American Journal of Plant Sciences
Vol.06 No.13(2015), Article ID:59176,6 pages
10.4236/ajps.2015.613209
Elevational Floral Size Variation in Prunella vulgaris
Shin Egawa1, Mitsuru Hattori1, Takao Itino1,2*
1Department of Biology, Faculty of Science, Shinshu University, Nagano, Matsumoto, Japan
2Institute of Mountain Science, Shinshu University, Nagano, Matsumoto, Japan
Email: *itinot@shinshu-u.ac.jp
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 3 April 2015; accepted 21 August 2015; published 27 August 2015
ABSTRACT
Floral size is potentially influenced by local pollinators’ body size. As pollinator fauna and size often change with elevation, correlation between elevational variation of pollinator size and floral size is expected. We investigated the variation of floral size in Prunella vulgaris L. (Laminaceae) and the variation of their pollinator fauna along an elevational gradient. We measured the floral size of four traits: corolla length (CL), corolla tube length (CTL), corolla width (CW), and calyx length (CAL), in 23 populations, and found that CL and CTL were negatively correlated with elevation, and CW and CAL were not. Six bumblebee species visited the flower, and the visiting bee fauna differed among populations; the smallest and the largest bumblebee species visited the high elevational range (above 1800 m a. s. l.) populations, and the largest and the second largest bumblebee species visited the middle elevational range (1400 - 1800 m a. s. l.). Although abiotic factors can potentially affect floral size, the fact that we do not find an elevational decrease in CW and CAL suggests that the elevational change in P. vulgaris’s CL and CTL reflects the local pollinator size.
Keywords:
Bumblebee, Flower Size, Geographic Trait Variation, Pollinator Fauna

1. Introduction
Geographical changes in floral size have been reported by many studies [1] -[3] . As the match between floral size and pollinator size is important for plants to achieve reproductive success [4] [5] , selection by differently- sized pollinators potentially causes variation in floral size [6] [7] . Thus, geographical changes in floral size are expected to be mediated by geographical changes in pollinator fauna (and the resultant changes in pollinator size) [8] -[10] . As changes in pollinator fauna along an altitudinal gradient can be potential selective pressures on floral size, not a few studies have investigated altitudinal variation of floral size in relation to pollinator size (e.g. [11] ) although the number of sampling sites and their altitudinal range are often limited.
In this study, we investigated the variation of floral size in Prunella vulgaris in 23 populations and the variation of its pollinator fauna along an elevational gradient. In the study area, six bumblebee species were observed to visit P. vulgaris flowers, from the largest to the smallest, Bombus consobrinus (Bc), B. diversus (Bd), B. ussurensis (Bu), B. honshuensis (Bh), B. beaticola (Bb), and B. hypocrita (Bhy). As these species have different body sizes and different elevational distributions, they are expected to exert selective pressure on P. vulgaris floral size in this system.
2. Materials and Methods
2.1. Prunella vulgaris and Study Sites
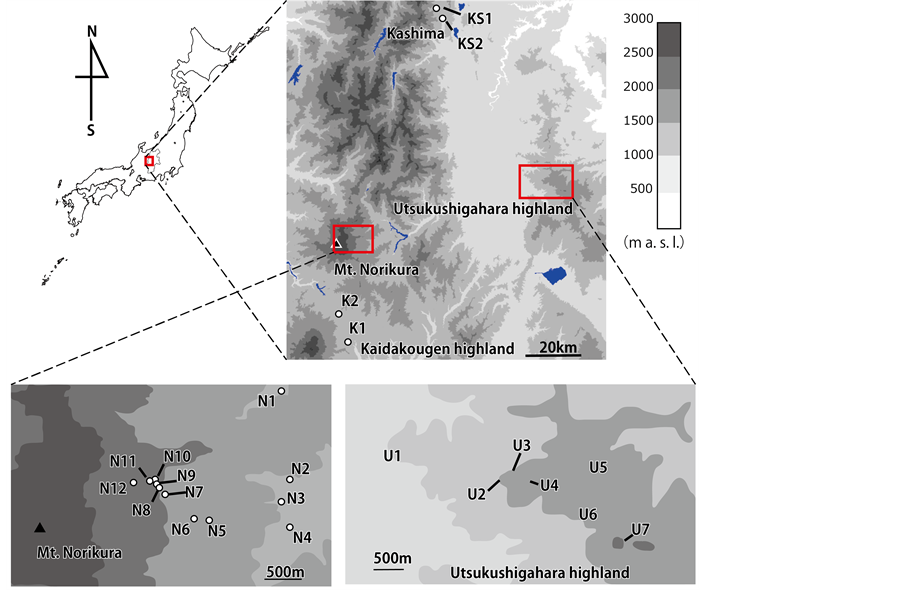
Prunella vulgaris L. (Lamiaceae) subsp. asiatica (Nakai) H. Harais is found throughout the temperate northern hemisphere, including Japan. In the study area, Nagano, Japan, P. vulgaris is found over a wide range of elevation (1300 - 2200 m a. s. l.). Prunella vulgaris is a perennial herb with protandrous purple flowers on inflorescences blooming in June to August (Figure 1). It grows in open habitat and is mainly pollinated by bumblebees. This study was carried out in wild populations of P. vulgaris on Mt. Norikura and in Utsukushigahara highland, Kaidakougen highland, and Kashima in Nagano, central Japan (Table 1, Figure 2). We grouped the study sites
Table 1. Study sites of Prunella vulgaris.
*Observations of bumblebee species (visiting frequency per flower per 10 h) were made between 09:00 and 14:00 on sunny days during the peak flowering season within an observation quadrat (1 m × 2 m) in each population for at least 1 h. Bc: Bombus consobrinus; Bd: B. diversus; Bu: B. ussurensis; Bh: B. honshuensis; Bb: B. beaticola; Bhy: B. hypocrita; ―: not censused; None, no bees were observed. According to Nagano et al. (2014), the pollinators’ mean mouthpart lengths (distance from the base of the foreleg to the tip of the galea) in the Mt. Norikura area are: Bc, 20.3 mm; Bd, 16.3 mm; and Bb, 10.9 mm.
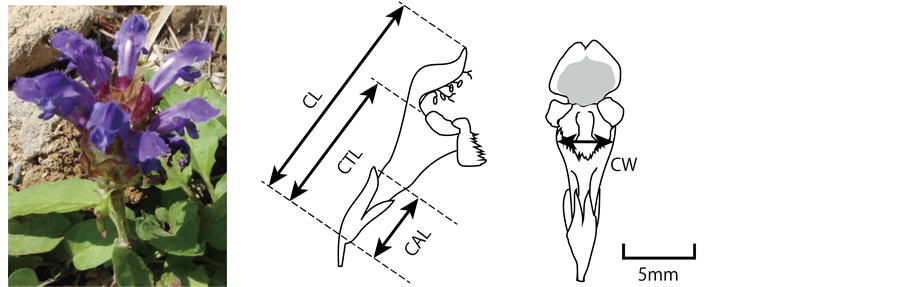
Figure 1. (a) An inflorescence of Prunella vulgaris. (b) The floral traits measured. CL, CTL, CW, and CAL indicate “corolla length”, “corolla tube length”, “corolla width” and “calyx length”, respectively.
Figure 2. Locations of the study sites.
into three elevational ranges (<1400 m a. s. l., 1400 - 1800 m a. s. l., and >1800 m a. s. l.). In the study area, tree vegetation zone in 800 - 1400 m a. s. l. include montane deciduous broad-leaved forests whereas the upper elevational ranges include subalpine coniferous forests, and the smallest pollinator bumblebee species occurr mostly in >1800 m a. s. l. (Table 1).
2.2. Measurement of Floral Size
During summer 2013, we measured the floral size of P. vulgaris in 23 populations (Table 1, Figure 2). In the peak flowering season of each population, 7 to 30 flowers were picked from each population (the lowest flower was picked from each randomly selected inflorescence) and stored in 70% ethanol. After one month preservation in 70% EtOH, we measured their corolla length (CL), corolla tube length (CTL), corolla width (CW), and calyx length (CAL) by digital caliper (0.01 mm precision) (Figure 1).
CW can potentially inhibit the bumblebees from entering the flower. CL and CTL may influence pollinator efficiency, because the match, or the lack of one, between flower and bee may affect pollen attachment to the bee’s body. CAL may not influence pollinator efficiency but can potentially inhibit nectar robbing from the base of the corolla.
We compared the four floral traits among the three elevational ranges (<1400 m a. s. l., 1400 - 1800 m a. s. l., and >1800 m a. s. l.), and among populations within each of the three elevational ranges by using one-way ANOVA and Tukey’s HSD post hoc test. These analyses were performed with JMP ver. 9.0.2 software (SAS Institute, Japan). We tested the correlation of the floral sizes against elevation by using Pearson’s correlation test.
2.3. Visiting Bumblebees
We observed visitors to P. vulgaris flowers in 14 populations (Table 1) in 2013. In each P. vulgaris population, we set an observation quadrat (1 m × 2 m), counted open flowers, and conducted observations for 1 to 2 h. The observations were made between 09:00 and 14:00 on fine days during the peak flowering season.
3. Results
3.1. Variation of P. vulgaris Floral Size along Elevational Gradient
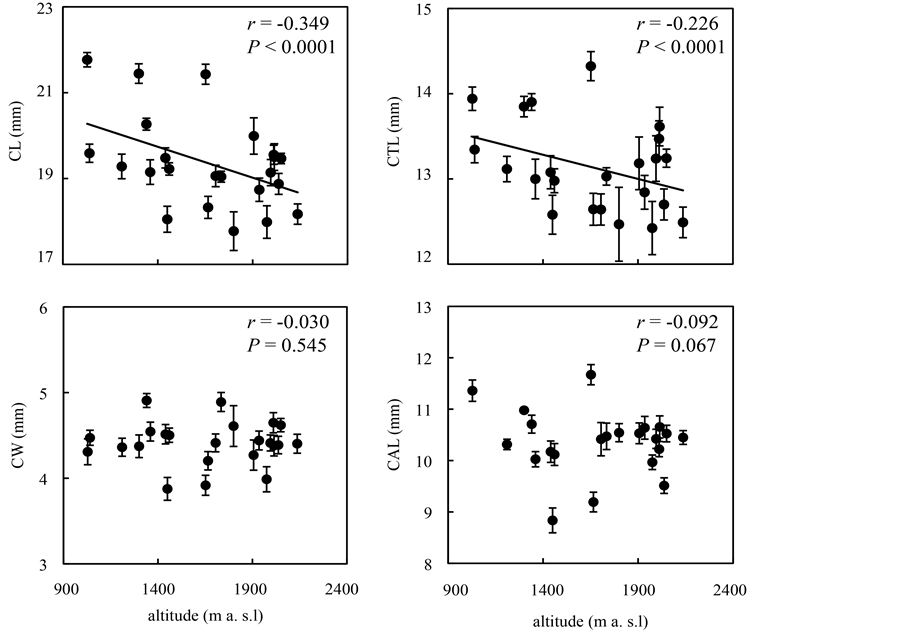
Floral sizes differed among populations (Figure 3). Corolla length and corolla tube length significantly correlated with elevation (CL, r = −0.349, P < 0.0001; CTL, r = −0.226, P < 0.0001), but corolla width and calyx length did not correlate with elevation (CW, r = −0.030, P = 0.545; CAL, r = −0.092, P = 0.067).
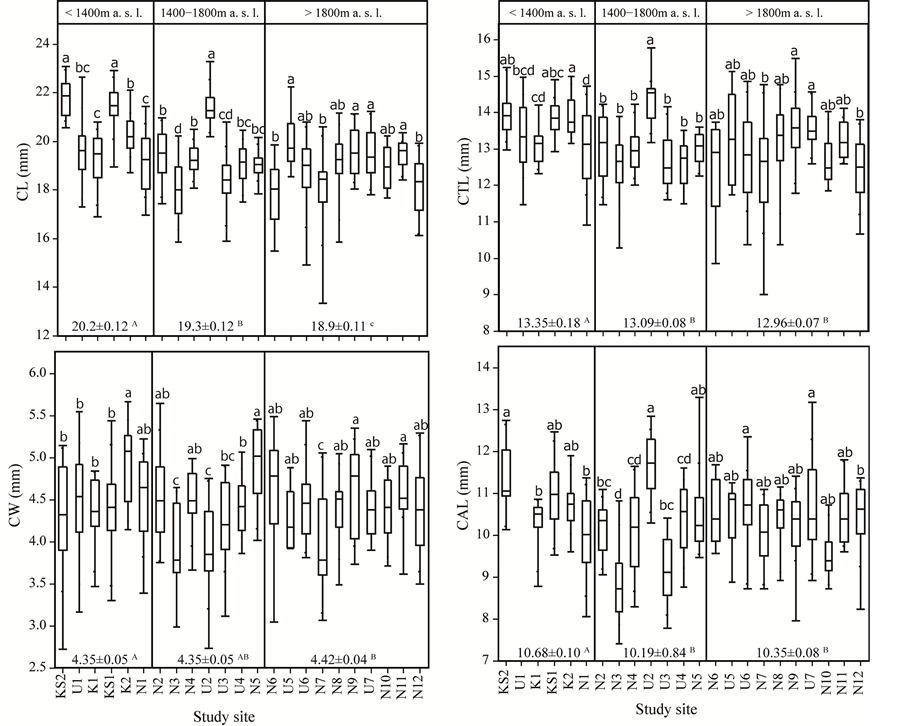
Each floral dimension varied among the three elevational ranges (<1400 m a. s. l., 1400 - 1800 m, and >1800 m) (Figure 4; CL: F = 34.76, P < 0.001; CTL: F = 15.55, P < 0.001; CW: F = 3.95, P = 0.0199; CAL: F = 7.61,
Figure 3. Elevational variation (mean ± SE) of floral sizes (CL, CTL, CW and CAL, see Figure 1). Each circle represents a population.
Figure 4. Box and whisker plots of Prunella vulgaris floral traits: CL, CTL, CW, and CAL indicate “corolla length”, “corolla tube length”, “corolla width” and “calyx length”, respectively. The bottom line in each box plot is the 25th percentile, the midline is the median, and the top is the 75th percentile. The whiskers extend to the lowest datum and the highest datum. Each floral dimension varied among the three elevational ranges (mean ± SD indicated at the bottom, P < 0.005; one-way ANOVA, see text for results). The different uppercase letters indicate significant differences between elevational ranges (P < 0.05; Tukey’s HSD post hoc pairwise comparisons). Each floral dimension also varied among populations within each of the three elevational ranges (P < 0.005; one-way ANOVA, see text for results). Different lowercase letters indicate significant differences between the values within each of the three elevational ranges (P < 0.05; Tukey’s HSD post hoc pairwise comparisons).
P < 0.001), and among populations within each of the three elevational ranges (Figure 4; CL: F = 21.94, P < 0.001 at < 1400 m; F = 30.37, P < 0.001 at 1400 - 1800 m; F = 5.47, P < 0.001 at >1800 m; CTL: F = 7.01, P < 0.001 at <1400 m; F = 16.75, P < 0.001 at 1400 - 1800 m; F = 3.17, P = 0.0016 at >1800 m; CW: F = 4.33, P = 0.0012 at <1400 m; F = 10.70, P < 0.001 at 1400 - 1800 m; F = 2.82, P = 0.0042 at >1800 m; CAL: F = 9.43, P < 0.001 at <1400 m; F = 25.00, P < 0.001 at 1400 - 1800 m; F = 2.93, P = 0.0031 at >1800 m).
3.2. Visiting Bumblebees
Bombus consobrinus (the largest bumblebee species in Japan) and B. diversus (the second largest species in Japan) were found at middle elevations (1400 - 1800 m a. s. l; N2, N3, N4, U3, and U4; Table 1). Bombus beaticola (the smallest species in Japan) and B. consobrinus were found at over 1800 m a. s. l. (N6, N7, N8, N9, N10, N11, N12, and U7; Table 1).
4. Discussion
The results showed that P. vulgaris displays elevational/geographic variation in floral size. The pattern of variation was different among the four floral traits: corolla length and corolla tube length were both negatively correlated with elevation, but corolla width and calyx length did not correlate with elevation (Figure 3).
Corolla length (CL) and corolla tube length (CTL) may be under a selection regime correlated with elevation. Abiotic factors such as temperature, soil moisture, and insolation can potentially affect floral size [12] . However, we did not find any elevational decrease or increase in corolla width (CW) and calyx length (CAL); this fact suggests that clinal changes in abiotic factors may not be responsible for the floral variation, so other factors may be involved. When a bumblebee is collecting floral nectar of P. vulgaris, pollen grains attach to the dorsal side of its thorax or head. Therefore, if the bee proboscis is too long or too short relative to the corolla length or the corolla tube length, the pollination efficiency may decrease. Thus, CL and CTL are expected to be affected by the local bee proboscis length. CL and CTL were smaller in the high elevation range (>1800 m a. s. l.), where the smallest bumblebee species B. beaticola with short proboscis and the largest bumblebee species B. consobrinus with long proboscis visited P. vulgaris flowers (Table 1). On the other hand, CL and CTL were larger in middle elevation range, where the smallest species, B. beaticola, was absent and the two large Bombus species (B. consobrinus and B. diversus) frequently visited P. vulgaris flowers (Table 1). These results suggest that the elevational change in P. vulgaris corolla length and corolla tube length are affected by the local pollinator size, although the relative importance of the largest and the smallest bumblebee species as P. vulgaris pollinators in the high elevation range is unknown and should be explored in future studies. Further, the among-population variations in floral sizes within the same elevational range (Figure 4) suggest that a fine-scale difference in pollinator fauna may influence local floral size.
5. Conclusion
Prunella vulgaris displays elevational/geographical variation in floral size. Corolla length (CL) and corolla tube length (CTL) were negatively correlated with elevation, while corolla width (CW) and calyx length (CAL) were not. Six bumblebee species visited the flower, and the visiting bee fauna differed among populations. The smallest and the largest bumblebee species visited the high elevational range (above 1800 m a. s. l.) populations and the largest and the second largest bumblebee species visited the middle elevational range (1400 - 1800 m a. s. l.). These results suggest that local pollinator size rather than elevational abiotic change affects corolla length of Prunella vulgaris.
Acknowledgements
We thank Y. Nagano for technical support, help with field survey, and helpful discussion. This study was supported by the Global Environmental Research Fund (D-0904) of the Ministry of the Environment, Japan, and by the Japanese Alps Inter-University Cooperative Project, Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. We thank the Chubu Regional Environmental Office (Ministry of the Environment), the Chushin District Forest Office (Forest Agency), and the Matsumoto Regional Office (Nagano Prefectural Government) for permission to work in the study area.
Cite this paper
ShinEgawa,MitsuruHattori,TakaoItino, (2015) Elevational Floral Size Variation in Prunella vulgaris. American Journal of Plant Sciences,06,2085-2091. doi: 10.4236/ajps.2015.613209
References
- 1. Medel, R., Valiente, A., Botto-Mahan, C., Carvallo, G., Pérez, F., Pohl, N. and Navarro, L. (2007) The Influence of Insects and Hummingbirds on the Geographical Variation of the Flower Phenotype in Mimulus luteus. Ecography, 30, 812-818.
http://dx.doi.org/10.1111/j.2007.0906-7590.05175.x - 2. Suzuki, K., Dohzono, I. and Hiei, K. (2007) Evolution of Pollinator Generalization in Bumblebee-Pollinated Plants. Plant Species Biology, 22, 141-159.
http://dx.doi.org/10.1111/j.1442-1984.2007.00187.x - 3. Herrera, C.M., Castellanos, M.C. and Medrano M. (2006) Geographic Context of Floral Evolution: Towards an Improved Research Programmein Floral Diversification. In: Harder, L.D. and Barrett, S.C., Eds., Ecology and Evolution of Flowers, Oxford University Press, Oxford, 278-294.
- 4. Galen, C. and Newport, M.E.A. (1987) Bumble Bee Behavior and Selection on Flower Size in the Sky Pilot, Polemonium viscosum. Oecologia, 74, 20-23.
http://dx.doi.org/10.1007/BF00377340 - 5. Noland, H.M. (2004) Flower Size Preferences of the Honeybee (Apis mellifera) Foraging on Mimulus guttatus (Scrophulariaceae). Evolutionary Ecology Research, 6, 777-782.
- 6. Stebbins, G.L. (1970) Adaptive Radiation of Reproductive Characteristics in Angiosperms, I: Pollination Mechanisms. Annual Review of Ecology and Systematics, 1, 307-326.
http://dx.doi.org/10.1146/annurev.es.01.110170.001515 - 7. Fenster, C.B., Armbruster, W.S., Wilson, P., Dudash, M.R. and Thomson, J.D. (2004) Pollination Syndromes and Floral Specialization. Annual Review of Ecology, Evolution, and Systematics, 35, 375-403.
http://dx.doi.org/10.1146/annurev.ecolsys.34.011802.132347 - 8. Arroyo, M.T.K., Primack, R. and Armesto, J. (1982) Community Studies in Pollination Ecology in the High Temperate Andes of Central Chile. I. Pollination Mechanisms and Altitudinal Variation. American Journal of Botany, 69, 82-97.
http://dx.doi.org/10.2307/2442833 - 9. Kearns, C.A. and Inouye, D.W. (1994) Fly Pollination of Linum lewisii (Linaceae). American Journal of Botany, 81, 1091-1095.
http://dx.doi.org/10.2307/2445470 - 10. Bingham, R.A. and Orthner, A.R. (1998) Efficient Pollination of Alpine Plants. Nature, 391, 238-239.
http://dx.doi.org/10.1038/34564 - 11. Nagano, Y., Abe, K., Kitazawa, T., Hattori, M., Hirao, A.S. and Itino, T. (2014) Changes in Pollinator Fauna Affect Altitudinal Variation of Floral Size in a Bumblebee-Pollinated Herb. Ecology and Evolution, 4, 3395-3407.
http://dx.doi.org/10.1002/ece3.1191 - 12. Galen, C. (2000) High and Dry: Drought Stress, Sex-Allocation Trade-Offs, and Selection on Flower Size in the Alpine Wildflower Polemonium viscosum (Polemoniaceae). The American Naturalist, 156, 72-83.
http://dx.doi.org/10.1086/303373
NOTES

*Corresponding author.