American Journal of Plant Sciences
Vol.08 No.06(2017), Article ID:76343,11 pages
10.4236/ajps.2017.86085
Seed Biology of Berberis manipurana Ahrendt: A Threatened Natural Dye Yielding Plant
Chitta Ranjan Deb*, Tsatingmong Lirola Sangtam, Nangshimeren Sakutemsu Jamir
1Affiliation Department of Botany, Nagaland University, Nagaland, India

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: April 9, 2017; Accepted: May 20, 2017; Published: May 23, 2017
ABSTRACT
Barberry has played a prominent role in herbal healing for more than 2500 years. Most of the berberis species have medicinal uses because of the presence of alkaloid “berberine” an isoquinoline alkaloid. The root and inner bark are also used for yellow dyeing of clothes. The objective of the present study was to evaluate the seed dormancy, effect of stratification, light conditions and planting bed quality on seed germination of berberis manipurana seeds. A set of freshly processed seeds were sowed in three light conditions i.e., normal light (~5000 lux), poly house ca. 50% shade (~2500 lux) and poly house ca. 75% shade (~1250 lux). In the laboratory, half of the collected seeds were packed in plastic bags, labeled and stored at 4°c (stratification treatment); another half was stored at room temperature of 25˚C. Stratified seeds from both the conditions (4˚C and 25˚C) were sowed in the seed bed at 15 days interval till 120th day (0 - 120 days) to check the viability and germination behaviors. Highest germination rate was observed from seeds stored at 4˚C, which registered 82.5% (1.5) germination in the poly-bags against 70% (2.5) from seeds stored at 25˚C. While 69% (2.0) and 56% (1.5) seeds germinated in the seeds beds from seeds stratified at 4˚C and 25˚C respectively.
Keywords:
Berberis Manipurana, Dye Yielding Plant, Seed Propagation

1. Introduction
A successful reforestation program depends on a continuous supply of healthy seedlings/planting stocks. This process begins with successful seed collection, storage, processing, and sowing operations, and then continues with the careful growing, lifting, and storage of seedlings. Most flowering plants reproduce by sexually and seed production and are the means by which plants produce genetically diverse offspring capable of surviving in variable and changing environments. The seed habit is the most complex and successful method of sexual reproduction in vascular plants. The seed plants (Spermatophyta) comprise two major groups: the Acrogymnospermae (also referred to as gymnosperms; c. 800 living species) and the Angiospermae (also referred to as angiosperms; c. 250,000 living species) [1] . The seed germination and the establishment of a normal seedling are determining features for the propagation of plant species, which are of both economic and ecologic importance. Because of high vulnerability to injury, disease and water/environmental stress, seed germination is considered to be the most critical phase in the plant life cycle. Seeds are the principal means of regeneration for most woody plants. They serve as the delivery system for the transfer of genetic materials from one generation to the next. The part of a plant’s life cycle that involves seed formation, maturation, dissemination, and germination is a complex yet fascinating chain of events, many of which are still poorly understood. Yet some knowledge of these events is necessary for successful collection and utilization of seeds to produce the means for artificial regeneration. Mature seeds remain dormant from few days to many years, and in this state they are able to tolerate adverse conditions and stressful environments (e.g. intense cold, heat, drought, darkness) that could not be endured by most plants [2] . In the seeds of many plant species, maturation is accompanied by the induction of a state of dormancy. Seed dormancy is a mechanism by which seeds can inhibit their germination in order to wait for more favorable conditions [3] . Sometimes seeds do not germinate because water and gases cannot permeate the seed coats. Various treatments, based on the physiological requirements of seeds, are used to stimulate and enhance germination. Different methods such as heating stratification, scarification [4] , and gibberellins application are well known to overcome with these problems depending on the type of plant species and dormancy. Stratification is the most consistently effective dormancy-release treatment. Moist chilling or cold stratification has been widely used as a pre-sowing treatment for breaking dormancy and enhancing the maximum rate and percentage germination of dormant seeds of many plant species [5] [6] .
Located in the North Eastern region of India, the state of Nagaland lies between the geographical coordinates of 25˚6' and 27˚4' North latitudes and 93˚20' and 95˚15' East longitude. Nagaland, though a small state in terms of area, has a rich and diverse heritage of biodiversity owing to its varying physiographic and geo-climatic conditions favorable for copious growth of vegetation. It varies from tropical rain forest to alpine vegetation and from evergreen forest to sub- tropical climatic region. Nagaland is located in one of the 25 hotspots of the world in terms of biodiversity. A few areas of the state are still untouched and harbor a wide variety of endemic species of plants. However, in recent times, the biodiversity of the state is facing serious threats due to escalating population, pressure on agriculture to bring more areas under cultivation and other developmental activities.
Berberis is a genus which is characterized by woody shrubs and small trees. It is the largest genus in Berberidaceae [7] and is commonly known as “Barberries” or “Pepperidge Bush”. Because of its ornamental potential owing to their durability, attractive coloured flowers, evergreen foliage and medicinal uses, Berberis is widely propagated and valued. The genus Berberis contains a complex of secondary metabolites having antitumor properties and is used as antimutagenic and is frequently used in the pharmaceutical industry [8] . Barberry has played a prominent role in herbal healing for more than 2500 years [10] . Most of the Berberis species have medical uses because of the presence of alkaloid, “Berberine” an isoquinoline alkaloid [9] [10] [11] known for its activity against cholera [12] , diarrhoea [13] . In traditional folk medicine, barberry has been used to treat diarrhea, reduce fever, improve appetite, relieve upset stomach, and promote vigour as well as a sense of well-being [14] .
Berberis manipurana, commonly known as Manipur barberry is an evergreen woody shrub, found in shady moist habitat. The limitation of its low population size, irregular fructification and consumption of seeds by birds has further overrated the problem of regeneration of this species. In view of these facts, an effort has been made to influence seed germination so as to bring about an improvement in regeneration of these species.
2. Materials and Methods
2.1. Site Description
The study was conducted in the Department of Botany, Nagaland University, Lumami located in Zunheboto district, Nagaland at an elevation of 1150 - 1200 m ASL, 26˚12'37''N and 95˚29'28''E. Plant identification was confirmed from Botanical Survey of India (BSI), Eastern regional Centre, Shillong, India.
2.2. Seed Collection and Storage
Matured and ripened fruits of Berberis manipurana were harvested from its natural habitat. The fruits were collected from Western Dzϋkou at an elevation of 2649 m ASL, 25˚36'36.3''N and 94˚00'03.0''E, during the month of September and October. The collected seeds were wrapped in damp cotton and packed in polybags and transported to the laboratory. Major attributes of seeds of the species was recorded (Table 1). The fleshy pericarpic layer of the fruit was discarded and the seeds were extracted. Each matured fruit had 3 - 4 seeds inside. Once the seed were brought to the laboratory, seed viability was checked using the float method [15] . All floating or damaged seeds were discarded. Settled down seeds were considered as viable and selected for the present study. The selected viable seeds were washed briefly with laboratory detergent (Labolene, 1%, v/v), air dried and stored in plastic bags until use. The processed seeds were divided into different groups for different germination experiment. For each treatment 20 seeds were sowed and repeated thrice. All the experiments were carried out in the laboratory and in the Departmental Experimental Garden, Department of Botany, Nagaland University, Lumami.
Table 1. Seed traits of Berberis manipurana.
2.3. Pre-Sowing Treatment and Experimental Process
A batch of the processed seeds was sowed immediately after harvest in the seed bed while rest of the seeds was treated differentially as described below:
1. A set of freshly processed seeds were sowed in three light conditions i.e.; normal light (~5000 lux), poly house ca. 50% shade (~2500 lux) and poly house ca. 75% shade (~1250 lux).
2. In the laboratory, half of the collected seeds were packed in plastic bags, labeled and stored at 4˚C (stratification treatment); another half was stored at room temperature of 25˚C. Seeds were kept in these conditions until germination trials were undertaken.
3. Stratified seeds from both the conditions (4˚C and 25˚C) were sowed in the seed bed at 15 days interval till 120th day (0 - 120 days) to check the viability and germination behaviors.
4. The seeds were sowed in prepared seed beds as well as poly bags. All the seed beds were prepared by combining a mixture of top soil, farmyard manure and sand in the ratio of 3:1:1 (v/v/v).
5. Seeds were sown 2.0 cm deep in polybags. In each poly bag only one seed was sowed.
6. While, distance between seed to seed and row to row in the seed bed was ~30 cm.
The polybags were kept in open air in all the three shade conditions as mentioned above, watered every alternate day till the experiments completed. Emergence of the radical was considered as the onset of seed germination. Data were recorded daily from the start of germination. All experiments were conducted in a randomized block design (RBD). The seedlings developed from the germinated seeds were maintained in the seed beds/poly bags for further growth. The well developed seedlings were transferred to the field after one year.
3. Results
For the present study seeds were collected from Western Dzϋkou valley, Nagaland, India. Data on flowering, seed setting, maturation etc were collected for three consecutive years. Seed traits of B. manipurana are given in Table 1. It was found that plant flowers during July-August, seed setting starts during September-October and matures during October-November. Different stages of seeds (young to mature) are shown in Figure 1. After collection of seeds from the field, seeds were extracted and processed as described in materials and methods. It was found that average number of seeds per fruits is 3 - 4 and the average seed weight is 0.027 gm.
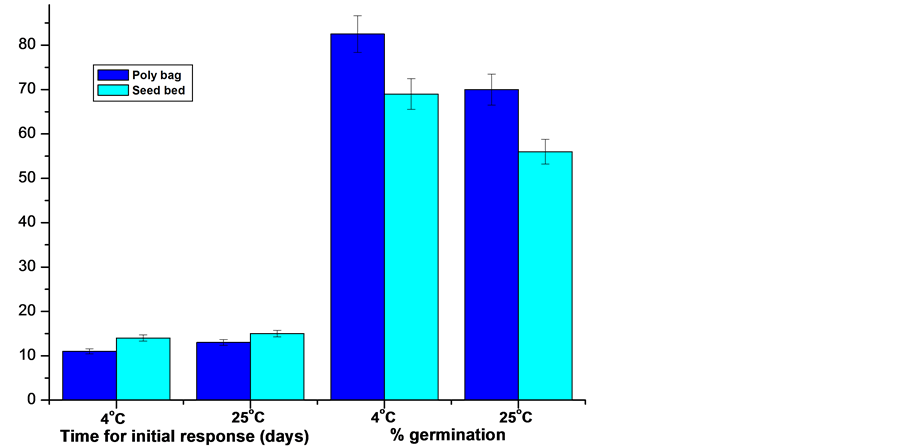
Collected mature seeds were processed and tested for dormancy, effect of stratification, light conditions and seed bed types on seed germination. The result thus obtained exhibited differential response under different conditions. The seed germination rate, germination time, seedling morphology was greatly influenced by illumination in the seed beds and seed bed quality, temperature of post harvest storage/stratification and duration. In the present study seeds were sowed in three different light conditions viz., normal light (5000 lux); 50% shade (2500 lux) and 75% shade (1250 lux). Of the different light conditions studied, optimum response was achieved from seeds sowed in poly-bags as well as in the seed beds and maintained at 50% shade (2500 lux), where radical emerged within 11 - 12 days of sowing and registered 82.5 and 69% germination in poly-bags and seed bed respectively (Figure 2, Figure 3(a), Figure 4(a)). Seeds sowed under normal light conditions delayed germination, while seeds sowed under 75% shade seedlings were healthy but comparatively etiolated in comparison to 50% shaded condition.
There was a significant variation in time taken for initial response of germination as well as per cent germination in both poly-bags as well as seed bed. It was found that with increase in stratification period, germination rate improved significantly in both the temperatures. In the present study with B. manipurana,
Figure 1. (a) Twig of Berberis manipurana bearing young fruits; (b) Harvested young fruits; (c) Extracted young seeds from fruits; and (d) Mature fruits and extracted seeds.
Figure 2. Effect of quantity of light on seed germination Berberis manipurana.
Figure 3. Different stages of B. manipurana seed germination in poly bags. (a) Seed sowed in poly bag showing the sign of germination; (b) Young seedling releasing the first set of leaf; (c) Established seedling ready for field transfer.
Figure 4. Different stages of B. manipurana seed germination in seed bed. (a) Seed sowed in seed bed showing the sign of germination; (b) Young seedlings releasing the first set of leaf; (c) Established seedling ready for field transfer.
germination percentage was affected by both stratification time and light conditions during germination. The seeds sowed immediately after harvest exhibited low germination frequency (7.5%) and longer germination time (40 days). With increase in stratification period, germination rate improved. Of the different stratification period tested in the present study under both 4˚C and 25˚C, 75 days post harvest storage prior to sowing found to be most suitable for germination (Table 2). Further, of the two temperatures tested for stratification, 4˚C was found to be better over 25˚C for both registering early initial germination response as well as germination rate. Seeds stored at 4˚C registered initial response of germination within 11th and 12th day in poly-bag and seed bed respectively against 13th and 15th day from the seeds stratified at 25˚C (Table 2). Besides this, seed germination rate was significantly higher from the seeds stratified at 4˚C against 25˚C in both poly-bag and seed bed. Seeds stored at 4˚C registered 82.5% germination in the poly-bags against 70% from seeds stored at 25˚C. While 69 and 56% seeds germinated in the seeds beds from seeds stratified at 4˚C and 25˚C respectively (Table 2; Figure 5).
Figure 5. A comparative representation of germination behaviours of Berberis manipurana seeds sowed in poly bags and seed bed.
Table 2. Effect of post harvest storage/stratification of Berberis manipurana seeds at 4˚C and 25˚C on seed viability and germination in the poly bag and seed bed.
First sign of germination was recorded as emergence of radical (Figure 3(a), Figure 4(a)). The germinating seeds progressed through their developmental process and converted into seedlings and released the first set of leaves (Figure 3(b), Figure 4(b)). Both poly-bags and seed beds were irrigated at regular interval for seedling growth. Seedlings were allowed to grow further in the respective germination beds for one year (Figure 3(c), Figure 4(c)) before transferring to the wild.
4. Discussion
Propagation via seed is nature’s most common method of plant reproduction. Presently there is an estimate of about 1500 seed or gene banks around the world containing over 6 million seed accessions. However, storage of seeds in seed banks for ex situ conservation needs a thorough understanding of post harvest seed physiology and seed storage as seeds exhibiting orthodox seed storage behaviour can only be stored in seed banks for a longer period of time without losing the seed viability. The storage of seeds in seed banks/gene banks is generally considered the safest, most inexpensive and most convenient method of conservation as seeds occupy little space, and also require little attention over considerable period of time [16] [17] .
For seed germination, emergence of radical’s germination rate, seedling morphology and their establishment is influenced by various factors. Depending on the plant species, there is a variation in their habitat preference, temperature requirements, post harvest storage, specific pre-treatment for seed germination, seedling emergence and survival. The light requirement conditions for successful seed germination and healthy seedling morphology appears to be species specific. According to [18] , light and temperature are the main factors that promote germination in soils with good water availability. Temperature may also determine the amount of dormant seeds in a population while concurrently modulating germination of non-dormant seeds [19] . The availability of light, water and nutrients are also the governing factors for the survival of seedling on the seed beds or forest floor [20] . Increase in light and nutrients stimulate the growth of the competing shrubs and fast growing herbaceous plants. Each species requires a range of temperatures for seed germination and seedling establishment [21] . Although seeds of a particular species may germinate over a wide range of temperatures, the time needed for maximum germination varies as temperatures fluctuate [22] . According to [23] , germination percentage was almost independent from light conditions. In the present study with Berberis manipurana, there was a reasonable germination in seeds sowed across different light conditions. In higher light conditions, germination was delayed compared to lower light intensities. From the observations recorded, it was noted that the seeds of B. manipurana are orthodox in nature like some species of Berberis. Seeds of Japanese and common barberries species have embryo dormancy that requires cold stratification to provide prompt germination. It was also reported that seeds of Japanese and common barberry species remained viable for at least 4 years when kept at 1˚C to 3˚C in sealed container [24] , which indicates that these species are orthodox in storage behavior.
In addition to light conditions, another form of controlling the biological activities is the use of low temperatures. In this experiment, seeds were stored at 4˚C and 25˚C .Seed stratification at lower temperature showed better response than seeds kept at room temperature. Storing the seeds at 4˚C gave slightly higher percentage of viability and germination in comparison to storage at 25˚C. Observations recorded showed that exposure to longer period of stratification has positive effect on the seeds of Berberis manipurana which germinated faster than those stratified for shorter period. Seeds from colder climates and higher altitude germinate quickly at low temperatures than seeds from warmer and lower altitude [25] . This is a characteristic showed by many temperate species; they require cold stratification to germinate [26] .
5. Conclusion
Based on the result obtained from this study, it can be assumed that the seeds of berberis manipurana, can endure desiccation, i.e., they are orthodox in nature and the seeds can be preserved for an extended period of time as compared to recalcitrant seeds. The seeds exhibited subjective difference when stored in two temperatures i.e., 4˚C and 25˚C. Seeds treated with cold stratification treatment (4˚C), gave better response in term of breaking dormancy. From this study, it is observed that chilling effect and light shows a role in breaking dormancy. Further studies on mechanism of seed dispersal, seed storage and temperature tolerance will help in developing the conservation strategies of this economically important medicinal and dye plant.
Acknowledgements
The authors are thankful to the Department of Biotechnology, Ministry of Science & Technology, Govt. of India, New Delhi for the financial facility for the present study through a research grant to Prof. C. R. Deb and Prof. N. S. Jamir. The facilities used from UGC-SAP (DRS-III) program to the Department of Botany, Nagaland University is duly acknowledged.
Cite this paper
Deb, C.R., Sangtam, T.L. and Jamir, N.S. (2017) Seed Biology of Berberis manipurana Ahrendt: A Threatened Natural Dye Yielding Plant. American Journal of Plant Sciences, 8, 1285-1295. https://doi.org/10.4236/ajps.2017.86085
References
- 1. Cantino, P.D., Doyle, J.A., Graham, S.W., Judd, W.S., Olmstead, R.G., Soltis, D.E., Soltis, P.S. and Donohue, M.J. (2007) Towards a Phylogenetic Nomenclature of Tracheophyta. Taxon, 56, 822-846.
https://doi.org/10.2307/25065865 - 2. Baskin, C.C. and Baskin, J.M. (1998) Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination. Academic Press, San Diego.
- 3. Finkelstein, R., Reeves, W., Ariizumi, T. and Steber, C. (2008) Molecular Aspects of Seed Dormancy. Annual Review of Plant Biology, 59, 387-415.
- 4. Herranz, J.M., Ferrandis, P. and Martínezm Sánchez, J.J. (1999) Influence of Heat on Seed Germination of Nine Woody Cistaceae Species. International Journal of Wildland Fire, 9, 173-182.
https://doi.org/10.1071/WF00014 - 5. Schopmeyer, C.S. (1974) Seeds of Woody Plants in the United States. USDA, Forest Service. Agriculture Handbook No. 450, US Government Printing Office, Washington DC.
- 6. Wang, B.S.P. and Berjak, P. (2000) Beneficial Effects of Moist Chilling on the Seeds of Black Spruce (Picea mariana [Mill.] B.S.P.). Annals of Botany, 86, 29-36.
https://doi.org/10.1006/anbo.2000.1150 - 7. Ahrendt, L.W.A. (1961) Berberis and Mahonia, a Taxonomic Revision. Botanical Journal of the Linnaean Society, 57, 1-410.
https://doi.org/10.1111/j.1095-8339.1961.tb00889.x - 8. Arayne, M.S., Sultana, N. and Bahadur, S.S. (2007) The Berberis Story: Berberis vulgaris in Therapeutics. Pakistan Journal of Pharmaceutical Sciences, 20, 83-92.
- 9. Kala, C.P. and Sajwan, B.S. (2007) Revitalizing Indian Systems of Herbal Medicine by the National Medicinal Plants Board through Institutional Networking and Capacity Building. Current Science, 93, 797-806.
- 10. Khosla, P.K. (1992) Status of Indian Forestry Problem and Perspective. Indian Society of Tree Scientists, University of Horticulture and Forestry, Solan (H.P.) India
- 11. Rastogi, R., Back, E., Schneiderbauer, A., Bowsher, C., Moffatt, G. and Rothstein, S.J. (1993) A 330 bp Region of the Spinach Nitrite Reductase Gene Promoter Directs Nitrate-Inducible Tissue-Specific Expression in Transgenic Tobacco. The Plant Journal, 4, 317-326.
https://doi.org/10.1046/j.1365-313X.1993.04020317.x - 12. Rabbani, M.G., Au, M.H. and Mondal, M.F. (1996) Effect of BAP and IBA on Micropropagation of Some Banana Cultivars. Bangladesh Horticulture, 25, 47-52.
- 13. Yamamoto, N., Mukai, Y., Matsuoka, M., Kano-Murakami, Y., Tanaka, Y., Ohashi, Y., Ozeki, Y. and Odani, K. (1991) Light-Independent Expression of cab and rbcS Genes in Dark-Grown Pine Seedlings. Plant Physiology, 95, 379-383.
https://doi.org/10.1104/pp.95.2.379 - 14. Bergner, P. (1996) Goldenseal and the Common Cold: Goldenseal Substitutes. Medical Herbalism: A Journal for the Clinical Practitioner, 8.
- 15. Gough, R.E. (1996) Growing Trees and Shrubs from Seed. Colorado State University, Fort Collins. Montguide 9604.
- 16. Linington, S.H. and Pritchard, H.W. (2001) Genebanks. In: Levin, S., Ed., Encyclopedia of Biodiversity, Vol. 3, Academic Press, New York, 165-181.
- 17. Engelmann, F. and Engels, J.M.M. (2002) Technologies and Strategies for ex Situ Conservation. In: Managing Plant Genetic Diversity, CABI, 89-103.
https://doi.org/10.1079/9780851995229.0089 - 18. Andrade, A.C.S. (1995) Effect of Light and Temperature on the Germination of Leandra breviflora Cong., Tibouchina benthamiana Cong., Tibouchina grandifolia Cong. and Tibouchina moricandia (DC.) Baill. (Melastomataceae). Brazilian Journal of Seeds, 17, 29-35.
- 19. Benech-Arnold, R.L. and Sánchez, R.A. (1995) Modeling Weed Seed Germination. In: Kigel, J. and Galili, G., Eds., Seed Development and Germination, Dekker, New York, 545-566.
- 20. Kitajima, K. (2007) Seed and Seedling Ecology. In: Pugnaire, F.I. and Valladares, F., Eds., Functional Plant Ecology, 2nd Edition, CRC Press, Taylor & Francis Group, Boca Raton, 549-580.
https://doi.org/10.1201/9781420007626.ch18 - 21. Bradbeer, J.W. (1988) Seed Dormancy and Germination. Chapman and Hall, New York, 27-54.
https://doi.org/10.1007/978-1-4684-7747-4 - 22. Bewley, J.D. and Black, M. (1994) Seeds: Physiology of Development and Germination. Plenum Press, New York, 199-262.
- 23. Onen, H. (1999) Studies on Biology and Control of Mugwort (Artemisia vulgaris L.). Ph.D. Thesis, Gaziosmanpasa University, Tokat, Turkey.
- 24. Heit, C.E. (1967) Propagation from Seed: 11. Storage of Deciduous Tree and Shrub Seeds. American Nurseryman, 126, 12-13, 86-94.
- 25. Fountain, D.W. and Outred, H.A. (1991) Germination Requirements of New Zealand Native Plants: A Review. New Zealand Journal of Botany, 29, 311-316.
https://doi.org/10.1080/0028825X.1991.10416609 - 26. Miriam, E.A. and Guillermo, M.P. (1994) Seed Propagation in Berberis buxifolia Lam. International Journal of Experimental Botany, 56, 59-63.