Advances in Aging Research
Vol.2 No.4(2013), Article ID:38599,6 pages DOI:10.4236/aar.2013.24022
Mortality and morbidity due to the failure to treat mild anemia unrelated to cancer in elderly Americans—Review of the literature and case presentation
![]()
Medical Research Division, New Terra Enterprises, Los Angeles, USA: allend.allen@yahoo.com
Copyright © 2013 Allen D. Allen. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 18 September 2013; revised 18 October 2013; accepted 25 October 2013
Keywords: Elderly; Geriatric; Iron Deficiency; Anemia; Morbidity and Mortality; Injectable Iron; US Public Health; American Health Maintenance Organizations
ABSTRACT
Even mild iron deficiency anemia, as defined by the World Health Organization, is associated with increased mortality and significant morbidity in elderly individuals who are cancer free. Yet, anemia in the elderly is often dismissed as a benign sign of aging. This problem is exacerbated by the fact that elderly individuals often suffer from gastrointestinal complaints that preclude treatment with iron supplements by mouth. The FDA has approved two brands of injectable iron for treating such patients. Nonetheless, a major American health maintenance organization refuses to treat elderly patients with injectable iron, even when it is indicated, unless their anemia is associated with cancer. This may well reflect a public health crisis afflicting many elderly residents of the United States.
1. INTRODUCTION
1.1. Non-Cancer Related Iron Deficiency Anemia
Elderly patients are at risk for non-cancer related iron deficiency anemia (NCR-IDA) [1-8]. Likewise, patients with chronic diseases, such as rheumatoid arthritis [9,10], are at risk for the anemia of chronic disease. Too often anemia is ignored as a sign of normal aging [1,2]. To the contrary, anemia in the elderly causes severe deterioration of quality of life, morbidity, decline in physical and cognitive function, and an increased risk of mortality [3,5-7,11]. It is associated with an increased risk of recurrent falls due to fatigue, weakness, and limited activity [5-6,11-14], and also increases cardiovascular risks [1,6-7]. Moreover, anemia in the elderly is associated with dementia [15-17]. In general it increases the risk of hospitalization and mortality [18-20]. In the elderly even mild anemia is associated with hospitalization and mortality [19]. Hence, as observed by Walsh [21] early on, “Anemia in the elderly should never be attributed to old age. Even a mild anemia in collusion with multiple physical and mental problems may tip the balance for those previously able to cope with their disabilities.” Pointedly, Guralnik, et al. [22] characterize anemia in the elderly as “A Public Health Crisis in Hematology”. In that vein, the present paper serves to review the relevant literature and to provide an example of the problem via an illustrative case report.
1.2. Treatment Options
An iron-rich diet and iron supplements taken by mouth may improve general health and fatigue in women of childbearing age [23,24]. However, elderly patients often have gastrointestinal problems such as constipation, irritable bowel syndrome, and diverticulosis [25-28] resulting in intolerance to iron by mouth. Intolerance naturally leads to non-compliance. Indeed, oral iron preparations that promote constipation can be contraindicated in such patients [29]. These contraindications must be recognized in elderly patients with NCR-IDA [30]. Fortunately, two brands of iron dextran have been approved in the United States “for treatment of patients with documented iron deficiency in whom oral administration is unsatisfactory or impossible” [31,32]: INFed© is given IM or IV in a single large dose infused slowly [31]. Dexferrum© is administered IV (only) in a single large dose infused slowly [32]. Yet, a major American health maintenance organization (HMO) refuses to prescribe injectable iron for NCR-IDA patients when indicated, even in the case of elderly patients at risk, as illustrated by the case report in the sequel. Since the HMO in question sets and reflects trends, this may well reflect a public health crisis in the United States that needs to be urgently addressed.
2. SUMMARIES OF SOME RELEVANT STUDIES
2.1. The Health and Anemia Population Based Study: Mild Anemia in the Elderly
Methods: Riva, et al. [19] conducted a prospective population-based study of all of the 65 to 84 years old residents of Biella, Italy from 2003 to 2007. Data were available from a total of 7536 elderly subjects whose blood tests could be used to estimate mortality. Full health information for evaluating health-related outcomes was available for 4501 of these elderly subjects. Mild grade anemia was defined as a hemoglobin concentration between 10.0 and 11.9 g/dL in women, and between 10.0 and 12.9 g/dL in men, as in the case report in the sequel, and consistent with the criteria used by the World Health Organization [5,10,14].
Results: The risk of hospitalization in the three years following recruitment was higher among the mildly anemic elderly subjects than among control subjects who were not anemic. The adjusted hazard ratio is 1.32 with 95% confidence over the interval 1.09 - 1.60. Mortality risk in the following 3.5 years was also higher among the mildly anemic elderly subjects. The adjusted hazard ratio is 1.86 with 95% confidence over the interval 1.34 - 2.53. Similar results were found when slightly elevating the lower limit of normal hemoglobin concentration to 12.2 g/dL in women and 13.2 g/dL in men.
2.2. Established Populations for Epidemiologic Studies of the Elderly Sponsored by the National Institutes of Aging: Anemia in Old Age Carries Increased Risks of Mortality and Hospitalization
Methods: In 2006 Penninx, et al. [18] reported data obtained from 3607 human subjects aged 71 years or older. Anemia was defined as a hemoglobin concentration below 12.0 g/dL in women and below 13 g/dL in men, consistent with the criteria of the World Health Organization. Data on subsequent mortality and hospital admissions over four years were obtained from death records and the US Medicare database.
Results: Anemia was present in 451 (12.5%) of the 3607 enrolled subjects. During the follow-up period, anemic subjects were more likely to die than were nonanemic controls (37.0% versus 22.1%, p < 0.001). In addition, anemic subjects were hospitalized more often (65.9% versus 54.6%, p < 0.001) and spent more days in the hospital (25.0 versus 13.7, p < 0.001). After adjustment for demographics and baseline comorbidities, anemia significantly predicted subsequent mortality and hospitalization. The relative risks, respectively, were 1.61 with 95% confidence over the interval 1.34 - 1.93, and 1.27 with 95% confidence over the interval 1.12 - 1.45. After excluding subjects with prevalent diseases at baseline, anemia remained significantly associated with increased risks of mortality and hospitalization. A higher hemoglobin level was significantly associated with lower risks of mortality and hospitalization (for both trends p < 0.001).
2.3. Prospective Cohort Study in the Netherlands: Anemia in the Elderly Carries an Increased Risk of Recurrent Falls
Methods: In 2005, Penninx, et al. [12] reported a prospective-cohort study of community-dwelling subjects in the Netherlands. They enrolled 394 subjects aged 65 to 88 from the Longitudinal Aging Study Amsterdam. As always, anemia was defined according to the criteria of the World Health Organization, i.e., a hemoglobin concentration less than 12 g/dL for women and less than 13 g/dL for men. Falls were prospectively determined using calendars that subjects filled out weekly for three years. Subjects were identified as having recurrent falls if, and only if, they fell at least two times within six months during the 3-year follow-up.
Results: Of the 394 enrolled subjects, 18 women and 29 men had anemia, a total of 11.9%. The incidence of recurrent falls was 38.3% for anemic subjects as compared to 19.6% for non-anemic control subjects (p = 0.004). After adjustment for sex, age, body mass index, and comorbidity, anemia was significantly associated with recurrent falls by a hazard ratio of 1.91 with 95% confidence over the interval 1.09 - 3.36. In other words, anemic subjects had almost twice the risk of recurrent falls as non-anemic controls.
2.4. Anemia and Decline in Physical Performance in the Elderly
Methods: Earlier, in 2003, Penninx, et al. [14] reported on a 4-year prospective study of 1146 subjects aged 71 years or older living in Iowa. As always, anemia was defined using the criteria of the World Health Organization (WHO). Physical performance was evaluated using standing balance, a timed 2.4 meter walk, and a timed test of five chair rises. These were combined to arrive at a score ranging from 0 (poor) to 12 (excellent).
Results: After adjustment for baseline performance score, health status, and demographic characteristics, anemia was associated with greater mean decline in physical performance over four years. The adjusted mean decline in subjects with anemia was 2.3 with 95% confidence over the interval 1.7 to 2.8. The adjusted mean decline in subjects without anemia was 1.4 with 95% confidence over the interval 1.2 to 1.5; p = 0.003. The association between anemia and greater physical decline was also present in participants who were free of diseases associated with anemia, such as cancer, infectious diseases and renal failure, and after adjustment for serum cholesterol, iron, and albumin levels. Persons with a borderline anemia who had a hemoglobin concentration within 1 g/dL above the WHO criteria also showed a greater mean physical decline of 1.8 than those with higher hemoglobin concentration with 95% confidence over the interval 1.5 to 2.2, p = 0.02.
2.5. The Health, Aging and Body Composition Study: Is Anemia a Greater Risk for American Whites Than for American Blacks?
Methods: In 2007, Patel, et al. [33] analyzed data on 1018 black community-dwelling adults and 1,583 white community-dwelling adults aged 71 to 82 years. Anemia, as defined by the World Health Organization (WHO), was used to predict mortality over six years and the incidence of mobility disability over four years.
Results: The age-adjusted hazard ratio for mortality in anemic white males was 1.96 with 95% confidence over the interval 1.35 - 2.83. For white women it was 2.86 with 95% confidence over the interval 1.69 - 4.82. In contrast, anemia was not associated with mortality in black men or women, the respective hazard ratios being 1.15 with 95% confidence over the interval 0.77 - 1.72, and 1.39 with 95% confidence over the interval 0.91 - 2.14. A higher mortality rate was observed only in black men with hemoglobin concentrations more than 2.0 g/dL below the WHO cutoff. In contrast, mortality rates were elevated in white men with hemoglobin concentrations of 1 - 20 and more than 20 g/dL below the WHO cutoff. In other words, anemia was significantly associated with an increased risk of death and mobility disability in community-dwelling older whites. But older blacks classified as anemic by the WHO criteria were not at risk for adverse events.
Discussion: At the same time, blacks are more likely to be anemic than whites [34]. Moreover, in 2006, Denny, et al. [11] reported increased mortality associated with anemia in elderly, community-dwelling African Americans. Likewise, in 2007, Agnihotri, et al. [4] reported improvements in fatigue and quality of life when anemic older African-American women were treated with epoetin alpha.
3. CASE PRESENTATION
3.1. History
DF is currently a 77 year-old white American male. At the age of 33 he had a gastric ulcer that was successfully treated with cimetidine. Due to persistent complaints of dyspepsia and his history of gastric ulcer, he was switched to a proton pump inhibitor when those drugs became available, specifically, pantoprazole 40 mg poqhs, which he has continued taking up until the present. By the age of 45, DF also had a history of NCR-IDA, probably due to a lupoid disease that was diagnosed with several differentials depending upon the rheumatologist. His NCRIDA was successfully treated with IM iron dextran. Even then he was found to be intolerant of iron by mouth due to irritable bowel syndrome. Later in life he would be diagnosed with diverticulosis, which one presumes could only have exacerbated his intolerance of iron by mouth.
By the age of 68, DF had multiple risk factors for an acute myocardial infarction (MI), including his age, familial history (his mother died of her first MI at the age of 65), a tenacious and refractory smoking addiction [35], a sedentary lifestyle, and Type 2 diabetes mellitus. As a consequence, he was started on a daily dose of 160 mg of aspirin by mouth. Despite the daily pantoprazole, the taking of aspirin every day gave DF a very slow, diffuse, gastrointestinal hemorrhage. This was discovered after about a year of aspirin therapy when DF’s iatrogenic NCR-IDA became symptomatic. In the Emergency Department (ED), his hemoglobin concentration was determined to be only 7.0 g/dL and he was treated with a transfusion (2 units of blood). Needless to say, DF discontinued aspirin at that time. His gastrointestinal bleeding resolved within a matter of days as determined by the absence of occult stool blood. Within a month, his hemoglobin concentration had rebounded to its premorbid level of 14.0 g/dL, the lower bound of the laboratory reference range.
3.2. The Heart Attacks and Sequelae
At the age of 76, DF had a pair of acute MIs, so unusual that the present author reported it in the literature. The details may be found elsewhere [36]. The salient fact for the present purposes is that DF had a drug-eluting stent implanted in his left anterior descending artery (Boston Scientific PROMUS Element® Plus). As a consequence, he had to resume taking 160 mg aspirin by mouth every day along with 75 mg clopidogrel bisulfate. Aware of his history of aspirin-induced hemorrhage and the resulting NCR-IDA, DF expressed a concern about taking aspirin to his physicians throughout the next year but to no avail. After 14 months, however, his hemoglobin concentration had fallen to <10 g/dL and he was again given a transfusion with two units of blood. Fortunately, his cardiologist advised DF that it was no longer necessary for him to take aspirin and he discontinued it. As before, his gastrointestinal hemorrhage resolved within a matter of days. This time, however, his hemoglobin concentration did not rebound to its premorbid level of 14.0 g/dL (WNL). Rather, he had a persistent mild anemia as defined by the WHO criteria, the laboratory reference range, and the pre-morbid baseline, as shown in Table 1.
DF’s persistent mild anemia was clinically correlated and by now was exacerbated by chronic restrictive lung disease. He reported pronounced exercise intolerance leading to weakness of his legs and requiring a wheelchair for any but trivial mobility. He also complained of marked fatigability that limited his time spent out of bed. This was despite the fact that DF maintained an iron-rich diet that included green leafy vegetables such as spinach. (The author has no dietary information on the human subjects enrolled in formal studies reported here retrospectively). Regrettably, no one but the HMO’s hematologists were permitted to administer injectable iron. Despite FDA approval of iron dextran for this indication, the patient’s history of tolerating that treatment, and the literature reviewed here, the HMO’s hematologists refused to provide the indicated treatment since they reserve it for cancer patients. Indeed, the HMO apparently considers hematology to be more of an adjunct to oncology than a separate medical specialty.
4. CONCLUSIONS
According to the current and prospective studies reviewed here, even mild NCR-IDA is associated with significant morbidity and an increased risk of mortality in elderly individuals who are cancer free. These studies define anemia using the criteria of WHO. Although anemia is more common in African-Americans than in white Americans, one study demonstrates that elderly African-
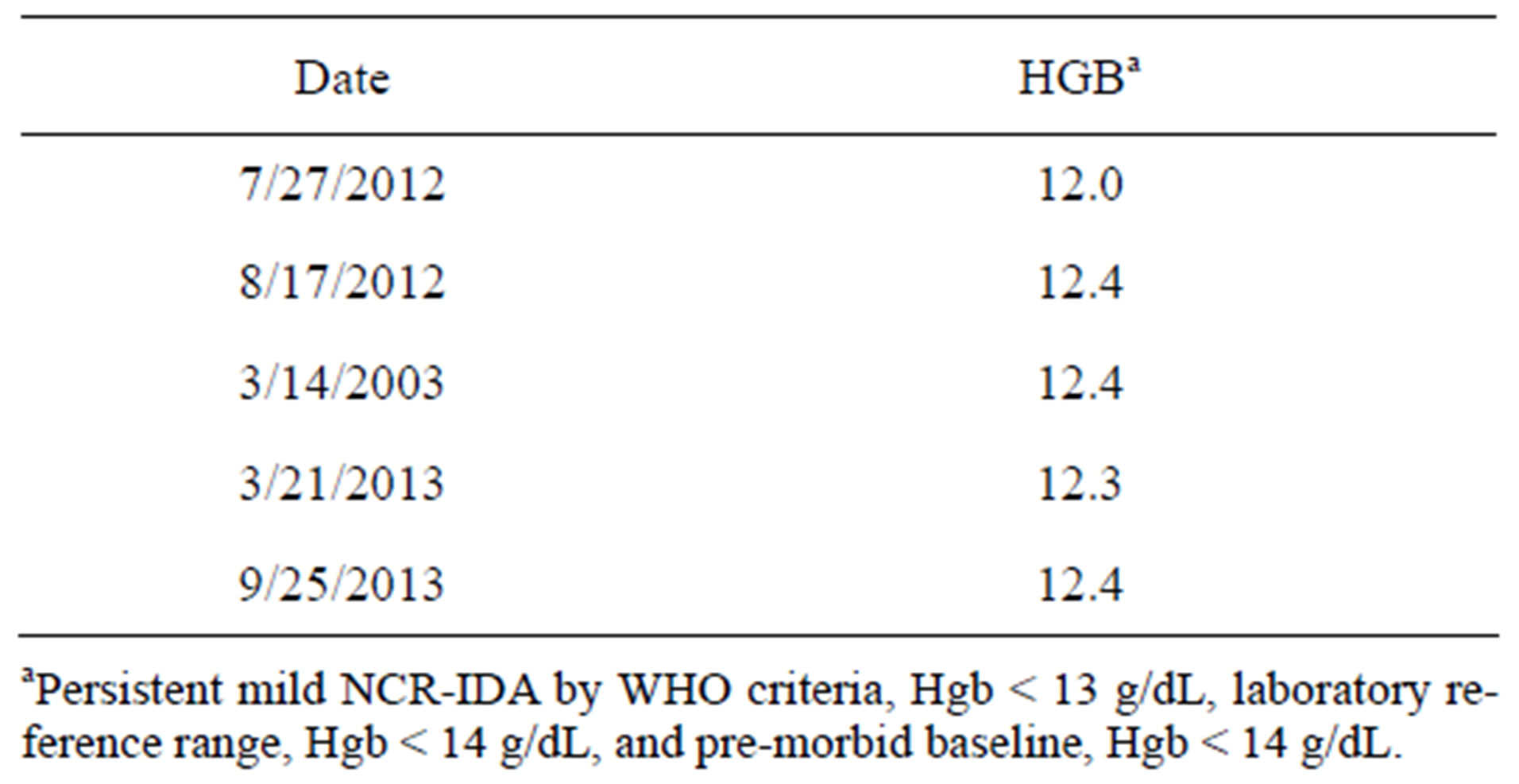
Table 1. Persistent mild anemia in the elderly male following discontinuation of aspirin and a transfusion used to treat a florid aspirin-induced anemia.
Americans are less at risk from anemia than elderly white Americans. This is contradicted by two other studies referenced here. One shows an equal increase in the risk of mortality for both races. The other shows an improvement in fatigue and quality of life following improved hemoglobin concentration in African-American women treated with epoetin alpha. However, there is no doubt but what even mild anemia in elderly white Americans is associated with significant morbidity, increased hospitalizations, and an increased risk of mortality. Signs and symptoms to expect in elderly individuals with untreated anemia include severe deterioration of quality of life, decline in physical and cognitive function, dementia, recurrent falling, fatigue, weakness, limited activity, and cardiovascular disease.
It is not unusual for elderly individuals to suffer from gastrointestinal complaints that preclude treatment with iron supplements by mouth. The FDA has approved two brands of injectable iron dextran for this indication. The author and others [37] have extensive experience with the treatment of anemia with injectable iron and find it safe and effective if properly administered. Nonetheless, a major American HMO refuses to prescribe injectable iron when indicated for elderly individuals if their anemia is unrelated to cancer.
There is no doubt that anemia associated with cancer and its treatment deserves careful attention and appropriate management [38-44]. However, this does not justify withholding injectable iron from elderly patients when it is otherwise indicated and their anemia is unrelated to cancer. There are, of course, other parenteral drugs for treating anemia in elderly patients who do not tolerate iron supplements by mouth, such as epoetin [45], both alfa and beta [46], and a novel erythropoiesis stimulating protein consisting of a hyperglycosylated analogue of recombinant human erythropoietin, which has an increased terminal half-life [47]. However, the cost of epoetintype drugs presents a financial obstacle for an HMO. But the practice of not offering injectable iron when indicated has no medical or financial rationale to justify the increased mortality and morbidity in the elderly. Since the major HMO in question sets and reflects trends in American medical practice, this may well be an urgent national problem in the United States adversely affecting millions of elderly patients.
REFERENCES
- Aapro, M.S., Cella, D. and Zagari, M. (2002) Age, anemia, and fatigue. Seminars in Oncology, 29, 55-59.
- Smith, D.L. (2002) Anemia in the elderly. American Family Physician, 62, 1565-1572.
- Balducci, L., Ershler, W.B. and Krantz, S. (2006) Anemia in the elderly—Clinical findings and impact on health. Clinical Reviews in Oncology/Hematology, 58, 156-165.
- Agnihotri, P., Telfer, M., Butt, Z., Jella, A., Cella, D., Kozma, C.M., Ahuja, M., Riaz, S. and Akamah, J. (2007) Chronic anemia and fatigue in elderly patients: Results of a randomized, double-blind, placebo-controlled, crossover exploratory study with epoetin alfa. Journal of the American Geriatrics Society, 55, 1557-1565.
- Artz, A.S. (2008) Anemia and the frail elderly. Seminars in Hematology, 45, 261-266. http://dx.doi.org/10.1053/j.seminhematol.2008.06.002
- Eisenstaedtr, R., Penninx, B.W.J.H. and Woodman, R.C. (2006) Anemia in the elderly: Current understanding and emerging concepts. Blood Reviews, 20, 213-226.
- Gabrilove, J. (2005) Anemia and the elderly: Clinical considerations. Best Practice & Research Clinical Haematology, 18, 417-422.
- Lipschitz, D. (2003) Medical and functional consequences of anemia in the elderly. Journal of the American Geriatrics Society, 51, 10-13. http://dx.doi.org/10.1046/j.1532-5415.51.3s.6.x
- Kaltwasser, J.P., Kessler, U., Gottschalk, R., Stucki, G. and Möller, B. (2001) Effect of recombinant human erythropoeietin and intravenous iron on anemia and disease activity in rheumatoid arthritis. The Journal of Rheumatology, 28, 2430-2436.
- Bloxham, E. Vagadia, V., Scott, K., Francis, G., Saravanan, V., Heycock, C., Rynne, M., Hamilton, J. and Kelly, C.A. (2011) Anemia in rheumatoid arthritis: Can we afford to ignore it? Postgraduate Medical Journal, 87, 596-600. http://dx.doi.org/10.1136/pgmj.2011.117507
- Denny, S.D., Kuchibhatia, M.N. and Cohen, H.J. (2006) Impact of anemia on mortality, cognition, and function in community-dwelling elderly. The American Journal of Medicine, 119, 327-334. http://dx.doi.org/10.1016/j.amjmed.2005.08.027
- Penninx, B.W.J.H., Pluijm, S.M.F., Lips, P., Woodman, R., Miedema, K., Guralnik, J.M. and Deeg, D.J.H. (2005) Late-life anemia is associated with increased risk of recurrent falls. Journal of the American Geriatrics Society, 53, 2106-2111.
- Penninx, B.W.J.H., Pahor, M., Cesari, M., Corsi, A.M., Woodman, R.C., Bandinelli, S., Guralnik, J.M. and Ferrucci, L. (2004) Anemia is associated with disability and decreased physical performance and muscle strength in the elderly. Journal of the American Geriatrics Society, 52, 719-724.
- Penninx, B.W.J.H., Guralnik, J.M., Onder, G., Ferrucci, L., Wallace, R.B. and Pahor, M. (2003) Anemia and decline in physical performance among older persons. The American Journal of Medicine, 115, 104-110. http://dx.doi.org/10.1016/S0002-9343(03)00263-8
- Hong, C.H., Falvey, C., Harris, T.B., Simonsick, E.M., Satterfield, S., Ferrucci, L., Metti, A.L., Patel, K.V. and Yaffe, K. (2013) Anemia and risk of dementia in older adults. Neurology, 81, 528-533. http://dx.doi.org/10.1212/WNL.0b013e31829e701d
- Terekeci, H.M., Kucukardali, Y., Onem, Y., Erikci, A.A., Kucukardali, B., Sahan, B., Sayan, O., Celik, S., Gulec, M., Sanisoglu, Y.S., Nalbant, S., Top, C. and Oktenli, C. (2010) Relationship between anaemia and cognitive functions in elderly people. European Journal of Medicine, 21, 87-90. http://dx.doi.org/10.1016/j.ejim.2009.12.005
- Atti, A.R., Palmer, K., Volpato, S., Zuliani, G., Winblad, B. and Fratiglioni, L. (2006) Anemia increases the risk of dementia in cognitively intact elderly. Neurobiology of Aging, 27, 278-284. http://dx.doi.org/10.1016/j.neurobiolaging.2005.02.007
- Penninx, B.W.J.H., Pahor, M., Woodman, R.C. and Guralnik, J.M. (2006) Anemia in old age is associated with increased mortality and hospitalization. The Journals of Gerontology: Series A, 61, 474-479.
- Riva, E., Tettamanti, M., Mosconi, P., Apolone, G., Gandini, F., Nobili, A., Tallone, M.V., Detoma, P., Giacomin, A., Clerico, M., Tempia, P., Guala. A., Fasolo, G. and Lucca, U. (2008) Association of mild anemia with hospitalization and mortality in the elderly: The health and anemia population-based study. Haematologica, 94, 22-28. http://dx.doi.org/10.3324/haematol.13449
- Zakai, N.A., Katz, R., Hirsch, C., Shlipak, M.G., Chaves, P.H.M., Newman, A.B. and Cushman, M. (2005) A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort. Archives of Internal Medicine, 165, 2214-2220. http://dx.doi.org/10.1001/archinte.165.19.2214
- Walsh, J.R. (1981) Hematologic disorders in the elderly. Western Journal of Medicine, 135, 446-454.
- Guralnik, J.M., Ershler, W.B., Schrier, S.L. and Picozzi, V.J. (2005) Anemia in the elderly: A public health crisis in hematology. American Society of Hematology Education Book, 2005, 528-532. http://dx.doi.org/10.1182/asheducation-2005.1.528
- Pattrson, A.J., Brown, W.J. and Roberts, D.C.K. (2001) Dietary and supplement treatment of iron deficiency results in improvements in general health and fatigue in Australian women of childbearing age. Journal of the American College of Nutrition, 20, 337-642. http://dx.doi.org/10.1080/07315724.2001.10719054
- Patterson, A.J., Brown, W.J., Roberts, D.C.K. and Seldon, M.R. (2001) Dietary treatment of iron deficiency anemia in women of childbearing age. American Journal of Clinical Nutrition, 74, 650-656.
- Firth, M. and Prather, C.M. (2002) Gastrointestinal motility problems in the elderly patient. Gastroenterology, 122, 1688-1700. http://dx.doi.org/10.1053/gast.2002.33566
- Camilleri, M., Lee, J.S., Viramontes, B., Bharucha, A.E. and Tangalos, E.G. (2000) Insights into the pathophysiology and mechanisms of constipation, irritable bowel syndrome, and diverticulosis in older people. Journal of the American Geriatrics Society, 48, 1142-1150.
- Comparato, G., Pilotto, A., Franzè, A., Franceschi, M. and Di Mario, F. (2007) Diverticular disease in the elderly. Digestive Diseases, 25, 151-159. http://dx.doi.org/10.1159/000099480
- Shoji, B.T. and Becker, J.M. (1994) Colorectal disease in the elderly patient. The Surgical Clinics of North America, 74, 293-316.
- van Geloven, A.A.W., Biesheuvel, T.H., Luitse, J.S.K., Hoitsma, H.F.W. and Obertop, H. (2000) Hospital admissions of patients aged over 80 with acute abdominal complaints. European Journal of Surgery, 166, 866-871. http://dx.doi.org/10.1080/110241500447254
- Pirzio-Biroli, G. and Finch, C.A. (1957) Treatment of iron deficiency anemia in the adult. Journal of Chronic Diseases, 6, 302-306. http://dx.doi.org/10.1016/0021-9681(57)90025-5
- http://www.pdr.net/drug-summary/infed?druglabelid=2087&id=1319
- http://www.pdr.net/drug-summary/dexferrum?druglabelid=1310&id=3102
- Patel, K.V., Harris, T.B., Faulhaber, M., Angleman, S.B., Conneley, S., Bauer, D.C., Kuller, L.H., Newman, A.B. and Guralnik, J.M. (2007) Racial variation in the relationship of anemia with mortality and mobility disability among older adults. Blood, 109, 4663-4670. http://dx.doi.org/10.1182/blood-2006-10-055384
- Zakai, N.A., McClure, L.A., Prineas, R., Howard, G., McClellan, W., Holmes, C.E., Newsome, B.B., Warnock, D.G., Audhya, P. and Cushman, M. (2009) Correlates of anemia in American blacks and whites. American Journal of Epidemiology, 169, 355-364. http://dx.doi.org/10.1093/aje/kwn355
- Allen, A.D. (1989) The realities of tobacco addiction. Journal of Clinical Psychiatry, 50, 148.
- Allen, A.D. (2012) State of the art in cardiac intervention: A case report. International Journal of Clinical Medicine, 3, 628-632. http://dx.doi.org/10.4236/ijcm.2012.37112
- González, Z.M., Barrasa, A.G., Lorenzo, L.R. and Renua, A.R. (2009) Hierro intravenoso (intravenous iron). Cirugia Española, 86, 196-203. http://dx.doi.org/10.1016/j.ciresp.2009.05.012
- Leitgeb, C., Pecherstorfer, M., Fritz, E. and Ludwig, H. (1994) Quality of life in chronic anemia of cancer during treatment with recombinant human erythropoietin. Cancer, 73, 2532-2542
- Cella, D. (1997) The functional assessment of cancer therapy-anemia (FACT-An) scale: A new tool for the assessment of outcomes in cancer anemia and fatigue. Seminars in Hematology, 34, 13-19.
- Yellen, S.B., Cella, D.F., Webster, K., Blendowski, C. and Kaplan, E. (1997) Measuring fatigue and other anemiarelated symptoms with the functional assessment of cancer therapy (FACT) measurement system. Journal of Pain and Symptom Management, 13, 63-74 http://dx.doi.org/10.1016/S0885-3924(96)00274-6
- Ludwig, H. and Fritz, E. (1998) Anemia in cancer patients. Seminars in Oncology, 25, 2-6.
- Cella, D. (1998) Factors influencing quality of life in cancer patients: Anemia and fatigue. Seminars in Oncology, 25, 43-46.
- Caro, J.J., Salas, M., Ward, A. and Goss, G. (2001) Anemia as an independent prognostic factor for survival in patients with cancer. Cancer, 91, 2214-2221. http://dx.doi.org/10.1002/1097-0142(20010615)91:12<2214::AID-CNCR1251>3.0.CO;2-P
- Knight, K., Wade, S. and Balducci, L. (2004) Prevalence and outcome of anemia in cancer: A systematic review of the literature. The American Journal of Medicine, 116, 11-26. http://dx.doi.org/10.1016/j.amjmed.2003.12.008
- Hecht, D. and Boujoukos, A. (2010) Anemia in the ICU: Are your patients needin’ erythropoetin? Critical Care, 14, 332.
- Halstenson, C.E., Macres, M., Katz, S.A., Schnieders, J.R., Watanabe, M., Sobota, J.T. and Abraham, P.A. (1991) Comparative pharmacokinetics and pharmacodynamics of epoetin alfa and epoetin beta. Clinical Pharmacology and Therapeutics, 50, 702-712. http://dx.doi.org/10.1038/clpt.1991.210
- MacDougall, I.C., Gray, S.J., Elston, O., Breen, C., Jenkins, B., Browne, J. and Egrie, J. (1999) Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. Journal of the American Society of Nephrology, 10, 2392-2395.

