCl2 (BaCO3).
3.4. Notations
Expression of results and some used formulas.
R defines the ratio between two isotopic species. Example:
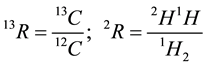
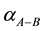 is the fractionation factor between phases or components A and B
is the fractionation factor between phases or components A and B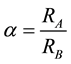 , if > 1, A is enriched in
, if > 1, A is enriched in
the heavy isotope respect B, conversely if < 1, A is depleted in the heavy isotope respect to B. Also is used the enrichment factor (defined as ).
).
Results are expressed in ‰ defined as: 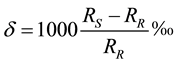 where S denotes sample, R reference standard
where S denotes sample, R reference standard
And the isotopic deviation in ‰, 2H or 13C respect the International reference Standard, V-Pee Dee Belemnitella (VPDB) for 13C and V-Standard Mean Ocean Water for (VSMOW) for δ2H [62] [63] .
Since 2H and 13C are values actually measured, fractionation factor are calculated from δ by means of the fol-
lowing expression: 
4. Conclusion
Principal processes leading to the production and sinking of CH4 in wetlands are presented and discussed mainly from the stable isotopes point of view. Isotope fractionation factors associated to production, diffusion, ebullition, bacterial oxidation, etc., allow using the isotopes of carbon and hydrogen as environmental tracers of these pro- cesses. So, it can be applied to study CH4 and CO2 generation, distribution and consume in wetlands. This review presents an updated list of bibliographic references to support its arguments and serve as a guide to researcher in the thematic of CH4 dynamic from wetland. Also it is presented guidelines of the most reliable field and laboratory methods used at present for the correct sampling and analyzing CH4 in different stages of occurrence.
Cite this paper
RominaSanci,Héctor O.Panarello, (2015) Carbon and Hydrogen Isotopes as Tracers of Methane Dynamic in Wetlands. International Journal of Geosciences,06,720-728. doi: 10.4236/ijg.2015.67058
References
- 1. IPCC (2015) Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, IPCC, Geneva.
- 2. Kirschke, S., Bousquet, P., Ciais, P., Saunois, M., Canadell, J.G., Dlugokencky, E.J., et al. (2013) Three Decades of Global Methane Sources and Sinks. Nature Geoscience, 6, 813-823.
http://dx.doi.org/10.1038/ngeo1955 - 3. Stenke, A., Deckert, R. and Gottschaldt, K. (2012) Methane modeling: From Process Modeling to Global Climate Models. In: Schumann, U., Ed., Atmospheric Physics: Background-Methods-Trends, Springer, Berlin, 781-797, http://dx.doi.org/10.1007/978-3-642-30183-4_47
- 4. IPCC (2007) The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the intergovernmental Panel on Climate Change. In: Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds., Climate Change 2007, Cambridge University Press, Cambridge and New York, 996.
- 5. Keppler, F., Hamilton, J.T.G., Brab, M. and Rockmann, T. (2006) Methane Emissions from Terrestrial Plants under Aerobic Conditions. Nature, 439, 187-191. http://dx.doi.org/10.1038/nature04420
- 6. Mikaloff Fletcher, S.E., Tans, P.P., Bruhwiler, L.M., Miller, J.B. and Heimann, M. (2004) CH4 Source Estimated from Atmospheric Observations of CH4 and Its 13C/12C Isotopic Ratios: 1. Inverse Modeling of Source Processes. Global Biogeochemical Cycles, 18, Article ID: GB4004.
- 7. Mikaloff Fletcher, S.E., Tans, P.P., Bruhwiler, L.M., Miller, J.B. and Heimann, M. (2004) CH4 Source Estimated from Atmospheric Observations of CH4 and Its 13C/12C Isotopic Ratios: 2. Inverse Modeling of CH4 Fluxes from Geographic Regions. Global Biogeochemical Cycles, 18, Article ID: GB4004.
- 8. Bousquet, P., Ciais, P., Miller, J.B., Dlugokencky, E.J., Hauglustaine, D.A., Prigent, C., et al. (2006) Contribution of Anthropogenic and Natural Sources to Atmospheric Methane Variability. Nature, 443, 439-443. http://dx.doi.org/10.1038/nature05132
- 9. Chen, Y.-H. and Prinn, R.G. (2006) Estimation of Atmospheric Methane Emissions between 1996 and 2001 Using a Three-Dimensional Global Chemical Transport Model. Journal of Geophysical Research, 111, Article ID: D10307. http://dx.doi.org/10.1029/2005jd006058
- 10. Neef, L., van Weele, M. and van Velthoven, P. (2010) Optimal Estimation of the Present-Day Global Methane Budget. Global Biogeochemical Cycles, 24, Article ID: GB4024.
http://dx.doi.org/10.1029/2009gb003661 - 11. Whiticar, M.J., Faber, E. and Schoell, M. (1986) Biogenic Methane Formation in Marine and Freshwater Environments: CO2 Reduction vs. Acetate Fermentation—Isotope Evidence. Geochimica et Cosmochimica Acta, 50, 693-709. http://dx.doi.org/10.1016/0016-7037(86)90346-7
- 12. Conrad, R. (1989) Control of Methane Production in Terrestrial Ecosystems, In: Andreae, M.O. and Schimel, D.S., Ed., Exchange of Trace Gases between Terrestrial Ecosystems and the Atmosphere, John Wiley, New York, 39-58.
- 13. Whiticar, M.J. (1999) Carbon and Hydrogen Isotope Systematics of Bacterial Formation and Oxidation of Methane. Chemical Geology, 161, 291-314. http://dx.doi.org/10.1016/S0009-2541(99)00092-3
- 14. Monteil, G., Houweling, S., Dlugockenky, E.J., Maenhout, G., Vaughn, B.H., White, J.W. and Rockmann, T. (2011) Interpreting Methane Variations in the Past Two Decades Using Measurements of CH4 Mixing Ratio and Isotopic Composition. Atmospheric Chemistry and Physics, 11, 9141-9153.
http://dx.doi.org/10.5194/acp-11-9141-2011 - 15. Fisher, R.E., Sriskantharajah, S., Lowry, D., Lanoiselle, M., Fowler, C.M.R., et al. (2011) Arctic Methane Sources: Isotopic Evidence for Atmospheric Inputs. Geophysical Research Letters, 38, Article ID: L21803. http://dx.doi.org/10.1029/2011gl049319
- 16. Khalil, M. (2000) Atmospheric Methane. Springer, New York. http://dx.doi.org/10.1007/978-3-662-04145-1
- 17. Wuebbles, D.J. and Hayhoe, K. (2002) Atmospheric Methane and Global Change. Earth-Science Reviews, 57, 177-210. http://dx.doi.org/10.1016/S0012-8252(01)00062-9
- 18. EPA (2001) Environmental Protection Agency of United States, Office of Water EPA 843-F-01-002b, Oceans and Watersheds (4502T). http://water.epa.gov/type/wetlands/types_index.cfm
- 19. MacDonald, J.A., Fowler, D., Hargreaves, K.J., Skiba, U., Leith, I.D. and Murray, M.B. (1998) Methane Emission Rates from a Northern Wetland: Response to Temperature, Water Table and Transport. Atmospheric Environment, 32, 3219-3227. http://dx.doi.org/10.1016/s1352-2310(97)00464-0
- 20. Christensen, T.R., Panikov, N., Mastepanov, M., Joabsson, M.A., Stewart, A., ?quist, M., Sommerkorn, M., Reynaud, S. and Svensson, B. (2003) Biotic Controls on CO2 and CH4 Exchange in Wetlands—A Closed Environment Study. Biogeochemistry, 64, 337-354. http://dx.doi.org/10.1023/A:1024913730848�
- 21. Olson, D.M., Griffis, T.J., Noormets, A., Kolka, R. and Chen, J. (2013) Interannual, Seasonal, and Retrospective Analysis of the Methane and Carbon Dioxide Budgets of a Temperate Peatland. Journal of Geophysical Research: Biogeosciences, 118, 226-238. http://dx.doi.org/10.1002/jgrg.20031
- 22. Le Mer, J. and Roger, P. (2001) Production, Oxidation, Emission and Consumption of Methane by Soils: A Review. European Journal of Soil Biology, 37, 25-50. http://dx.doi.org/10.1016/S1164-5563(01)01067-6
- 23. Whalen, S.C. (2005) Biogeochemistry of Methane Exchange between Natural Wetlands and the Atmosphere. Environmental Engineering Science, 22, 73-94. http://dx.doi.org/10.1089/ees.2005.22.73
- 24. Lai, D.Y. (2009) Methane Dynamics in Northern Peatlands: A Review. Pedosphere, 19, 409-421. http://dx.doi.org/10.1016/S1002-0160(09)00003-4
- 25. Baird, A., Belyea, L.R., Comas, X., Reeve A.S. and Slater, L.D. (2013) Carbon Cycling in Northern Peatlands. American Geophysical Union (AGU), Washington DC.
- 26. Glaser, P.H., Chanton, J.P., Morin, P., Rosenberry, D.O., Siegel, D.I., Ruud, O., Chasar, L.I. and Reeve, A.S. (2004) Surface Deformations as Indicators of Deep Ebullition Fluxes in a Large Northern Peatland. Global Biogeochemical Cycles, 18, Article ID: GB1003. http://dx.doi.org/10.1029/2003gb002069
- 27. Clark, I.D. and Fritz, P. (1997) Environmental Isotopes in Hydrogeology. Lewis Publishers, New York.
- 28. Coleman, D.D., Liu, C.L., Hackley, K.C. and Benson, L.J. (1993) Identification of Landfill Methane Using Carbon and Hydrogen Isotope Analysis. Proceedings of 16th International Madison Waste Conference, Municipal & Industrial Waste, Department of Engineering Professional Development, University of Wisconsin Madison, Madison, 22-23 September 1993, 303-314.
- 29. Hackley, K.C., Coleman, D.D. and Liu, C.L. (1996) Environmental Isotope Characteristics of Landfill Leachates and Gases. Groundwater, 34, 827-836. http://dx.doi.org/10.1111/j.1745-6584.1996.tb02077.x
- 30. Conrad, R. (2005) Quantification of Methanogenic Pathways Using Stable Carbon Isotopic Signatures: A Review and a Proposal. Organic Geochemistry, 36, 739-752.
http://dx.doi.org/10.1016/j.orggeochem.2004.09.006 - 31. Megonigal, J.P., Hines, M.E. and Visscher, P.T. (2004) Anaerobic Metabolism: Linkages to Trace Gases and Aerobic Processes. In: Schlesinger, W.H., Ed., Biogeochemistry, Elsevier-Pergamon, Oxford, 317-424.
- 32. Hornibrook, E.R.C, Longstae, W.S. and Fyfe, W.S. (1997) Spatial Distribution of Microbial Methane Production Pathways in Temperate Zone Wetland Soils: Stable Carbon and Hydrogen Isotope Evidence. Geochimica et Cosmochimica Acta, 61, 745-753.
- 33. Dorodnikov, M., Marushchak, M., Biasi, C. and Wilmking, M. (2013) Effect of Microtopography on Isotopic Composition of Methane in Porewater and Efflux at a Boreal Peatland. Boreal Environment Research, 18, 269-279.
- 34. Walter, K.M., Chanton, J.P., Chapin, F.S., Schuur, E.A. and Zimov, S.A. (2008) Methane Production and Bubble Emissions from Arctic Lakes: Isotopic Implications for Source Pathways and Ages. Journal of Geophysical Research, 113, Article ID: G00A08. http://dx.doi.org/10.1029/2007JG000569
- 35. Yamamoto, S., Alcauskas, J.B. and Crozier, T.E. (1976) Solubility of Methane in Distilled Water and Seawater. Journal of Chemical & Engineering Data, 21, 78-80. http://dx.doi.org/10.1021/je60068a029
- 36. Simpkins, W.W. and Parkin T.B. (1993) Hydrogeology and Redox Geochemistry of Methane in a Late Wisconsinan Till and Loess Sequence in Central Iowa. Water Resources Research, 29, 3643-3657. http://dx.doi.org/10.1029/93WR01687
- 37. Beckwith, C.W. and Baird, A.J. (2001). Effect of Biogenic Gas Bubbles on Water Flow through Poorly Decomposed Blanket Peat. Water Resources Research, 37, 551-558.
http://dx.doi.org/10.1029/2000WR900303 - 38. Baird, A.J. and Waldron, S. (2003). Shallow Horizontal Groundwater Flow in Peatlands Is Reduced by Bacteriogenic Gas Production. Geophysical Research Letters, 30, Article No. 2043.
http://dx.doi.org/10.1029/2003GL018233 - 39. Strack, M., Kellner, E. and Waddington, J.M. (2005) Dynamics of Biogenic Gas Bubbles in Peat and Their Effects on Peatland Biogeochemistry. Global Biogeochemical Cycles, 19, Article ID: GB1003.
http://dx.doi.org/10.1029/2004GB002330 - 40. Kellner, E., Baird, A.J., Oosterwoud, M., Harrison, K. and Waddington, J.M. (2006) Effect of Temperature and Atmospheric Pressure on Methane (CH4) Ebullition from Near-Surface Peats. Geophysical Research Letters, 33, Article ID: L18405. http://dx.doi.org/10.1029/2006GL027509
- 41. Comas, X., Slater, L. and Reeve, A. (2005). Spatial Variability in Biogenic Gas Accumulations in Peat Soils Is Revealed by Ground Penetrating Radar (GPR). Geophysical Research Letters, 32, Article ID: L08401. http://dx.doi.org/10.1029/2004gl022297
- 42. Tokida, T., Miyazaki, T., Mizoguchi, M., Nagata, O., Takakai, F., Kagemoto, A. and Hatano, R. (2007) Falling Atmospheric Pressure as a Trigger for Methane Ebullition from a Peatland. Global Biogeochemical Cycles, 21, Article ID: GB2003. http://dx.doi.org/10.1029/2006GB002790
- 43. Beckmann, M., Sheppard, S.K. and Lloyd, D. (2004) Mass Spectrometric Monitoring of Gas Dynamics in Peat Monoliths: Effects of Temperature and Diurnal Cycles on Emissions. Atmospheric Environment, 38, 6907-6913. http://dx.doi.org/10.1016/j.atmosenv.2004.08.004
- 44. Fechner-Levy, E.J. and Hemond, H.F. (1996) Trapped Methane Volume and Potential Effects on Methane Ebullition in a Northern Peatland. Limnology and Oceanography, 41, 1375-1383.
http://dx.doi.org/10.4319/lo.1996.41.7.1375 - 45. Coulthard, T.J., Baird, A.J., Ramirez, J. and Waddington, J.M. (2009) Methane Dynamics in Peat: Importance of Shallow Peats and a Novel Reduced-Complexity Approach for Modeling Ebullition. In: Carbon Cycling in Northern Peatlands. Geophysical Monograph Series, 184, 173-185.
http://dx.doi.org/10.1029/2008gm000811 - 46. Siegel, D.I., Chanton, J.E., Glaser, E.H., Chasar, L.C. and Rosenberry, D.O. (2001) Estimating Methane Production Rates in Bogs and Landfills by Deuterium Enrichment of Pore-Water. Global Biogeochemical Cycles, 15, 967-977. http://dx.doi.org/10.1029/2000GB001329
- 47. Romanowicz, E.A., Siegel, D.I., Chanton, J.P. and Glaser, P.H. (1995) Temporal Variations in Dissolved Methane Deep in the Lake Agassiz Peatlands, Minnesota. Global Biogeochemical Cycles, 9, 197-212. http://dx.doi.org/10.1029/95GB00634
- 48. Walter, B.P., Heimann, M. and Matthews, E. (2001) Modeling Modern Methane Emissions from Natural Wetlands: 1. Model Description and Results. Journal of Geophysical Research, 106, 34189-34206.
http://dx.doi.org/10.1029/2001JD900165 - 49. De Visscher, A., De Pourcq, I. and Chanton, J. (2004) Isotope Fractionation Effects by Diffusion and Methane Oxidation in Landfill Cover Soils. Journal of Geophysical Research, 109, Article ID: D18111.
http://dx.doi.org/10.1029/2004jd004857 - 50. Happell, J.D., Chanton, J.P. and Showers, W.J. (1995) Methane Transfer across the Water-Air Interface in Stagnant Wooded Swamps of Florida: Evaluation of Mass-Transfer Coefficients and Isotopic Fractionation. Limnology and Oceanography, 40, 290-298. http://dx.doi.org/10.4319/lo.1995.40.2.0290
- 51. Chanton, J.P., Martens, C.S., Kelley, C.A., Crill, P.M. and Showers, W.J. (1992) Methane Transport Mechanisms and Isotope Fractionation in Emergent Macrophytes of an Alaskan Tundra Lake. Journal of Geophysical Research, 97, 16681-16688. http://dx.doi.org/10.1029/90JD01542
- 52. Bhullar, G.S., Iravani, M., Edwards, P.J. and Venterink, H.O. (2013) Methane Transport and Emissions from Soil as Affected by Water Table and Vascular Plants. BMC Ecology, 13, 32.
http://dx.doi.org/10.1186/1472-6785-13-32 - 53. Chanton, J.P. (2005) The Effect of Gas Transport on the Isotope Signature of Methane in Wetlands. Organic Geochemistry, 36, 753-768. http://dx.doi.org/10.1016/j.orggeochem.2004.10.007
- 54. Bergamaschi, P. (1997) Seasonal Variation of Stable Hydrogen and Carbon Isotope Ratios in Methane from a Chinese Rice Paddy. Journal of Geophysical Research, 102, 25383-25393.
http://dx.doi.org/10.1029/97JD01664 - 55. Coleman, D.D., Risatti, J.B. and Schoell, M. (1981) Fractionation of Carbon and Hydrogen Isotopes by Methane-Oxidizing Bacteria. Geochimica et Cosmochimica Acta, 45, 1033-1037.
http://dx.doi.org/10.1016/0016-7037(81)90129-0 - 56. Gebert, J., Streblow, C. and Pfeiffer, E.M. (2011) Impact of Gas Transport on Fractionation of Carbon Stable Isotope Related to the Microbial Oxidation of Methane in Soils. EGU General Assembly. Geophysical Research Abstracts, 13, Article No. 14108.
- 57. Happell, J., Chanton, J.P. and Showers, W. (1994) The Influence of Methane Oxidation on the Stable Isotope Composition of Methane Emitted from Florida Swamp Forests. Geochimica et Cosmochimica Acta, 58, 4377-4388. http://dx.doi.org/10.1016/0016-7037(94)90341-7
- 58. Sanci, R., Panarello, H. and Ostera, H. (2009) Assessment of Soil Moisture Influence on CO2 Flux: A Laboratory Experiment. Environmental Geology, 58, 491-497. http://dx.doi.org/10.1007/s00254-008-1522-7
- 59. Sanci, R., Panarello, H. and Ostera, H. (2012) CO2 Emissions from a Municipal Site for Final Disposal of Solid Waste in Gualeguaychú, Entre Ríos Province, Argentina. Environmental Earth Sciences, 66, 519-528. http://dx.doi.org/10.1007/s12665-011-1260-0
- 60. Wassmann, R., Neue, H.U., Alberto, M.C.R., Lantin, R.S. Bueno, C., Llenaresas, D., Arah, J.R.M., Papen, H., Seiler, W. and Rennenberg, H. (1996) Fluxes and Pools of Methane in Wetlands Rices Oils with Varying Organic Inputs. Environmental Monitoring and Assessment, 42, 163-173.
http://dx.doi.org/10.1007/BF00394048 - 61. McAuliffe, C. (1971) Gas Chromatographic Determination of Solutes by Multiple Phase Equilibrium. Chemical Technology, 1, 46-51.
- 62. Gonfiantini, R. (1978) Standards for Stable Isotope Measurements in Natural Compounds. Nature, 271, 534-536. http://dx.doi.org/10.1038/271534a0
- 63. Coplen, T. (1994) Reporting of Stable Hydrogen, Carbon, and Oxygen Isotopic Abundances. Pure and Applied Chemistry, 66, 273-276. http://dx.doi.org/10.1351/pac199466020273
NOTES
*Corresponding author.



