Neuroscience & Medicine
Vol.4 No.2(2013), Article ID:33251,7 pages DOI:10.4236/nm.2013.42015
Alpha Rhythms Response to 10 Hz Flicker Is Wavelength Dependent
![]()
1L. D. University, Verona and Prosenectute, Vicenza, Italy; 2CRIRMANMEC University, Padova, Italy; 3Ocular Department Hospital, Vicenza, Italy.
Email: *fferromilone@gmail.com
Copyright © 2013 Francesco Ferro Milone et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 3rd, 2013; revised April 5th, 2013; accepted May 9th, 2013
Keywords: Alpha Rhythms; Light Stimulation; Wavelength
ABSTRACT
Since Adrian and Metthew [1], light may be considered the dominant stimulus for the brain. This statement is confirmed after the discovery of the suprachiasmatic nucleus (SCN) that regulates the master biological clock [2]. In 1998 the discovery of photopigment melanopsin in the ganglion cells of the retina, give new insight in the importance of the light in the pathophysiology of the brain [3]. We have studied the effect of flashing at 10 Hz with LED light of different wavelength on the response of the alpha system. We have shown that this response, consistent with the drive of the frequency and the augmentation of the voltage of the alpha rhythms, is far more significant with the RED-LED than GREEN-LED or BLUE-LED or WHITE-LED (three-chrome) light flashing. We stem the hypothesis that the amplitude increase and phase reset of the alpha waves produced by RED-LED flashing at 10 Hz may be due either to photobiomodulation on the cytochromo c oxidase [4,5] and/or of the photopigment melanopsin, at the level of the retinal ganglion cells, that reinforce the incoming cone-LHC signal and therefore the projection to the SCN [6] or to reinforcement of postsynaptic short term responsiveness, in retinal cone-LHC synapse, due to repetitive stimulation [7,8] or both. We may speculate that the increase of amplitude and phase reset of alpha rhythms, due to flashing at 10 Hz, is primarily modulated in the retina.
1. Introduction
Since Adrian [1] and Walter [9] the EEG “activation methods” with light is a well known method in electroencephalography. Adrian conclude that light is a dominant stimulus in the central nervous system. More recently correlational evidence has linked alpha rhythms to memory formation in healthy people [10] and to memory problems in Alzheimer’s disease [11]. Going beyond mere correlation, brain stimulation at 10 Hz can enhance Long Term Potentiation (LTP), the neural basis of memory, in rats. This suggest that alpha rhythms may subserve memory formation in man and that 10 Hz flicker enhances memory in healthy people and may have therapeutic potential in memory disorders [11].
We have studied since 10 years the effect of light stimulation, in the frequency range of the alpha rhythms, in EEG phenomenology simulation, in controls and in patients affected by cognitive impairment. The findings are consistent with an improvement of behavioural memory performances in aged subjects produced by means of prolonged intermittent light stimulation [12-15].
Initially light stimulation was performed by means of xenon 6 W lamp 100 - 700 mJ intensity (MICROMED apparatus). More recently we have adopted a suitable and handy device (google shaped) into which we have assembled three-chrome LED lamp: RED-LED (InGaAIP), GREEN-LED (InGaN) and BLUE-LED (InGaN).
2. Methods
It is well established that Light Emission Diode (LED light) is a monochromatic source of light that has distinctive propriety that appears useful in the stimulation of the retina.
LED-Light is a monochromatic but not coherent light and therefore with low energy. Radiant power at a distance of 1.5 cm (viewing angle 120˚) were calculated; RED-LED = 11 μW/cm2, BLUE-LED = 29 μW/cm2, and GREEN-LED= 20 μW/cm2.
We have adopted Kingbright Ltd. LED lamp: HypeerRED (InGaAIP), peak λ 650 nm; BLUE-LED (InGaN), peak λ 468 nm; GREEN-LED (InGaN) peak λ 520 nm.
Voluntary subject (10) and patients (24) are involved in the study having subscribed informed consent declaration. The patient’s protocol was submitted to the Ethic Committee of the ULS (Vicenza) [12].
Intermittent light stimulation was performed on patient awake, sited on a confortable chair. The EEG was recorded by means of a casque with 10 leads (10 - 20 system reduced) on the electroencephalograph MICROMED (Mogliano, Italy), connected wireless to the PC by the System Plus software. The on/off for the light stimulation is performed through the electroncefalograph (sited into the casque) by wireless software. So that there are no manouvre around the head of the subject/patient during the change of colour for differential stimulation. For instance it is possible stimulate: on red 1 min off 1 min, on blue 1min., off 1 min. etc. Parameter’s modification (duty cycle, duration and flicker frequency) are easy achievable through a computer program, as five independent steps.
EEG recorder WSPY, a device developed by MICROMED (Mogliano, Italy), is a wireless 10 channel recorder specifically designed to operate without any connection to the acquisition system. EEG wet electrodes and the casque are carefully described and discussed in a paper held in the Conference on Bioinformatics and Biomedical Enginering in Shanghai 2011 [13].
3. Results
First phase: Light Intermittent Stimulation (ILS) 10 Hz was performed with white flash from a xenon 6 W 100 - 200 mJ lamp (MICROMED Ltd.) Figures 1-3.
As seen in Figure 1, ILS with white (standard) lamp provokes drive of alpha rhythm and the appearance of harmonic that belong to the beta rhythms. Whereas in Figure 2, we see the resettimg of the phase during ILS and the augmentation of the voltage (synchronizing effect of the ILS). Figure 3 shows the effect of ILS in different clinical conditions: the comment is that during the decline of the EEG activity, due to aging and/or neurode-
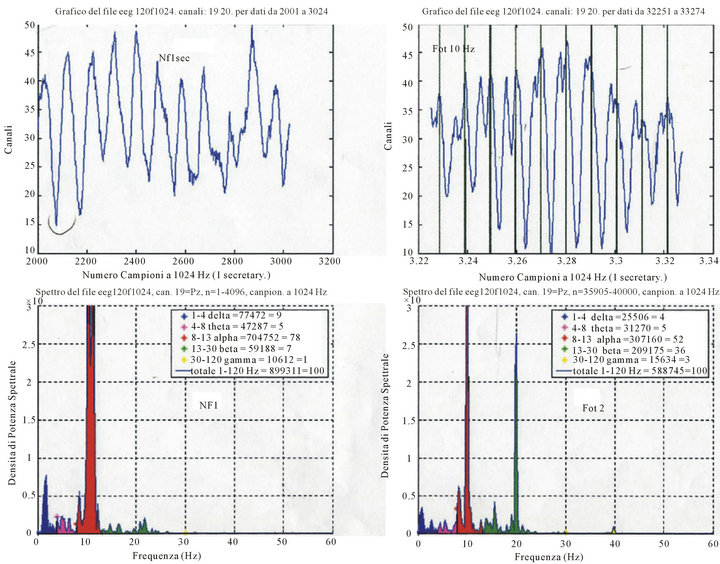
Figure 1. Up-left = row EEG O2, non photostimulated (NF); Up-right = EEG photostimulated 10 Hz; Down-left = Spectral Density (μV2/Hz) at rest; Down right = Spectral Density photo-stimulated (μV2/Hz). NOTICE: the converging of the spectral density around the peak of the alpha rhythm at 10 Hz and the appearance of harmonic at 20 Hz, during flicker.
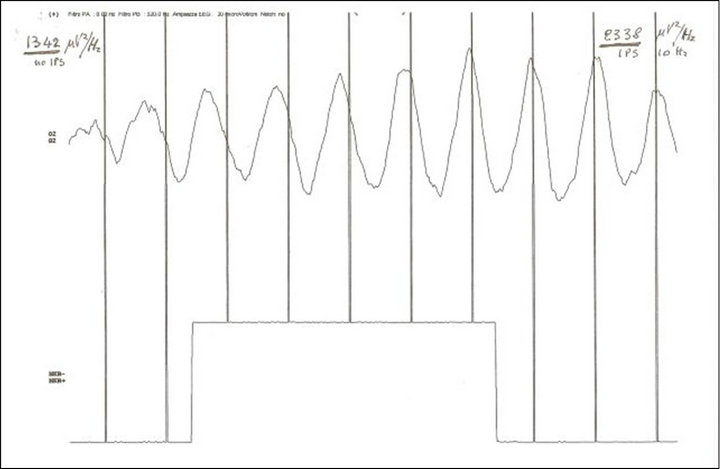
Figure 2. Phase and amplitude drive (time 1 second) of one alpha spindle (EEG) in O2 at the start of flicker 10 Hz (vertical bars) with standard xenon lamp 200 mJ intensity (MICROMED Ltd). Spectral density (epoch 10 seconds): basal 1342 μV2/Hz and during stimulation 2338 μV2/Hz. NOTICE: drive of phase and amplitude of alpha waves.
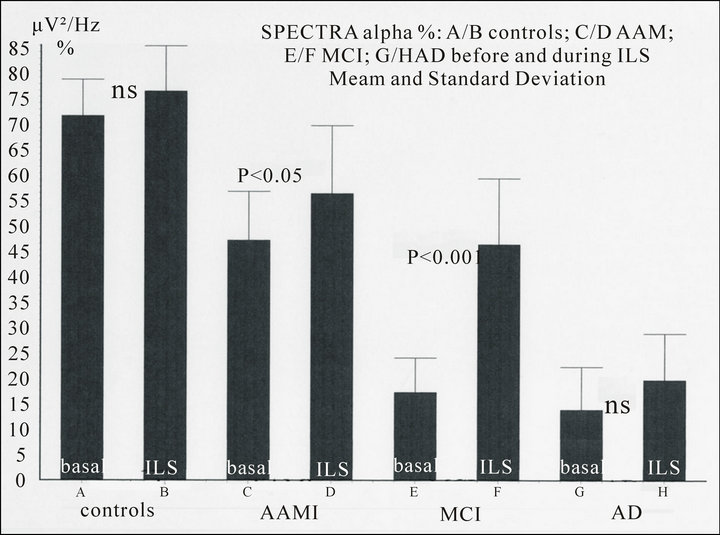
Figure 3. Spectral density (μV2/Hz %, O2, 7 - 14 Hz) in controls and patients in basal conditions and during Intermittent Light Stimulation (kind permission Ferro Milone et al., Psicogeriatria, 2009). NOTICE: progressive decrease of the alpha spectral density (per cent value) from controls to patients affected by Alzheimer’s disease (AD). The driving effect of the Intermittent Light Stimulation (ILS) at 10 Hz on the alpha waves during flicker is non significant (ns) in controls and AD but become significant in patients with AAMI (Age Associated Memory Impairment) and more in MCI (Mild Cognitive Impairment).
generation, there is a phase in which the recruiting response of the light is more evident and significant. We speculate that there are synapses, in the contest of the synaptic decay, “silent “and/or out of phase that may be recruited and give rise to the augmented response.
Prolonged ILS shows increase of amplitude and reset of the phase of the alpha rhythms and the appearance of resonance phenomena. In controls and patients the increase of amplitude of alpha, as calculated by power spectral density (μV2/Hz), in absolute as well as in relative (per cent) value, shows an increase of the density during ILS non significant in controls and Alzheimer’s disease and poorly significant (p < 0.05) in AAMI (Age Associated Memory Impairment), but very significant (p < 0.001) in MCI (Mild Cognitive Impairment) [12,14].
Second phase: ILS was performed with HyperrREDLED (InGaAIP, peak λ 650 nm) mounted on a google (without lens) alone or in sequence with GREEN-LED (InGaN, peak λ 520 nm), BLUE-LED (InGaN, peak λ 468 nm), WHITE-LED (three-chrome). Radiant power at a distance of 1.5 cm was: Red 11 μW/cm2, Blue 29 μW/cm2, Green 20 μW/cm2, Figures 4-6.
As seen in those figures the ILS with RED-LED flash results in a marked increase of the voltage in absolute value, or in confront to blue and green or white colour stimulation, despite the energy of RED-LED is reduced in respect to blue, green, and white. The difference between non stimulated and stimulated is very significant (ANOVA p < 0.001) instead of the non significant (ns) in controls as seen in Figure 3, where the stimulation was with white standard lamp. The differential increase of voltage of the alpha rhythms with RED-LED in confront to BLU-LED light is evident also in the respective harmonics (Figure 6).
4. Discussion
In the last 10 years we have studied the effect of flickering in aged patients with the aim to understand the feature of the EEG deterioration during aging in normal and pathologic conditions [12,14]. We observed that the increase of amplitude and phase reset are constant features of almost all individuals with few exception. The increase of amplitude is far well evident in a period of the aging brain where the synapses are unstable may be for Ca control derangement, or AMPA-NMDA receptors overload [16].
But in a second phase of our work ILS was performed by means of LED flash with different colours. We are surprised by the difference in power augmentation during ILS with RED-LED Light in respect to WHITE, BLUELED and GREE-LED Light stimulation. The difference must have an explanation only in term of an effect of the light inside the ganglion cells of the retina. We presume it because the difference of the intensity of the response to RED-LED, but not of the response to BLUE-, GREENLED, stem the hypothesis that there is an effect of photobiomodulation on cytochromo c oxidase [17] and/or a direct activation of the photopigment melanopsin, an opsin recently discovered in the ganglion cells of the retina (intrinsecally photosensituve Retinal Ganglion Cells, ipRGCs). Activation of ipRGCs increases their sensitivity to light stimulation. Melanopsin mediates nonvisual photoreceptive tasks, and is important in
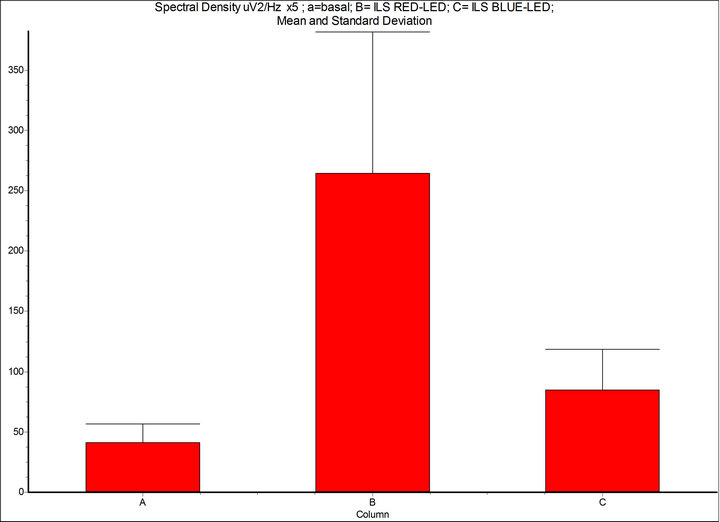
Figure 4. Spectral density (μV2/Hz, 7 - 14 Hz): A = basal condition; B = Intermittent Light Stimulation (ILS) 10 Hz, with RED-LED flash (inGaAIP) λ 650 nm, duty cycle 50%, intensity 11 μW/cm2; C = BLUE-LED flash (InGaN) λ 468 nm, intensity 29 μW/cm2. InSTAT (neighbor columns): A-B = p < 0.001; A-C = p < 0.05; B-C = p < 0.001. NOTICE: the drive of phase and amplitude of the alpha rhythm is more significant with RED-LED light than BLUE-LED light despite energy (eV) of red light is 1.9 and blue 2.7. InSTAT(neighbor columns): A-B = p < 0.001.
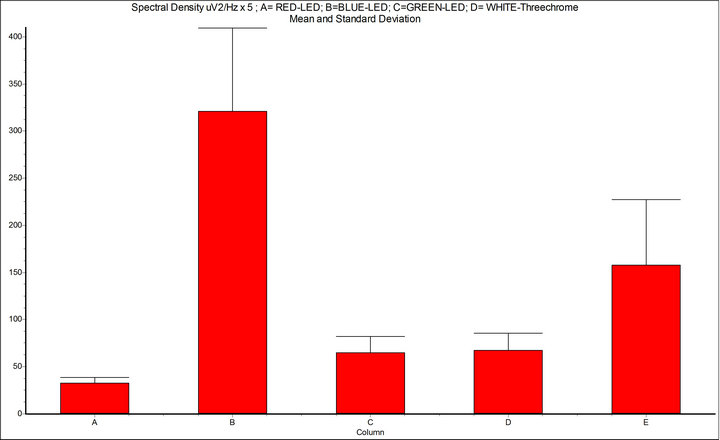
Figure 5. Spectral density (μV2/Hz, 7 - 14 Hz); A = basal condition; B = Intermittent Light Stimulation (ILS) 10 Hz, with RED-LED flash (InGaAIP, λ 650 nm); C = ILS with BLUE-LED flash (InGaN, λ 468 nm); D = ILS with GREEN-LED flash (InGaN, λ 520 nm); E = WHITE-LED three-chrome flashing; InSTAT (between columns) A-B = p < 0.001; A-C = ns; A-D = ns; A-E = p < 0.001; B-C = p < 0.001; B-D = p < 0.001; B-E = p < 0.01; NOTICE: ILS with RED-LED flash is powerful mean to provoke drive of alpha rhythm.
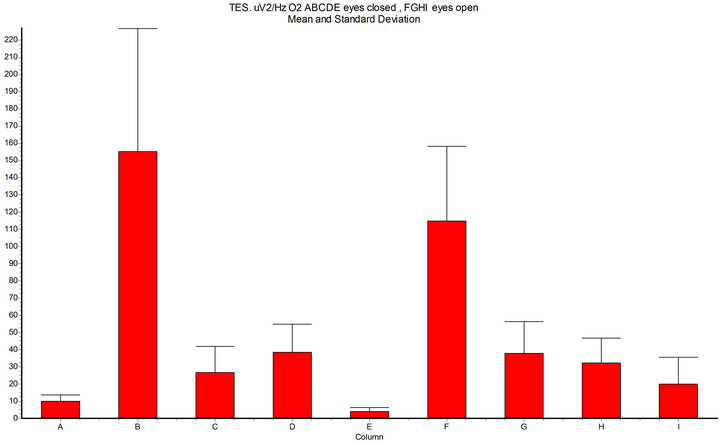
Figure 6. Spectral density (x axis μV2/Hz O2, 7 - 14 Hz, epoch 10 sec); A-B-C-D-E = eyes closed; F.G.H-I = eyes open; A = basal condition; B = during Intermittent Light Stimulation (ILS) 10 Hz with RED-LED light (InGaAIP, peak λ 650 nm) eyes closed; C = harmonics-red; D = BLUE-LED light (InGaN peak λ 468 nm); E = harmonics-blue; F = ILS RED-LED eyes open; G = harmonics-red; H = ILS BLUE-LED eyes open; I= harmonics-blue; InSTAT (between columns): A-B = p < 0.001; A-D = ns; B-D = p < 0.001; A-F = p < 0.001; F-H = p < 0.001; NOTICE: RED-LED light ILS shows significant increase of the alpha spectral density (and harmonics) in confront to BLUE-LED light ILS (and harmonics), both with eyes closed and with eyes open.
entrainement, through the retinohypothalamic tract, of many regulatory function in the hypothlamus and one before all: of the master pacemaker of circadian rhythms [3,18].
There is lot of literature about the importance of RED and Near Infrared light effect. RED and NIR-LED (600 - 1000 nm) light. In addition to the ability of photons to produce electronic excitation in chromophores, the REDNIR-LED induce wave-like alternating electric field that modifies the enzymatic activity i.e. NaKAT-Pase pump and enhances metabolism, due to mitochondrial ATP increase. Cytochromo c oxidase and ATP are key mechanism in the energy feature of the cells. [19-22].
The localization of the effect of the RED-LED light at the level of the ganglion cells of the retina increases their sensitivity to the signals incoming from the cone and enhances the output to the SNC [6]. In the pathologic field, many researchers support the theory that at the origin of many optic as well as neurodegenerative diseases is a dysfunction (loss) of the energy metabolism of the cell [20]. RED-LED light pulsed is well suited to restore the metabolic energy of the cell in the retina and the optic nerve [19].
Melanopsin in the ganglion cells appear most sensitive to blue light [3]; the shorter wavelength (λ 488 nm) are suitable for the stimulation of the protein part of the molecule and more efficient in the melanopsin signalling system, whereas the part of the enzyme containing metal absorb visible RED and NIR wavelength (λ 529 nm) that enhances metabolism through mitochondrial ATP production and carries the photobiomodulatory effect [3,22].
5. Conclusions
The flicker stimulation at 10 Hz with RED-LED light λ 650 nm give rise to a cascade of events in the retina and in the brain that may be summarized as follows:
1) Retina: activation of cytochromo coxidase and/or melanopsin in the ganglion cell, a novel site of phototransduction recently discovered (the third eye). This stimulation of the mitochondrial ATP in ganglion cells results is an increase of their sensitivity to the incoming cone-LHC (Light Horizontal Cells) signals. The end result is a reinforcement of the output to the suprachiasmatic nucleus (SCN), and therefore to the thalamus and the hypothalamus [3,6,18]; the reinforcement may be also due, in addition, to a possible mechanism of flicking-induced short term plasticity in retinal cone-LHC synapses [7,8].
2) Brain: sub a) cognitive function (thalamo/cortical level): the synchronization of oscillatory phases between different brain regions, support the enhancement of both working memory and long term memory, and acts by facilitating neural communication and by promoting neural plasticity [12,23].
Sub b) regulatory function, (hypothalamic-pineal gland-hypophysis gland level), acts on brain’s master clock (i.e. melatonin), reproductive cycle (i.e. prolactin), and many different autonomic functions [24-26].
At least ILS with 10 Hz RED-BLUE-GREEN-LED photostimulation of the retina is a powerful mechanism for differential stimulation of the synaptic plasticity: a) at the level of the brain cognitive function through an effect in the vast phenomenology of the alpha rhythm, and b) at the level of the hypothalamic wide regulatory function. The involvement of alpha rhythms may promote a deeper understanding of haw phase synchronization support the flexibility of interaction between memory systems and may yield new insight into the function of phase synchronization in general [12,23]. Whereas the stimulation at the hypothalamic level, offer the possibility to interfere with many regulatory disfunction as insomnia, jet lag, (desynchronization of the biological clock), reproductive function, metabolism and seasonal affective disorder (SAD) [3,27,28].
REFERENCES
- E. D. Adrian and B. H. C. Matthews, “The Berger Rhythm, Potential Changes from the Occipital Lobes in Man,” Brain, Vol. 57, No. 2, 1934, pp. 355-385.
- M. S. Reppert and D. B. Weaver, “Coordination of Circadian Timing in Mammals,” Nature, Vol. 418, 2002, pp. 935-941. doi:10.1038/nature00965
- J. Provencio, G. Jiang, W. J. De Grip, W. P. Hayes and M. D. Rollag, “Melanopsin: An Opsin in Melanophores,” The Proceedings of the National Academy of Sciences, Vol. 95, No. 1, 1998, pp. 340-345. doi:10.1073/pnas.95.1.340
- T. I. Karu and S. F. Kolyakov, “Exact Action Spectra for Cellular Responses Relevant to Phototherapy,” Photomedicine and Laser Surgery, Vol. 23, No. 4, 2005, pp. 355-361. doi:10.1089/pho.2005.23.355
- T. I. Karu, “Mitochondrial Signaling in Mammalian Cells Activated by Red and Near-IR Radiation,” Photochemistry and Photobiology, Vol. 84, No. 5, 2008, pp. 1091- 1099.
- J. Hannibal, P. Hindersson, S. M. Knudsen, B. Georg and J. Fahrenkrug, “The Photo-Pigment Melanopsin Is Exclusively Present in Pituitary Adenilate Cyclase-Activating Polypeptide-Containing Retinal Ganglion Cells of the Retinohypothalamic Tract,” The Journal of Neuroscience, Vol. 22, No. 1, 2002, pp. 191-198.
- J-F. Hu, Y. Liu and P. J. Liang, “Stimulus Pattern Related Plasticity of Synapses between Cones and Horizontal Cells in Carp Retina,” Brain Research, Vol. 857, No. 1-2, 2000, pp. 321-326. doi:10.1016/S0006-8993(99)02472-5
- X. Jin, J-F. Hu and P-J. Liang, “Possible Mechanism of Flicking-Induced Short-Term Plasticity in Retinal ConeLHC Synapse: A Computational Study,” Biological Cybernetics, Vol. 90, No. 5, 2004, pp. 360-367. doi:10.1007/s00422-004-0478-2
- W. G. Walter, V. J. Dovey and H. Shipton, “Analysis of the Electrical Response of the Human Ortex to Photic Stimulation,” Nature, Vol. 158, 1946, pp. 540-543. doi:10.1038/158540a0
- W. Klimesch, “EEG-Alpha Rhythms and Memory Processes,” International Journal of Psychophysiology, Vol. 26, No. 1-3, 1997, pp. 319-340. doi:10.1016/S0167-8760(97)00773-3
- J. R. Williams, “Frequency Specific Effects of Flicker on Recognition Memory,” Neuroscience, Vol. 104, No. 2, 2001, pp. 283-286. doi:10.1016/S0306-4522(00)00579-0
- F. Ferro Milone, T. A. Minelli and L. Turicchia, “Neuron Synchronization and Human EEG Phenomenology Simulation,” Nonlinear Dynamics Psychology and Life Science, Vol. 2, No. 1, 1998, pp. 212-33.
- F. Ferro Milone, T. A. Minelli, A. Porro, F. Binda, “Ritmo Alfa (EEG) e Memoria Comportamentale (Test di Rivermead): Studio di una Possibile Interdipendenza in Soggetti Normali e con Deficit Cognitivo Lieve,” Psicogeriatria, Vol. 4, No. 2, 2009, pp. 59-65.
- F. F. Milone, F. A. Pozzato, G. Bortolan, E. Meneghesso, G. Moresco, G. P. Favaro, M. Sarto, G. Salemi, R. Z anini and G. Manente, “A New Device for Repeated EEG Records with Reduced Leads and Light Stimulator Assembled in a Casque (Technical Note),” International Conference on Bioinformatics and Biochemical Engineering (iCBE2012), Shanghai, 2011, No. 72171.
- F. F. Milone, T. A. Minelli and F. Binda, “Alpha Rhythms and Memory Processes: An Intriguing Question,” Chaos and Complexity Letters, Vol. 4, No. 3, 2008, pp. 1-3.
- H. Z. Shouval, M. F. Bear and L. N. Cooper, “A Unified Theory of NMDA Receptor Dependent Bidirectional Synaptic Plasticity,” The Proceedings of the National Academy of Sciences, Vol. 99, No. 16, 2002, pp. 10831- 10836. doi:10.1073/pnas.152343099
- T. J. Karu, “Mitochondrial Mechanisms of Photobiomodulation in Context of New Data about Multiple Roles of ATP,” Photomedicine and Laser Surgery, Vol. 28, No. 6, 2010, pp. 159-160. doi:10.1089/pho.2010.2789
- D. M. Berson, “Strange Vision, Ganglion Cells as Circadian Photoreceptors,” Trends in Neurosciences, Vol. 26, No. 6, 2003, pp. 314-320. doi:10.1016/S0166-2236(03)00130-9
- J. T. Eells, M. M. Henry, P. Summerfelt, M. T. T. Wong-Riley, E. V. Buchmann, M. Kane, N. T. Whelan and H. T. Whelan, “Therapeutic Photobiomodulation for Methanol-Induced Retinal Toxicity,” PNAS, Vol. 100, No. 6, 2003, pp. 3439-3444. doi:10.1073/pnas.0534746100
- P. Pizzo, M. Brini, S. Leo, C. Fotino, P. Pinton and R. Rizzuto, “Mitochondria, Calcium and Cell Death: A Deadly Triad in Neurodegeneration,” Biochimica et Biophysica Acta (BBA)—Bioenergetics, Vol. 1787, No. 5, 2009, pp. 335-344. doi:10.1016/j.bbabio.2009.02.021
- K. D. Desmet, B. S. Desmet, D. A. Paz, J. J. Corry, J. T. Eelles, M. T. T. Wong-Riley, M. M. Henry and H. T. Whelan, “Clinical and Experimental Application of NIR-LED Photobiomodulation,” Photomedicine and Laser Surgery, Vol. 24, No. 2, 2006, pp. 121-128. doi:10.1089/pho.2006.24.121
- A. Amat, J. Rigau, R. W. Waynant, I. K. Ilev and J. J. Anders, “The Electric Field Induced by Light Can Explain Cellular Responses to Electromagnetic Energy: A Hypothesis of Mechanism,” Journal of Photochemistry and Photobiology B: Biology, Vol. 82, No. 2, 2006, pp. 152-160. doi:10.1016/j.jphotobiol.2005.10.001
- F. Fell and N. Azmacher, “The Role of Phase Synchronization in Memory Processes,” Nature Reviews, Neuroscience, Vol. 12, No. 2, 2011, pp. 105-114.
- S. J. Aton and E. D. Herzog, “Come Together Right Now: Synchronization of Rhythms in a Mammalian Circadian Clock,” Neuron, Vol. 48, No. 5, 2006, pp. 631-634.
- S. Benedicenti, I. M. Pepe, F. Angero and A. Benedicenti, “Intracellular ATP Level Increases in Lynphocyte Irradiated with Infrared Laser Light of Wavelength 904 nm,” Photomedicine and Laser Surgery, Vol. 26, No. 5, 2008, pp. 451-453. doi:10.1089/pho.2007.2218
- J. P. Card, W. Larry and R. Y. Moore, “An Overview of the Regulatory System,” In: M. J. Ziegmond, F. E. Bloom, S. C. Landis, J. L. Roberts and L. R. Squire, Eds., Fundamental Neuroscience, Academic Press, San Diego, 1999, pp. 1013-1187.
- R. T. Moore, “Circadian Timing,” In: M. J. Ziegmond, F. E. Bloom, S. C. Landis, J. L. Roberts and L. R. Squire, Eds., Fundamental Neuroscience, Academic Press, San Diego, 1999, pp. 1189-1206.
- K. A. Roecklein, K. J. Rohan, W. C. Duncan, M. D. Rollag, N. E. Rosenthal, R. H. Lipsky and J. Provencio, “A Missense Variant (PIDL) of the Melanopsin (OPN$) Gene in Seasonal Affective Disorder,” Journal of Affective Disorders, Vol. 114, No. 1, 2009, pp. 279-285. doi:10.1016/j.jad.2008.08.005
NOTES
*Corresponding author.

