Food and Nutrition Sciences
Vol.4 No.8A(2013), Article ID:35257,7 pages DOI:10.4236/fns.2013.48A007
Nutritional, Antioxidant and Anti-Inflammatory Properties of Cyclanthera pedata, an Andinean Fruit and Products Derived from Them
![]()
1INQUINOA (CONICET), Tucumán, Argentina; 2Cátedra de Botánica Sistemática y Fitogeografía, Facultad de Ciencias Agrarias, Universidad Nacional de Jujuy, Alberdi, San Salvador de Jujuy, Argentina; 3Cátedra de Elementos de Química Orgánica y Biológica, Facultad de Ciencias Naturales e Instituto Miguel Lillo, Tucumán, Argentina; 4Cátedra de Fitoquímica, Facultad de Bioquímica, Química y Farmacia, Universidad Nacional de Tucumán, (4000) S.M. de Tucumán, Tucumán, Argentina; 5Fundación Miguel Lillo, Tucumán, Argentina.
Email: *misla@tucbbs.com.ar
Copyright © 2013 Marisa Rivas et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received April 8th, 2013; revised May 8th, 2013; accepted May 16th, 2013
Keywords: achojcha Fruit; achojcha Flour; Cyclanthera pedata; Nutritional Properties; Functional Properties
ABSTRACT
There is an increasing interest in food plant with health-giving effects. The aim of this research was to evaluate the phytochemical and nutraceutical profiles of Cyclanthera pedata fruits, a native fruit of the Andean region used by Incas communities called achojcha. Soluble protein (SP), total sugar (TS), free phenolic compounds (FPC), ascorbic acid (AA), total monomeric anthocyanin (TMA), condensed and hydrolizable tannin (CT, HT) were evaluated by sprectrophotometric methods in fresh fruits with and without heating and in flour obtained from fruits. Multielemental composition by ICP-MS was done. Antioxidant activity (AOA) was determined by ABTS and Linoleic acid-b-carotene methods. The inhibitory capacity of LOX, a pro-inflammatory enzyme was also analyzed. The achojcha fresh fruits and achojcha flour showed low amount calories. The flour was high in potassium (7400 mg∙K/100 g) and low in sodium (77 mg Na/100 g). High levels of FPC (670 mg GAE/100 g) were found together with elevated levels of AA (123 mg AA/ 100 g). The TMA (0.6 mg C3-GE/100 g) as well as HT (3.4 mg PB2E/100 g) were also detected in flour. All preparations obtained with fresh and dried fruits showed AOA with SC50 values between 1.8 to 14.5 μg GAE/mL. Inhibitory capacity on LOX was also demonstrated (IC50 values of 40 µg GAE/mL). The fresh fruits and flour showed nutraceutical characteristics that are demanded by functional food and could be used as dietary supplement.
1. Introduction
South America offers a wide diversity of plants and unique seasonal crops mainly due to the presence of natural areas such as the Andean mountains or the Amazon rainforest. Several scientific reports have pointed out the therapeutic potential of certain food plants from Andean mountains such as “maca” (Lepidium meyenii) [1] and “yacon” (Smallantus sonchifolius) [2] have been linked to multi-pharmacological properties. Cyclanthera pedata Schrad is of South American origin, where it is known by the common name of “achojcha”, “achocha”, “caygua”, “caihua”, “achuqcha” (quechua name). It is thought to be native to the Andean region or “Sierra”, and was cultivated by the Incas who used its fruits as food [3-6]. The fruit is a berry (10 - 20 cm length) with irregular surface, soft spines and longitudinal grooves. Its color varies from dark green to white. The mesocarp (edible part) is thin and succulent. The endocarp is white and fluffy. Its seeds are roughly quadrangular and rough black. Actually, the “achojcha” fruits are largely used in South America to make salad or soup for their medicinal properties popularly attributed, such as anti-inflammatory, hypoglycemic and hypocholesterolemic [7]. It thus represents an example of a plant used for medicinal purposes, and can appropriately be considered within the above-described context of food plant with health-giving effects. For this reason, C. pedata has a commercial interest in the functional food market. The nations involved in promoting the diffusion of this species are Peru, Ecuador (in particular the southern part), Bolivia, Colombia, Venezuela and north of Argentina. Fruits and seeds are rich in cucurbitacins, which are important as chemotaxonomic markers [8]. A number of studies have highlighted the presence of saponins [9,10] in fruits and seeds and Oand C-glycosides of chrysin and apigenin in fruits [6,11]. It was recently described inhibitory activity of angiotensin I-converting enzyme (ACE) [12]. The aim of this research was to evaluate the phytochemical and nutraceutical profiles of Cyclanthera pedata fruits (fresh fruits with and without heating) and in flour obtained from them by lyophilization process.
2. Materials and Methods
2.1. Plant Material
The fruits of C. pedata (Figure 1(a)) were purchased in local markets in the province of Jujuy, Argentina. A voucher sample is deposited in the Muestrario de Plantas útiles de la Cátedra de Botánica Sistemática y Fitogeografía de la Facultad de Ciencias Agrarias, Universidad Nacional de Jujuy (M-CBSF-072).
2.2. Product Derived from Fresh Ripe Fruits
Extractions without heating: Fresh fruits (100 g) were homogenized with 100 mL of distilled water in a blender during 20 minutes. Then the preparation was filtered through Whatman N˚4 paper. The filtrate was named aqueous extract (AE) without heating. The residues were extracted with 96% ethanol during 24 h at room temperature. Then, the preparation was filtered through Whatman N˚4 paper. The extract was named as maceration (M) without heating.
Extraction with heating: Fresh fruits (100 g) were decocted in 100 mL of distilled water for 20 min. The decoction was left to cool at room temperature and filtered through Whatman N˚4 filter paper. The extracted material was named AE with heating. The residues were extracted with 96% ethanol for 20 min at 70˚C and then were filtered through Whatman N˚4 filter paper. The extract was named as M with heating.
The extraction yield was calculated as % phenolic


Figure 1. (a) “Achojcha” fruits, (b) “Achojcha” fruits flour.
compounds per 100 g of fruits dry weight.
2.3. Extracts Obtained from Flour (Lyophilized Fruits)
Ripe fruits were lyophilized to obtain flour from “achojcha” (Figure 1(b)).
Acetone-water extract (AWE): Flour (1 g) was extracted with 10 mL acetone: water (70:30, v:v) in an ultrasonic bath for 30 min at room temperature and then centrifuged at 9000 × g during 10 min. The suspension obtained was filtered and the remaining solids were extracted exhaustively with the same solvent system. All organic extracts were combined and the acetone was evaporated, then the final volume was adjusted to 5 mL. After that, a fraction was subjected to acid hydrolysis by adding sulfuric acid (2N) to the aqueous fraction. The solution was maintained at 100˚C during 26 h.
Ethanolic extract (EE): Flour (1 g) was extracted with 12.5 mL ethanol 96% in an ultrasonic bath for 30 min at room temperature and then centrifuged at 9000 × g during 10 min. The suspension obtained was filtered and the remaining solids were extracted exhaustively with the same solvent system. All organic extracts were combined, evaporated and the final volume adjusted to 5 mL.
2.4. Chemical Composition Determination
2.4.1. Sugar
Sugar extraction: Flour (1 g) or fresh fruits (1 g) was extracted with ethanol 80% (4 mL) at 75˚C during 10 min and then centrifuged at 9000 × g during 5 min [13]. The remaining solids were extracted exhaustively with the same solvent system. All organic extracts were combined and then evaporated.
Sugar determination: The phenol-sulphuric acid method [14] was used to determine total neutral sugars in aqueous and ethanolic preparations. Reducing sugars were measured using the Somogyi-Nelson method [15,16]. Results were expressed as g of glucose (GE)/100 g dry weight.
2.4.2. Protein
Soluble protein concentration in all preparations was determined by the method of Bradford [17] using bovine serum albumin (BSA) as standard. Results were expressed as mg of BSA/100 g dry weight (mg BSA/100 g DW). The total Nitrogen (N) content of lyophilized fruits was determined by Kjeldahl method [18]. Crude protein content was calculated as % N × 6.25.
2.4.3. Total Polyphenols and Non-Flavonoid Phenols
Total phenolic content of the samples was determined using the Folin-Ciocalteu reagent [19]. Results were expressed in mg of gallic acid equivalents per 100 g dry weight (mg GAE/100gDW). Non-flavonoid phenols were measured by determination of total phenol content remaining after precipitation of the flavonoids with acidic formaldehyde [20]. Results are expressed in mg GAE/ 100 g DW.
2.4.4. Flavones and Flavonols
The AlCl3 method [21] was used for the determination of the flavones and flavonols content of the fruit extract. 0.5 mL of ethanolic 2% AlCl3∙6H2O was added to equal volumes of each extract. The mixture was shaken and the absorbance read at 420 nm after 60 min incubation at room temperature. Flavonoid content was expressed as mg quercetin (Q) equivalents per 100 g dry weight (mg QE/100 g DW).
2.4.5. Flavanone and Dihydroflavonols
Flavanone and dihydroflavonol content was determined using DNP in acid media according to Popova et al. [22]. An aliquot of each extract sample was diluted with 96˚ ethanol to a volume of 0.25 mL. Next, 0.5 mL of DNP solution (1 g DNP in 2 mL 96% sulfuric acid, diluted to 100 mL with methanol) was added and heated at 50˚C for 50 min. After cooling to room temperature, 0.3 mL of the previous mixture was diluted to 1 mL with 10% KOH. The resulting solution was centrifuged at 1500 × g for 10 min and 0.25 mL of the supernatants was diluted to 1.5 mL with methanol. Absorbance was measured at 492 nm. Flavanone and dihydroflavonol content was estimated using a calibration curve of naringenin with a concentration range of 30 - 120 µg. Results were expressed as mg naringenin equivalent per 100 g dry weight (mg NGE/100 g DW).
2.4.6. Proanthocyanidins
The total proanthocyanidins (PACs) content was determined with 4-dimethylaminocinnamaldehyde (DMAC) according to Prior et al. [23]. 450 μL of DMAC solution (0.1% in acidified ethanol) was added to 150 μL of AWE. The absorbance was measured at 640 nm after 20 min at 25˚C. Data were expressed as mg of procyanidin B2 equivalents per 100 g dry weight (mg PB2E/100 g DW).
2.4.7. Gallotanins
The AWE (2 mL) was first hydrolyzed with 4 mL of 2 N H2SO4 at 100˚C for 26 h and the gallic acid released was determined with the rhodanine method [24]. The hydrolyzed AWE (HAWE) and non hidrolyzed AWE were dried under nitrogen and resuspended in 200 μL of 0.2 N H2SO4. Two hundred μL of 0.2 N H2SO4 and 300 μL rhodanine (0.667% methanol) were added to the diluted extracts. After 5 min, 200 μL of 0.5 N potassium hydroxide and 4 mL distilled water were added and the absorbance at 520 nm was determined. Gallotannin concentrations were expressed as mg gallic acid equivalents per 100 g dry weight (mg GAE/100 g DW).
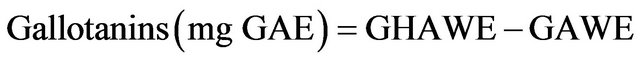
where GHAWE is the amount of gallic acid present in HAWE and GAWE is the amount of gallic acid present in the sample without hydrolysis.
2.4.8. Total Monomeric Anthocyanins (TMA)
Flour (1 g) was extracted with 5 mL 1% HCl in methanol overnight at 5˚C and then filtered through Whatman N˚1 filter paper and the remaining solids were extracted three times with the same solvent. All extracts were combined, vacuum-concentrated and resuspended with 5 mL MILLIQ water to obtain the total monomeric anthocyanin extract (TMA).
Total anthocyanins were evaluated by the pH differential method [25]. The TMA in 25 mM potassium chloride solution (pH 4.5) and 400 mM sodium acetate buffer (pH 1.0) were measured simultaneously at 520 nm and 700 nm, respectively. The content of total anthocyanins was expressed as mg cyanidin-3-glucoside equivalents per 100 g of dry weight (mg C3-G E/100 g DW).
2.4.9. Total Carotenoids
Samples (1 g flour) were extracted with 10 mL of hexane:acetone: ethanol (50:25:25, v/v). After centrifugation at 13000 × g for 10 min at 4˚C, the top hexane layer was recovered and the absorbance was measured at 450 nm. Total carotenoid content was calculated as mg of β-carotene equivalents per 100 g of dry weight (g β-CE/ 100 g DW), [26].
2.4.10. Ascorbic Acid
Flour (0.5 g) were extracted with 1.35 mL of H3PO4 2% according to Barros et al. [27]. After centrifuging at 12,000 × g for 10 min, the supernatant was reserved to determine the ascorbic acid content using 2.6 dichloroindophenol sodium salt hydrate (IDF). Different dilutions was added to 125 µL of sodium acetate buffer 400 mM, pH 4; 40 µL of IDF and distilled water until 1 mL. After mixing, the absorbance was measured at 515 nm. Vitamin C was calculated and expressed as mg L-ascorbic acid per 100 g dry weight (mg L-AA/100 g DW).
2.4.11. Elemental Analyses
The analysis was carried out by quadrupole inductively plasma mass spectrometry (Q-ICPMS). A Thermo-Elemental X7 series (Thermo Fisher Scientific, Bremen, Germany), equipped with an ASX-100 autosampler model (CETAC Technologies, Omaha, NE), was used (Instituto Superior de Investigación Desarrollo y Servicios en Alimentos, ISIDSA).
2.5. Measurement of Antioxidant Capacity
2.5.1. ABTS Free Radical Scavenging Activity
The antioxidant capacity assay was carried out by the improved ABTS●+ method [28]. The ABTS●+ solution (7 mM ABTS and 2.45 mM potassium) was diluted to obtain an absorbance of 0.70 at 734 nm in ethanol (for EE), in acetone/water (for AWE) or in buffer PBS pH 7.4 (for AE). ABTS●+ solution (1 mL) was added to AE, AWE and EE (1 to 30 µg GAE/mL) or commercial antioxidant (BHT, AA and Q) and mixed thoroughly. Absorbance was recorded at 734 nm during 6 min. The concentration of “achojcha” extract required to scavenge 50% of ABTS●+. (SC50 values) was calculated as µg GAE/mL
2.5.2. β-Carotene Bleaching Assay
Antioxidant activity was determined using the system linoleic acid-β-Carotene [29]. The initial absorbance at 470 nm was registered at zero time (t0) and during 120 min. Antioxidant activity (AA%) was calculated as percent inhibition relative to control without “achojcha” extract. IC50 values denote the µg GAE/mL required to inhibit 50% β-carotene bleaching.
2.6. Anti-Inflammatory Activity of “Achojcha” Flour
Lipoxygenase Enzyme Assay
Lipoxygenase (LOX) activity was determined using a spectrophotometric method, based on the enzymatic oxidation of linoleic acid to the corresponding hydroperoxide [30]. The reaction mixture contained substrate (50 µM linoleic acid in 0.2 M borate buffer pH 9), enzyme (0.9 nM soy LOX-1, Sigma-Aldrich) and different concentrations of “achojcha” extracts. A control without extracts was realized. Absorption at 234 nm was recorded as a function of time during 4 min. The concentration of “achojcha” extract that produce 50% inhibition of hydroperoxide-release (IC50) was calculated from the concentration-inhibition response curve by regression analysis. Caffeic acid was used as reference anti-inflammatory compounds.
3. Results
3.1. Nutritional Values of “Achojcha” Fruits
Fruits are most commonly consumed raw; however, their jams and liquors are also prepared to preserve them for longer periods of time. Physicochemical changes may occur during processing fruits that affect the functional properties. Preparations simulating domestic processing from fresh fruits, an aqueous preparation without and with cooked (by boiling during 20 min) and an alcoholic beverage with and without heating and lyophilized fruits (achojcha flour) were evaluated to determine the impact of processing on the chemical composition and its potential health benefits.
Sugar contents in fresh fruit were significantly higher in aqueous preparation than in tincture. The total carbohydrate and reducing carbohydrates were low (0.52% and 0.57%, respectively) as well as soluble protein (0.031% and 0.023%) (Table 1). The content of sugar and proteins was not affected by heating. The contained of total sugar and reducing sugar in flour was 4.74 g/100 g and 3.12 g/100 g of flour, respectively. The total protein content was of 0.4%. According with these results, the “achojcha” fresh fruits and “achojcha” flour showed low calories and could be incorporated in the hypocaloric diet. The mineral elements composition of “achojcha” flour was determined. The “achojcha” flour was high in potassium (7400 mg K/100 g) and low in sodium (77 mg Na/ 100 g). The flour had the highest contents in most of the elements, especially in calcium (480 mg Ca/100 g), magnesium (199 mg Mg/100 g) and iron (5.2 mg Fe/100 g). The daily mineral requirements of the Andean population could be cover partially with Andean fruits.
3.2. Quantification of Bioactive Compounds
The aqueous preparations from fresh fruits showed 2.5 to 3 folds higher content of total phenolic compounds (18.04 and 21.65 mg GAE/100 g DW) than ethanolic preparations (7.15 and 7.82 mg GAE/100 g DW), being principally non flavonoids compounds (Table 2).
On the other hand, the extractions of phenolic compounds were not affected by heat (Table 2).
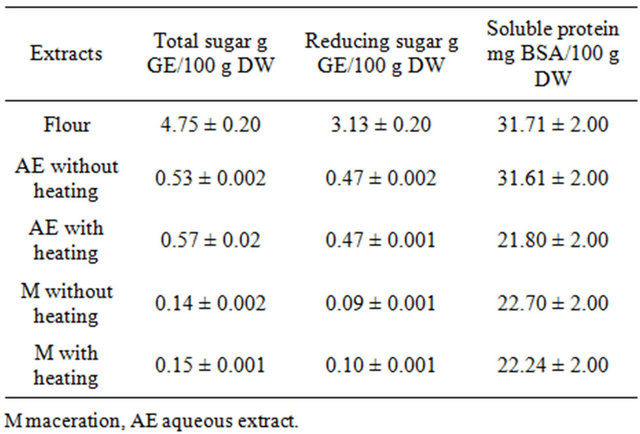
Table 1. Macronutrients in aqueous and ethanolic preparations obtained from “achojcha” fresh fruits and flour obtained from lyophilized fruits.
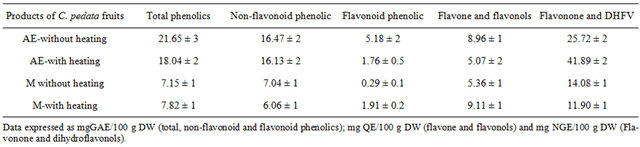
Table 2. Phytochemicals in fresh fruits.
The phenolic extractions of powered dried fruits (670 mg GAE/100 g DW) were more efficient than the extractions obtained from fresh fruits (Tables 2 and 3). Vasco et al. [31] classified the fruits on dry matter according to the polyphenolic content in low (<100 mg GAE/100 g), medium (100 - 500 mg GAE/100 g) and high (>500 mg GAE/100 g) category, Type I, II and III, respectively. The “achojcha” flour could be considered as Type III (high content of phenolic compound). Flavonoids and hydrolizable tannins were the dominant phenolics in the flour (266 mg/100 g DW and 3.4 mg PB2E/100 g DW, respectively). Other phenolic compounds such as anthocyanins were also detected (Table 3). The AA content was 122.82 mg/100 g DW. The values were higher than the reported for fruits like kiwi [32]. The ascorbic acid daily intake recommended in packaged food is 45 mg. For this reason, the consumption of 35 g of achojcha flour may be necessary to cover these requirements [33]. The carotenoid content (1.23 mg β-CE/100 g) was lower than the report for other Cucurbitaceas (10 to 20 mg β- CE/100 g) [34].
3.3. Antioxidant Activity of Preparations Obtained from “Achojcha” Fruits
The antioxidant activity of extracts obtained from “achojcha” fruits were analyzed in the present study. All preparations exhibited ABTS reducing capacity (Figure 2) with SC50 values between 1.7 to 4.82 μg GAE/mL for ABTS. The aqueous extracts with and without heating showed the same SC50 (2 μg GAE/mL) while the beverage with heating (3.4 μg GAE/mL) was more active as antioxidant than beverage obtained without heating (4.82 μg GAE/mL). The extract enriched with hydrolysable tannins (SC50 = 1.7 μg GAE/mL) was more active than the polyphenolic extract (SC50 = 4 μg GAE/mL). In general all the preparations obtained both fresh fruit and flour showed higher antioxidant capacity than natural and synthetic antioxidants (Quercetin: SC50 = 18 μg/mL, AA: SC50 = 54 μg/mL and BHT: SC50 = 55 μg/mL).
Furthermore, all extracts were able to protect lipids from oxidation with IC50 values similar to BHT (IC50 = 4
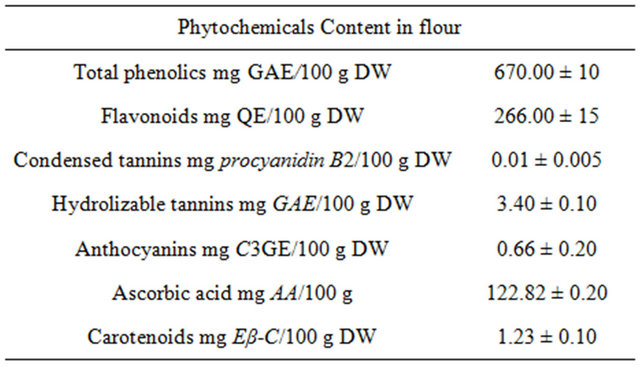
Table 3. Phytochemicals in flour of C. pedata fruits.
µg/mL) and higher than Quercetin (IC50 = 20 µg/mL) and AA (IC50 = 52 µg/mL).
The ethanolic extract obtained from fresh fruits without heating (IC50 = 7 μg GAE/mL) and from flour (8.2 μg GAE/mL) has similar antioxidant activity and less than the decoctions (IC50 = 1.8 μg GAE/mL) and maceration (IC50 = 5 μg GAE/mL) of fresh fruit. The extract enriched with hydrolysable tannins was the least active (IC50 = 14.5 μg GAE/mL). In all cases, polyphenols showed a dose-response relationship until 2 or 3 μg GAE/mL (R2 > 0.90) with antioxidant capacity.
3.4. Anti-Inflammatory Activity of Preparations Obtained from “Achojcha” Fruits
Products of the 5-LOX pathway are important mediators of inflammation. LOX and its reaction products are shown to play an important role in tumor formation and cancer metastasis [35]. Inhibitors of the 5-LOX pathway, therefore, have a therapeutic potential in a variety of inflammatory and allergic diseases as well as in cancer therapy. The polyphenolic extract obtained from “achojcha” flour showed an inhibitory effect on LOX activity with an IC50 value of 40 μg GAE/mL (Figure 3). The activity was similar to obtained for caffeic acid (IC50 = 45 GAE/mL), a phenolic compound with demonstrated activity on LOX.
The “achojcha” fruits can play an important economic role, either in the international market or locally in certain countries of tropical America for its nutraceutical
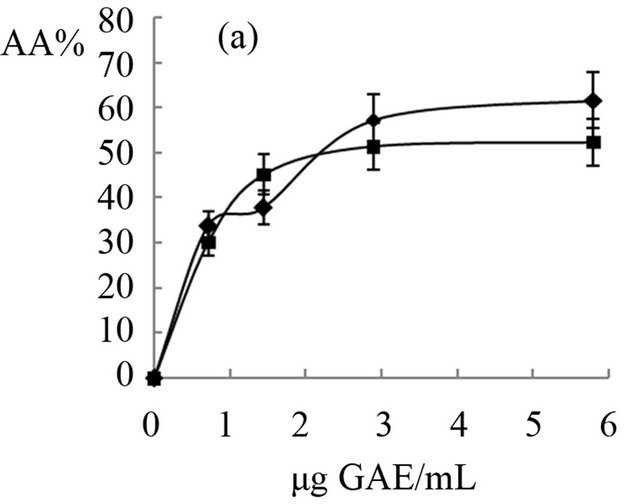
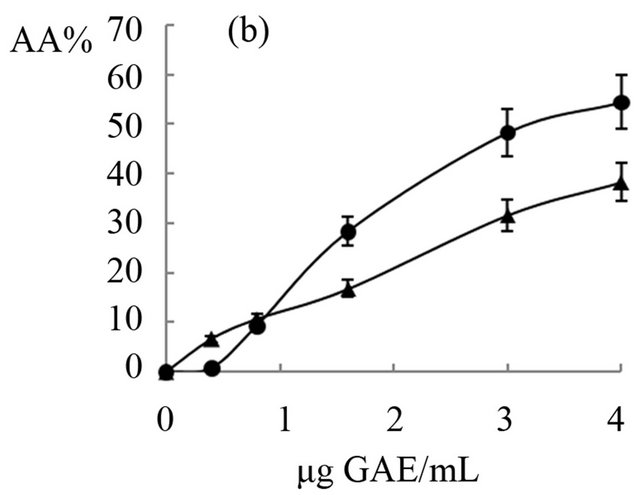
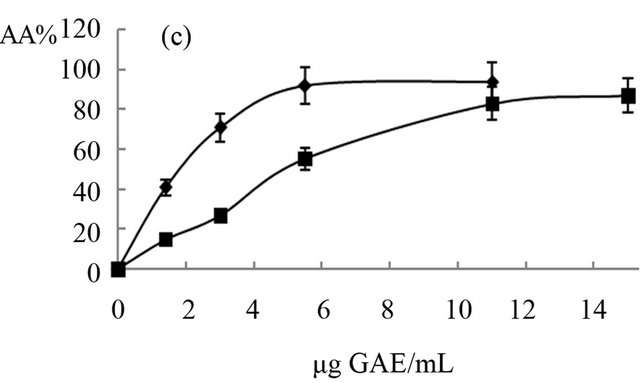
Figure 2. Antioxidant effects of “achojcha” preparation on ABTS●+. A) ●-Aqueous extract (AE) without heating -■-AE with heating B) ▲-Maceration (M) without heating -•-M with heating C) ¨-Acetonic extract (AWE); -■-Ethanolic extract (EE).

Figure 3. Effect of polyphenolic extractions from “achojcha” flour on LOX activity.
characteristics that are demanded by functional food. The lyophilized form obtained from them could be used as dietary supplement (antioxidant and anti-inflammatory) for its content of bioactive compounds (PC, F, AA and minerals) and low sugar content and used in hypocaloric diet. The bioactive food components could be administered in encapsulated forms to overcome the drawbacks of their instability, alleviate unpleasant tastes or flavors, and improve the bioavailability and half-life of the bioactive compound in vivo.
4. Acknowledgements
The authors thank the inhabitants of the areas of study for their cooperation and acknowledge the financial support from Consejo de Investigación de la Universidad Nacional de Tucumán (CIUNT 26 D-430), Argentina and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET PIP-704), Argentina.
REFERENCES
- Y. Wang, Y. Wang, B. McNeil and L. M. Harvey, “Maca: An Andean Crop with Multi-Pharmacological Functions,” Food Research International, Vol. 40, No. 7, 2007, pp. 783-792. doi:10.1016/j.foodres.2007.02.005
- J. Lachman, E. C. Fernández and M. Orsák, “Yacon [Smallanthus sonchifolia (Poepp. et Endl.) H. Robinson] Chemical Composition and Use—A Review,” Plant Soil Environmental, Vol. 49, No. 6, 2003, pp. 283-290.
- H. Dietschy, “L’antica Medicina Peruviana,” Ciba. Milano, No. 40, 1953, pp. 1318-1345.
- H. Popenoe and D. C. Washington, “Lost Crops of the Incas: Little-Known Plants of the Andes with Promise for Worldwide Cultivation,” Vol. 428, 1990, pp. 206-209.
- J. F. Macbride, “Cucurbitaceae Flora of Peru,” In: Field Museum of Natural History, Botanical Series, 1937, pp. 321-383.
- P. Montoro, V. Carbone, F. de Simone, C. Pizza and N. de Tommasi, “Studies on the Constituents of Cyclanthera pedata Fruits: Isolation and Structure Elucidation of New Flavonoid Glycosides and Their Antioxidant Activity,” Journal of Agriculture and Food Chemistry, Vol. 49, No. 11, 2002, pp. 5156-5161. doi:10.1021/jf010318q
- M. Monigatti, R Bussmann and C. Weckerle, “Medicinal Plant Use in Two Andean Communities Located at Different Altitudes in the Bolıvar Province, Peru,” Journal of Ethnopharmacology, Vol. 145, No. 2, 2012, pp. 450-464. doi:10.1016/j.jep.2012.10.066
- L. Dinan, J. Harmatha and R. Lafont, “Chromatographic Procedures for the Isolation of Plant Steroids,” Journal of Chromatographic A, Vol. 935, No. 1-2, 2001, pp. 105- 123. doi:10.1016/S0021-9673(01)00992-X
- N. De Tommasi, F. De Simone, G. Speranza and C. Pizza, “Studies on the Constituents of Cyclanthera pedata (Caigua) Seeds: Isolation and Characterization of Six New Cucurbitacin Glycosides,” Journal of Agriculture and Food Chemistry, Vol. 44, No. 8, 1996, pp. 2020-2025. doi:10.1021/jf950532c
- N. De Tommasi, F. De Simone, G. Speranza and C. Pizza, “Studies on the Constituents of Cyclanthera pedata Fruits: Isolation and Structure Elucidation of New Triterpenoid Saponins,” Journal of Agriculture and Food Chemistry, Vol. 47, No. 11, 1999, pp. 4512-4519. doi:10.1021/jf9900128
- V. Carbone, P. Montoro, N. De Tommasi and C. Pizza, “Analysis of Flavonoids from Cyclanthera pedata Fruits by Liquid Chromatography/Electrospray Mass Spectrometry,” Journal of Pharmaceutics and Biomedical Analysis, Vol. 34, No. 2, 2004, pp. 295-304.
- L. Galvez Ranilla, Y. Kwon, E. Apostolidis and K. Shetty, “Phenolic Compounds, Antioxidant Activity and in Vitro Inhibitory Potential against Key Enzymes Relevant for Hyperglycemia and Hypertension of Commonly Used Medicinal Plants, Herbs and Spices in Latin America,” Bioresource Technology, Vol. 101, No. 12, 2010, pp. 4676-4689. doi:10.1016/j.biortech.2010.01.093
- F. E. Prado, J. A. Gonzalez, C. Boero and A. R. Sampietro, “A Simple and Sensitive Method for Determining Reducing Sugars in Plant Tissues. Application to Quantify the Sugar Content in Quinoa (Chenopodium quinoa Willd.) Seedlings,” Phytochemical Analysis, Vol. 9, No. 2, 1998, pp. 58-62.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. A., Rebers and F. Smith, “Colorimetric Method for Determination of Sugars and Related Substances,” Analytical Chemistry, Vol. 28, No. 3, 1956, pp. 350-356. doi:10.1021/ac60111a017
- M. Somogyi, “A New Reagent for the Determination of Sugar,” Journal of Biological Chemistry, Vol. 160, No. 1, 1945, pp. 61-68.
- N. Nelson, “A Photometric Adaptation of the Somogyi Method for the Determination of Glucose,” Journal of Biological Chemistry, Vol. 153, 1944, pp. 375-380.
- M. M. Bradford, “Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding,” Analytical Biochemistry, Vol. 72, No. 1-2, 1976, pp. 248-254. doi:10.1016/0003-2697(76)90527-3
- AOAC, “Official Methods of Analysis,” 16th Edition, Association of Official Analytical Chemists, Arlington, 1998.
- V. L. Singleton, R. Orthofer and R. M. Lamuela-Raventos, “Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent,” Method in Enzymology, Vol. 299, 1999, pp. 152- 178. doi:10.1016/S0076-6879(99)99017-1
- B. W. Zoecklein, K. C. Fuelsang B. H. Gump and F. S. Nury, “Phenolic Compounds and Wine Color,” In: Van Nostrand Reinhold, Ed., Production Wine Analysis, New York, 1990, pp. 129-168. doi:10.1007/978-1-4615-8146-8_7
- J. L. C. Lamaison and A. Carnet, “Teneurs en Principaux Flavonoides des Fleurs de Crataegus monogyna Jacq et de Crataegus laevigata (Poiret D. C) en Fonction de la Vegetation,” Pharmaceutica Acta Helvetia, Vol. 65, 1990, pp. 315-320.
- M. Popova, S. Silici, O. Kaftanoglu and V. Bankova, “Antibacterial Activity of Turkish Propolis and Its Qualitative and Quantitative Chemical Composition,” Phytomedicine, Vol. 12, No. 3, 2005, pp. 221-228.
- R. L. Prior, E. Fan, H. Ji, A. Howell, C. Nico, M. J. Payne and J. Reed, “Multilaboratory Validation of a Standar Method for Quantifying Proanthocyanidins in Cranberry Powders,” Journal of the Science of Food and Agriculture, Vol. 90, No. 9, 2010, pp. 1473-1478. doi:10.1002/jsfa.3966
- K. H. Inoue and A. E. Hagerman, “Determination of Gallotannins with Rhodanine,” Analytical Biochemistry, Vol. 169, 1988, pp. 363-369.
- J. Lee, R. W. Durst and R. E. Wrolstad, “Determination of Total Monomeric Anthocyanin Pigment Content of Fruits Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study,” Journal of AOAC International, Vol. 88, No. 5, 2005, pp. 1269-1278.
- D. B. Rodríguez-Amaya, “A Guide to Carotenoid Analysis in Foods,” ILDI Press, Washington DC, 1999.
- L. Barros, S. Heleno, A. Carvalho and I. Ferreira, “Lamiaceae Often Used in Portuguese Folk Medicine as a Source of Powerful Antioxidants: Vitamins and Phenolics,” Food Science and Technology, Vol. 43, No. 3, 2010, pp. 544- 550. doi:10.1016/j.lwt.2009.09.024
- R. Re, N. Pellegrini, A. Proteggente, A. Pannala, M. Yang and C. Rice-Evans, “Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay,” Free Radical Biology and Medicine, Vol. 26, No. 9-10, 1999, pp. 1231-1237. doi:10.1016/S0891-5849(98)00315-3
- A. A. Ordoñez, D. Gomez, M. A. Vattuone and M. I. Isla, “Antioxidant Activity of Sechium edule (Jacq) Swartz,” Food Chemistry, Vol. 97, No. 3, 2006, pp. 452-458. doi:10.1016/j.foodchem.2005.05.024
- I. B. Taraporewala and J. M. Kauffman, “Synthesis and Structure-Activity Relationship of Anti-Inflammatory 9, 10-Dihydro-9-oxo-2-acridine-alkanoic Acids and 4-(2-Carboxyphenyl) Aminobenzenealkanoic Acids,” Journal of Pharmaceutical Sciences, Vol. 79, No. 2, 1990, pp. 173- 178. doi:10.1002/jps.2600790219
- C. Vasco, J. Ruales and A. Kamal-Eldin, “Total Phenolic Compounds and Antioxidant Capacities of Major Fruits from Ecuador,” Food Chemistry, Vol. 111, No. 4, 2008, pp. 816-823. doi:10.1016/j.foodchem.2008.04.054
- A. Valente, T. Albuquerque, A. Sanchez-Silva and H. Costa, “Ascorbic Acid Content in Exotic Fruits: A Contribution to Produce Quality Data for Food Composition Databases,” Food Research International, Vol. 44, No. 7, 2011, pp. 2237-2242. doi:10.1016/j.foodres.2011.02.012
- Expert Consultation Bangkak, “Human Vitamin and Mineral Requirements,” Report 07 a Joint FAO/OMS, 2001.
- D. Konopacka, A. Seroczyńska, A. Korzeniewska, K. Jesionkowska, K. Niemirowicz-Szczytt and W. Płocharski, “Studies on the Usefulness of Cucurbita Maxima for the Production of Ready-to-Eat Dried Vegetable Snacks with a High Carotenoid Content,” LWT—Food Science and Technology, Vol. 43, No. 2, 2010, pp. 302-309. doi:10.1016/j.lwt.2009.08.012
- A. Waldiceu, Verri Jr., T. M. C. Vicentini, M. Baracat, SR. Georgetti, D. R. Cardoso, T. M. Cunha, H. Ferreira, F. Q. Cunha, M. J. V. Fonseca and R. Casagrande, “Flavonoids as Anti-Inflammatory and Analgesic Drugs: Mechanisms of Action and Perspectives in the Development of Pharmaceutical Forms,” Bioactive Natural Products, Vol. 36, 2012, pp. 297-330.
NOTES
*Corresponding author.

