Neuroscience & Medicine
Vol.2 No.1(2011), Article ID:4279,4 pages DOI:10.4236/nm.2011.21007
Effects of Ginkgo Biloba Special Extract EGb 761(R) Very Mild Cognitive Impairment (vMCI)
![]()
1Alexian Hospital Krefeld, Krefeld, Germany; 2Adoria Clinic, Riga, Latvia; 3Alma Clinic, Riga, Latvia; 4Dr. Willmar Schwabe GmbH & Co. KG Pharmaceuticals, Karlsruhe, Germany; 5University of Osnabrueck, Osnabrueck, Germany.
E-mail:robert.hoerr@schwabe.de
Received January 12th, 2011; revised March 5th, 2011; accepted March 10th, 2011.
Keywords: Ginkgo Biloba, EGb 761®, Mild Cognitive Impairment, Memory, Concentration, Randomised Controlled Trial
ABSTRACT
Objective: To assess effects of EGb 761® on cognition and quality of life in subjects with very mild cognitive impairment. Methods: We randomized 300 subjects aged 45 to 65 with cognitive complaints and low functioning (more than one standard deviation below appropriate norm) in at least one cognitive test to double-blind treatment with once daily 240 mg EGb 761® or placebo for 12 weeks. Results: The exploratory intention-to-treat analysis showed significant improvement (p < 0.025, one-sided) beyond practice effects for EGb 761® in a measure of attention (Vienna Test SystemWork Performance Series) and trends in favour of EGb 761® in measures of memory (Wechsler Memory Scale IIIFaces I, Appointments Test—delayed recall), and perceived physical health (SF36-factor score Physical Health). Cognitive effects were more pronounced and more consistent (p < 0.025 in 4 of 5 tests) in subjects with lower memory function at baseline. Specifically, practice effects in the more demanding tests were attenuated or absent in these subjects. Conclusion: Ginkgo biloba extract EGb 761® improved cognitive functioning and aspects of quality of life in subjects with very mild cognitive impairment.
1. Introduction
The clinical efficacy of the standardised Ginkgo biloba extract EGb 761® in Alzheimer disease with or without cerebro-vascular disease and in vascular dementia has been demonstrated in a series of clinical trials (summarized by [1-3]).
Mild cognitive impairment (MCI) delineates a condition characterised by subjective complaints of deficiencies in cognitive performance and objective evidence of cognitive impairment, frequently associated with mild (sub-syndromal) behavioural and psychological symptoms, but widely preserved overall cognitive performance and no evidence of dementia. There have been a number of attempts to define an intermediate or transitional stage between successful aging and dementia, such as Age-Associated Memory Impairment (AAMI) [4], Late-Life Forgetfulness (LLF) [5], Aging-Associated Cognitive Decline (AACD) [6], Cognitive Impairment No Dementia (CIND) [7], and various concepts of Mild Cognitive Impairment [8-11]. Longitudinal studies have shown that part of the patients diagnosed as having any of these conditions will become demented, another part will remain in the stage of mild impairment for years and a third part will return to normal [7,12-14]. The likelihood for each of these possible courses seems to depend on the diagnostic criteria and the patients’ age. Hence, none of these conditions does necessarily represent a transitional stage, but an intermediate condition between successful ageing without any apparent cognitive decline and dementia, associated with a more or less elevated risk for progressing to dementia [15].
Memory impairment, in particular when evidenced by a delayed recall task, is generally considered the first sign of incipient dementia [16,17]. It may precede the stage of overt dementia by 15 to 20 years [16,18]. However, impairment in other domains of cognitive function, such as reasoning or visuo-constructive abilities may also be present in the stage between normal aging and dementia [16,18]. Employing a test battery designed specifically for MCI patients, Dwolatzky and co-workers [19] found significant deficiencies in tasks of executive function, visuo-spatial skills, attention and information processing. Therefore, a broadening of the concept of MCI beyond the purely amnestic form appears to be justified.
Subjective memory complaints, even in the absence of objective memory impairment, appear to be a strong predictor of future cognitive decline and dementia [20-23]. This emphasises the importance of subjective complaints within the diagnostic framework of MCI, although little is known about subjective complaints concerning other domains of cognitive function. In a cross-sectional perspective, subjective memory complaints were associated with depression rather than with objective cognitive impairment, but this did not affect their predictive value for cognitive decline in the longitudinal perspective [20]. Of note, depressive symptoms have been found to be elevated in preclinical Alzheimer’s disease, and this elevation was not merely a by-product of self-perceived cognitive difficulties [24]. Insulin resistance and dysregulation within the hypothalamus-pituitary-adrenal (HPA) axis have been described as a common feature of affective disorders and Alzheimer’s disease [25]. Depression in the elderly may thus be a risk factor for or even a harbinger of dementia.
Neuropsychiatric symptoms are frequently observed in association with cognitive complaints or impairment. Lyketsos and co-workers [26] reported from the Cache County Study total prevalence rates of neuropsychiatric symptoms in the past month of 43% for subjects with MCI, 16% for the general population and 75% for dementia patients. Depression (20%), apathy (15%), irritability (15%) and sleep disturbances (14%) were the symptoms reported most frequently. Anxiety was present in 10% of MCI subjects. Similar figures were reported from another population-based study [27] with prevalence rates of 47% for any psychiatric or behavioural symptom, 25% for agitation and 16% for depression. From the Kungsholmen Project Forsell and coworkers [28] reported a significantly elevated frequency in anxiety syndromes among subjects with MCI (10%). Social withdrawal was found in 15% and suspiciousness in 10% of trial participants.
Neither of the major classification systems provides consensus diagnostic criteria for MCI, and there are no generally accepted criteria, so far. A variety of criteria have been employed by different groups of researchers [9,10,13,14,26,27] to describe MCI, but less attention has been paid to the very early stage of mental decline.
The aim of this clinical trial was to assess the clinical efficacy and tolerability of EGb 761® in subjects below the age of retirement with very mild cognitive impairment (vMCI). It appears to be appropriate for a clinical trial in vMCI not only to focus on memory impairment, but to take into account other domains of cognitive function and neuropsychiatric symptoms as well. In the absence of consensus diagnostic criteria for the very early stage of mental decline a set of criteria was used that encompasses a broad spectrum of cognitive abilities, requiring both subjective complaints and evidence from cognitive tests of mild impairment.
2. Materials and Methods
2.1. Design
A randomised, double-blind, placebo-controlled, parallel-group, multi-centre (5 sites in Latvia) trial design was used. The trial was approved by the Independent Ethics Committee for Investigation of Drugs and Pharmaceutical Products of Latvia and performed in accordance with the Declaration of Helsinki and the Guideline for Good Clinical Practice (GCP) issued by the International Conference on Harmonisation (ICH). Written informed consent was obtained from all patients before enrolment.
2.2. Subjects
Male and female outpatients aged 45 to 65 years (both inclusive) were eligible for the study. All subjects suffered from very mild cognitive impairment as defined by the diagnostic criteria:
a) subjective complaints of impairment (reported spontaneously or upon inquiry), perceived as a decline from former level of functioning, in at least one of the following cognitive functions: memory, attention/concentration, speed of functioning, efforts required to complete complex tasks, general performance/efficiency;
b) low functioning (at least one standard deviation worse than the mean of the most appropriate normative group) in at least one of the cognitive tests administered at screening and baseline (WMS III Faces I + II, Vienna Test System ALS (S1), Vienna Test System DT (S16), Appointments Test TT);
c) perceived impairment present for at least 3 months;
d) widely preserved general cognitive function, as evidenced by a total score above 23 in the MMSE;
e) intact activities of daily living, as evidenced by inquiry (subtle difficulties, in particular slowing or increased efforts, in complex tasks is acceptable);
f) no indication of dementia.
All subjects had sufficient language skills to understand the test instructions and to perform the cognitive tests.
Subjects were excluded, if they had suffered an ischaemic stroke with sequelae within 3 months before randomisation, if cognitive impairment was due to a specific neurological or psychiatric disorder, if they had a history of recurrent major depression or anxiety disorder, if they had any severe somatic disease or a marked loss of vision or hearing that could have compromised neuropsychological testing. Patients were not allowed to take any psychoactive drugs, cognition-enhancing drugs or drugs acting on haemorrheology. Females were required to be under safe hormonal contraception or in a non-fertile state.
2.3. Recruitment, Randomisation and Treatment
Most subjects were recruited by general practitioners, a small proportion by neurologists. Randomisation, stratified by centres, was performed by the sponsor’s biometrics unit using a validated computer program. The length of the balanced blocks was described in a separate document that was withheld from the study sites. Drug boxes containing active drug or placebo were labelled uniformly and numbered in accordance with the randomisation list. Random treatment allocation was performed by handing each patient the drug box with the lowest yet unassigned number. The investigators received sealed emergency-envelopes for individual patients, all of which returned unopened after completion of the trial. Patients were instructed to take one tablet containing 240 mg of EGb 761® or placebo every morning for a period of 12 weeks. EGb 761®1 is a dry extract from Ginkgo biloba leaves (35-67:1), extraction solvent: acetone 60% (w/w). The extract is adjusted to 22.0% - 27.0% ginkgo flavonoids calculated as ginkgo flavone glycosides and 5.0% - 7.0% terpene lactones consisting of 2.8% - 3.4% ginkgolides A, B, C and 2.6% - 3.2% bilobalide and contains less than 5 ppm ginkgolic acids. Active drug and placebo were of identical appearance.
2.4. Procedures
Psychometric testing was performed at three visits: At baseline, after four and after 12 weeks of treatment. Investigators and study nurses who administered the computerized tests were trained by an experienced neuropsychologist during an investigators' meeting at the beginning of the study.
Wechsler Memory Scale III—Faces I and II:
These tasks are standardised subtests of the Wechsler Memory Scale III (WMS III) [29]. Faces I is composed of 24 stimulus photographs of human faces that are individually presented to participants at a rate of one face every 2 seconds. For immediate recognition (Faces I) participants are presented a second series of 48 photographs of human faces, 24 targets and 24 distracters. After 30 minutes, Faces II (delayed recognition) is administered following the same procedure.
Vienna Test System—Work Performance Series (Arbeitsleistungsserie, WTS-ALS):
The ALS [30] is a computerised test of concentration and fatigability under continued demand (additions of one-digit numbers and entry of the sum, or the second digit of the sum in case of a two-digit result, during a 20-minute period). The test is segmented in order to show longitudinal trends of performance. Parameters of interest were the total number of completed tasks, increase (or decrease) in completed tasks over time, percentage of errors, and total number of corrections.
Vienna Test System—Determination Test (WTS-DT):
The DT [31] is a computerised test for attention, reaction time, speed of processing, motor coordination, endurance, and overall performance/efficiency. It requires differential reactions to a variety of optic and acoustic stimuli. For this study a reactive form was used, i.e. the duration of stimulus presentation was fixed during each testing interval.
Appointments Test (Termine Test, TT):
The Appointments Test [Kaschel, publication pending] is a real life-orientated memory test with a free recall and a delayed recall (45 minutes) condition. Eight appointments typical for daily life are presented to the subject. Each appointment consists of four components: A twopart time information, a location and an action/purpose. There are 6 parallel forms for repeated measurements, and norms are available for different age groups. The test is designed in a way that even cognitively intact persons do not achieve the maximum possible score, thus excluding ceiling effects.
Mental Balance Scale (Befindlichkeitsskala, BfS’):
The BfS’ is a validated self-rating scale consisting of 28 pairs of adjectives with opposite meanings. It reflects general well-being and mental balance, encompassing a broad range of possible mood states. The lower the total score, the better and more balanced is a subject's mood and well-being [32].
SF-36 Health Survey:
The SF-36 is a self-rating scale to describe an individual’s current overall health status. It can be used as a measure of health-related quality of life. The 36 questions cover 11 domains of assessment, such as physical functioning, perceptions of health and vitality, social and emotional functioning, and mental well-being. The instrument is not disease-specific, so it may be used for healthy subjects as well as for patients irrespective of their disease. The scale has been validated extensively and translated into many different languages [33].
2.5. Sample Size Calculation and Statistical Analysis
As this was the first clinical trial with EGb 761® in patients with very mild cognitive impairment, it was exploratory in nature, and a sample size calculation based on former experience was not possible. To calculate a reasonable sample size two cognitive efficacy variables (WMS III—Faces II, WTS-DT) and a variable concerning mental balance (BfS’) were chosen as efficacy variables of major interest. Evaluation of these outcome variables was done one-sided under control of the type-I error rate α = 0.025 while considering centre effects and the baseline-value of the corresponding variable in the analysis (ANCOVA). To further investigate the data, p-values based on one-sided t-tests were calculated for all efficacy variables which are presented in this publication. The statistical analysis—although explorative in nature—was conceptually based on the assumption that EGb 761® would show superior effects compared to placebo, and therefore a directed (one-sided) approach was chosen. As no adjustment of the type-one error-rate was performed, the p-values have to be considered as explorative.
Considering that there is very limited room for improvement in very mild impairment, separate analyses for a subgroup with conspicuous memory impairment, as specified prospectively in the statistical analysis plan, were performed. This subgroup was defined by a baseline score below the median (i.e. <33) in the WMS III subtest Faces II which consists of a visual delayed recognition task. Taking into account that visual episodic memory impairment is one of the earliest indicators of imminent Alzheimer’s disease [18] and that low functioning even in the easiest of the memory tests administered seems to be a more reliable indicator of veritable impairment than low functioning in the very demanding tests, the so defined subgroup appears to deserve particular interest.
3. Results
A total of 300 subjects were randomised, subject disposition is shown in Table 1. The treatment groups were similar with respect to age, weight, and height. The patients of the EGb 761â group had a slightly higher level of education (p = 0.03, chi-square test, two-sided) than the patients in the placebo group. All study participants were Caucasians. There were no relevant treatment group differences regarding BMI, smoking habits, alcohol consumption and vital signs at screening. The baseline values for the main efficacy variables were comparable in the two treatment groups (Table 2).
The mean baseline scores of all neuropsychological tests were between the age-adjusted norms and one standard deviation below, indicating that—on average—the cognitive impairment was very mild. The MMSE score of the more distinctly impaired patients (EGb 761®: 27.9 ± 1.2; placebo: 27.9 ± 1.4) was comparable to the MMSE score of the whole sample, indicating that their overall
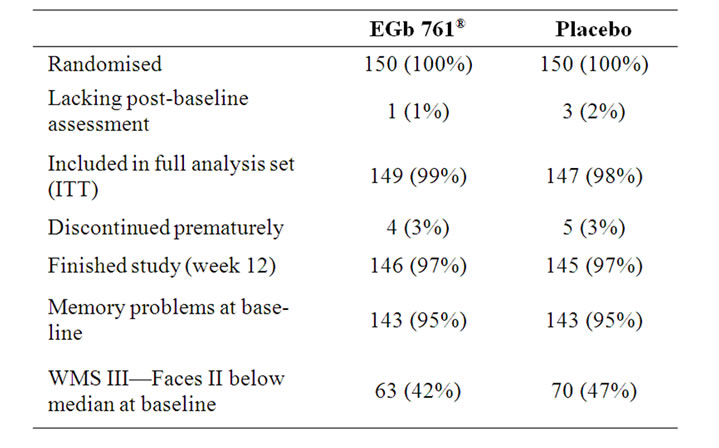
Table 1. Patient Sample, absolute numbers (percent).
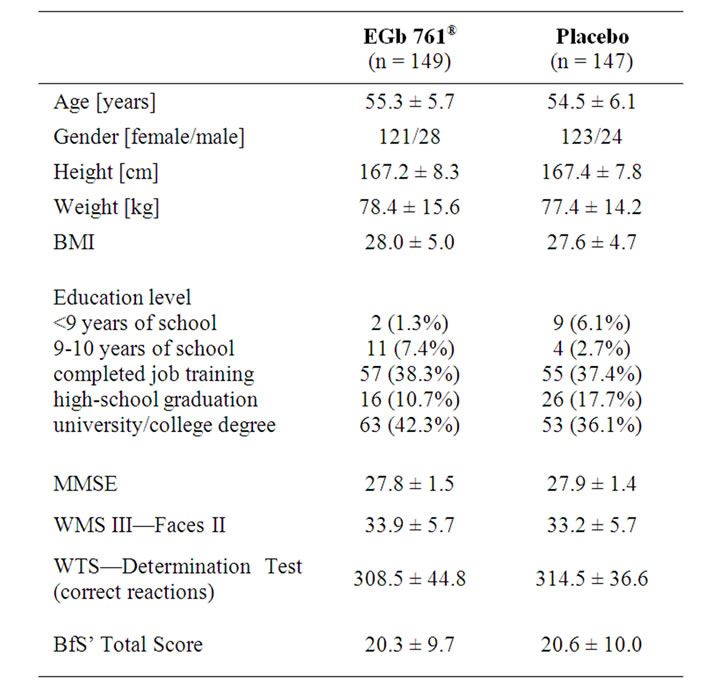
Table 2. Baseline characteristics (mean ± standard deviation or number (percent) of patients).
cognitive functioning was still widely intact. The results are provided for both the total patient sample and the subgroup scoring below median on the Faces II subtest at baseline.
There was consistent numerical superiority of EGb 761® in cognitive tests, indicating that there is a signal for improved memory and concentration in the EGb 761®-treated patients (Table 3). For the subgroup of more distinctly impaired patients larger drug-placebo differences were observed in all cognitive tests, with most p-values being below 0.025 (Table 4).
3.1. Wechsler Memory Scale III—Faces I and II
Subjects treated with EGb 761® tended to show a better performance compared to those receiving placebo in
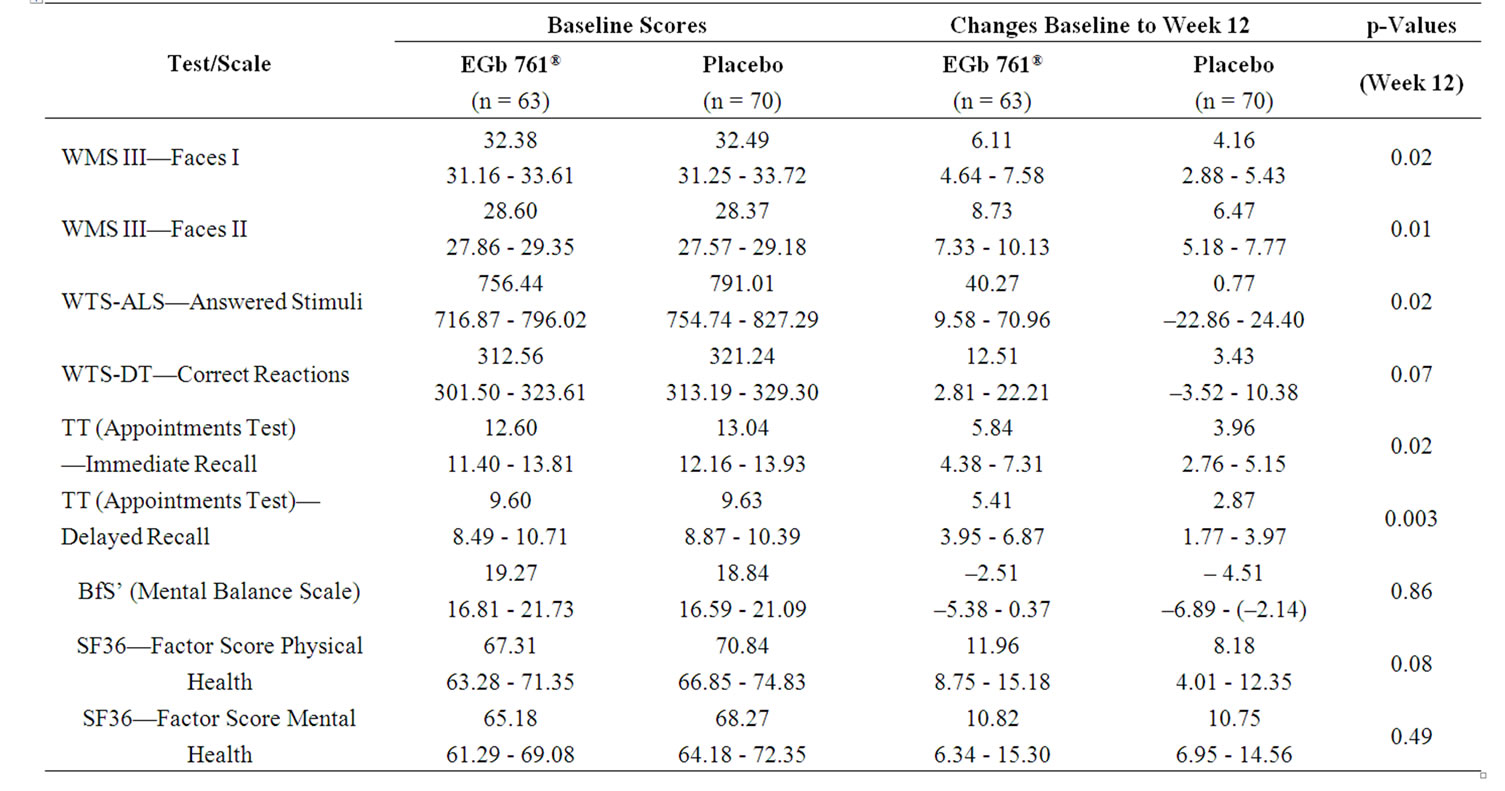
Table 3. Baseline scores (means, 95%-CI) and changes from baseline (means, 95%-CI) for total patient sample, one-sided p-values for between-group comparisons of changes (t-test).
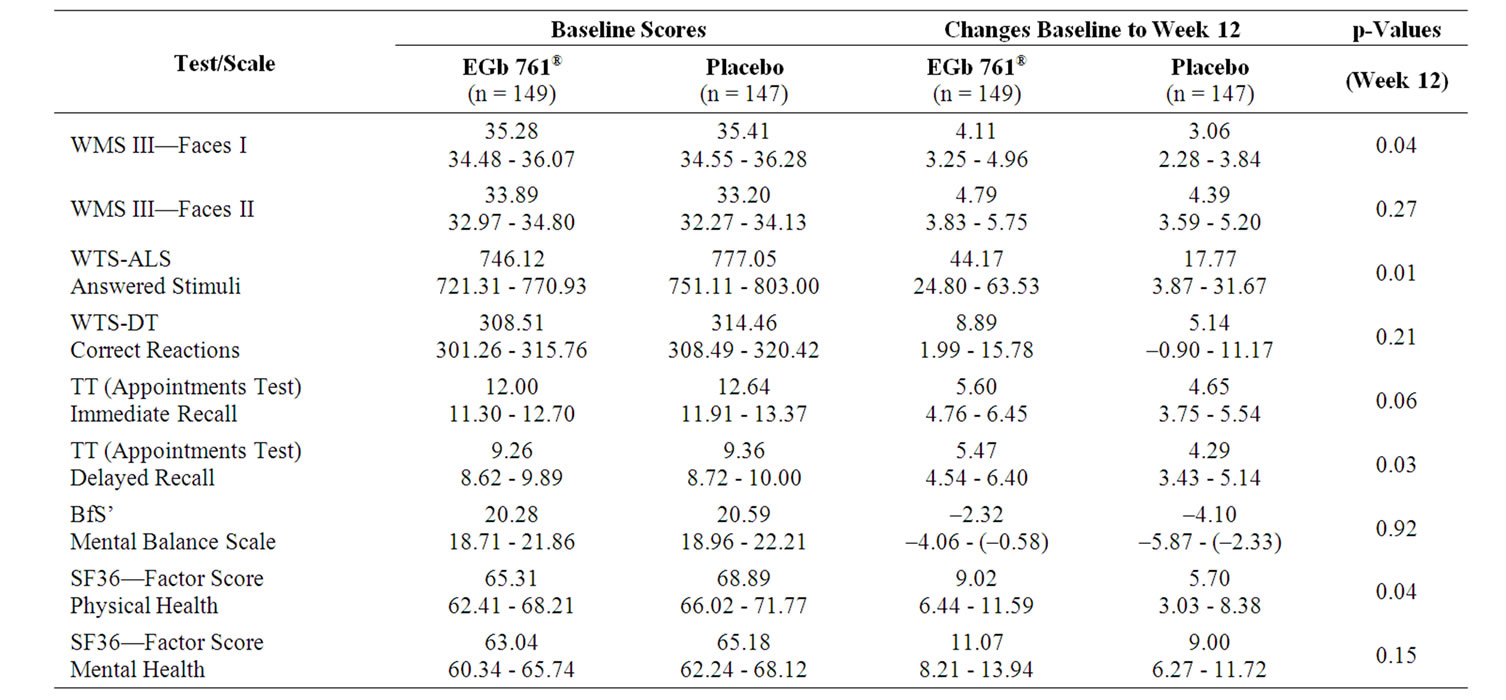
Table 4. Baseline scores (means, 95%-CI) and changes from baseline (means, 95%-CI) for more markedly impaired subsample (Faces II at baseline < 33), one-sided p-values for between-group comparisons of changes (t-test).
immediate recognition (mean change 4.11 vs. 3.06, Table 3). For the delayed recognition there was no relevant difference between groups. Significant superiority of active treatment was seen in the subgroup analysis of the more distinctly impaired subjects for the immediate recognition (p = 0.02) as well as for the delayed recognition (p = 0.01, Table 4).
3.2. Vienna Test System—Work Performance Series (ALS)
Subjects under active treatment showed a significantly
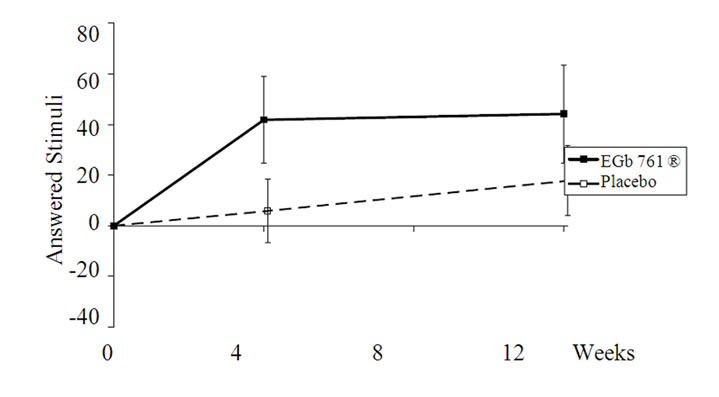
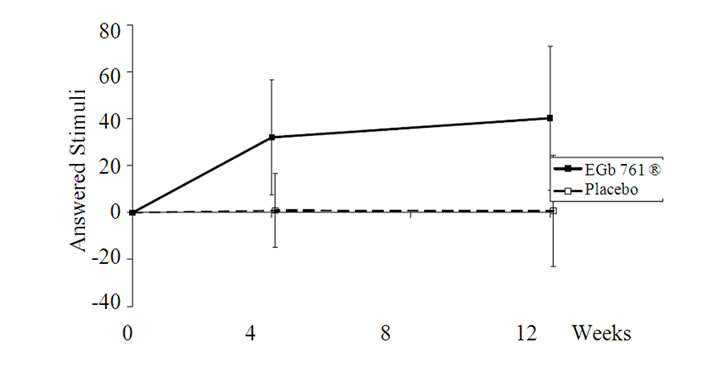
Figure 1. Changes from baseline in the ALS-task for the total sample (upper part) and for more distinctly impaired subjects (lower part); means and 95% CI (p < 0.05 for both comparisons). The total sample showed a minor practice effect under placebo intake, which was completely absent in the subgroup with conspicuous memory impairment.
higher quantity of additions in the whole sample (p =0.01; Table 3, Figure 1) as well in the subgroup analysis of the more distinctly impaired subjects (p = 0.02; Table 4, Figure 1). There was only a slight practice effect in the total sample and none in the subjects with conspicuous memory impairment. The error percent were comparable for active drug and placebo.
3.3. Vienna Test System—Determination Test (DT)
Concerning the Determination Test there was a trend in advantage of EGb 761® in the total sample (mean of change 8.89 vs. 5.14, p = 0.21, Table 3) and more pronounced in the more distinctly impaired subjects (mean of change 12.51 vs. 3.43, p = 0.07, Table 4). However, the performance in this task showed great variance in all groups.
3.4. Appointments Test (Termine Test, TT)
In the Appointments Test subjects treated with Egb 761® were able to save numerically more information for immediate as well as for delayed recall (Table 3). The effect was both larger and statistically significant for the more distinctly impaired subjects regarding the immediate recall (p = 0.02) and even more pronounced regarding the delayed recall (p = 0.003, Table 4). The number of errors was not influenced by either treatment.
3.5. Mental Balance Scale (Befindlichkeitsskala, BfS’), SF-36 —Factors Physical and Mental Health
There was no drug-placebo difference for the general well-being scale BfS’ and the factor score mental health of the quality-of-life scale SF-36, but a trend towards more pronounced improvement in the factor score physical health of the SF-36 (p = 0.04, Table 3).
Interestingly, the pre-specified subgroup with highest education (university/college degree) experienced significant improvement in the SF-36 domains physical functioning, physical role and vitality under EGb 761® treatment, which results in a significant drug-placebo difference in favour of EGb 761® in the factor score physical health (p < 0.005).
3.6. Safety
Overall, during the active treatment period, a total of 64 adverse events were reported for 46 patients. In each treatment group, 32 adverse events occurred in 23 subjects. In both treatment groups the majority of adverse events were reported during the first four weeks of treatment. No serious adverse events occurred during the study. The most frequently reported types of adverse events were gastrointestinal symptoms (EGb 761®: 9 events; placebo: 3 events) and infections and infestations (EGb 761®: 6 events; placebo: 8 events).
4. Discussion
The study shows a consistent pattern of improvement over placebo in the EGb 761®-treated subjects in concentration and working memory (WTS-ALS, WTS-DT, WMS III—Faces I, TT immediate recall) as well as in memory tasks relevant to everyday life (WMS III —Faces II, TT delayed recall). Such improvements are markedly larger in those patients who had conspicuous memory impairment at baseline as indicated by a score below the median in the WMS III—Faces II subtest. Interestingly, increases in the numbers of reactions in the tests of attention and concentration as well as increases in the numbers of remembered items in the memory tests were not associated with increases in errors. That means, performance was not enhanced at the cost of accuracy, but was veritably improved.
In the full patient sample discrimination between drug and placebo by the relatively easy recognition tests was hampered by both ceiling effects, which were particularly remarkable for the Faces tests (one quarter of the subjects scored close to the maximum of 46), and considerable practice effects. Due to the absence of ceiling effects, significant superiority of EGb 761® over placebo could be demonstrated by both recognition tasks for the patients with conspicuous memory impairment.
In the more demanding tests (WTS-ALS, WTS-DT, Appointments Test—immediate and delayed recall) the majority of subjects scored in the lower range and there was less evidence of ceiling effects. In these tasks however the patients with more distinct memory impairment at baseline benefited less from repeated test administration, i.e. practice effects were smaller or even absent. This is intriguing, because Zehnder and co-workers [34] found that patients with very mild Alzheimer's disease can be distinguished reliably from healthy elderly controls by the absence of practice effects upon repeated testing. It seems therefore likely that by splitting the patient sample along the median of the baseline Faces II scores, we in fact obtained a subgroup with conspicuous impairment and increased risk for progression to dementia.
It is conceivable that in subjects with very mild cognitive impairment, who score minimally below the normal range in just one cognitive test, there is limited room for improvement and that the tests administered are not sensitive enough to detect the improvement in cognitive performance apparently experienced by these patients under EGb 761® treatment. Hence, the larger and statistically significant drug-placebo differences in those patients who had more distinct cognitive impairment at baseline do not necessarily mean that EGb 761® is effective only in such patients. Improvements experienced by those who have already developed conspicuous impairment may however be clinically more relevant.
There were slight improvements in mental balance and quality of life for both treatment groups, but no difference between EGb 761® and placebo with respect to BfS’ scores and the mental health factor of the quality-of-life scale (SF-36). The latter may imply that the patients do not perceive their memory problems as mental problems but ascribe them to organic factors. There was a trend towards larger improvement in the physical health factor under EGb 761® treatment. Since perceived physical health usually correlates with mood rather than the patients’ actual physical condition, this may indicate some mood-balancing activity of EGb 761® as observed in earlier clinical trials [35,36]. The more pronounced effect of EGb 761® on quality of life in subjects with higher education may reflect an increased sensitivity of such mentally alert people to subtle changes in their condition.
Furthermore, BfS’ as well as SF-36 are complex scales with various questions to cover one’s well-being. Since the cognitive problems in our sample were really mild, it might have been difficult to judge their severity and their change on a complex scale: A small problem divided into various aspects might in the end be not noticeable. Thus, a global judgement might have shown clearer results.
The exploratory nature of the present study shows both a strength and a difficulty: a difficulty in that no a priori defined hypotheses were proven and p-values are descriptive in nature; a strength in that the large number of patients representing a range from very mild to mild but distinct memory and attention deficits and the array of more and less demanding tests administered allowed quite an enlightening picture of the differential effects of EGb 761® to emerge.
The safety and tolerability of EGb 761® was excellent. There were only few and minor adverse events that were perfectly balanced between active drug and placebo. This safety profile is in line with findings from former clinical trials and long-standing clinical experience.
In our sample of subjects with very mild cognitive impairment, EGb 761® led to a consistent pattern of improvement in cognitive functioning and aspects of subjective well-being. Further studies are recommended to confirm these findings with special attention to reliable scales with less ceiling effects in the evaluation of very mild cognitive impairment.
Against the background of these results, EGb 761® may be a reasonable option to try for subjects with very mild cognitive impairment.
REFERENCES
- S. Kasper and H. Schubert, “Ginkgo Biloba Extract EGb 761® in the Treatment of Dementia: Evidence of Efficacy and Tolerability [Ginkgo-Spezialextrakt EGb 761® in der Behandlung der Demenz: Evidenz für Wirksamkeit und Verträglichkeit],” Fortschritte der Neurologie Psychiatrie, Vol. 77, No. 9, September 2009, pp. 494-506.
- S. Weinmann, S. Roll, C. Schwarzbach, C. Vauth and S. N. Willich, “Effects of Ginkgo Biloba in Dementia: Systematic Review and Meta-Analysis,” BMC Geriatrics, Vol. 10, 2010, p.14. http://www.biomedcentral.com/1471-2318/10/14
- B. S. Wang, H. Wang, Y. Y. Song, H. Qi, Z. X. Rong, L. Zhang and H. Z. Chen, “Effectiveness of Standardized Ginkgo Biloba Extract on Cognitive Symptoms of Dementia with a Six-Month Treatment: A Bivariate Random Effect Meta-Analysis,” Pharmacopsychiatry, Vol. 43, No. 3, May 2010, pp. 86-91.
- T. Crook, R. T. Bartus, S. H. Ferris, P. Whitehouse, G. D. Cohen and S. G. Gershon, “Age-Associated Memory Impairment: Proposed Diagnostic Criteria and Measures of Clinical Change: Report of a National Institute of Mental Health Work Group,” Developmental Neuro -psychology, Vol. 2, No. 4, 1986, pp. 261-276.
- R. C. Blackford and A. La Rue, “Criteria for Diagnosing Age-Associated Memory Impairment: Proposed Improvement from the Field,” Developmental Neuropsychology, Vol. 5, No. 4, 1989, pp. 295-306.
- R. Levy and the Working Party of the International Psychogeriatric Association in collaboration with the World Health Organization, “Report: Aging-Associated Cognitive Decline,” International Psychogeriatrics, Vol. 6, No. 1, 1994, pp. 63-68.
- E. M. Ebly, D. B. Hogan and I. M. Parhad, “Cognitive Impairment in the Nondemented Elderly; Results from the Canadian Study of Health and Aging,” Archives of Neurology, Vol. 52, No. 6, June 1995, pp. 612-619.
- M. Zaudig, “A New Systematic Method of Measurement and Diagnosis of ‘Mild Cognitive Impairment’ and De-Mentia according to Icd-10 and DSM-III-R Criteria,” International Psychogeriatrics, Vol. 4, No. S2, 1992, pp. 203-219.
- R. C. Petersen, G. E. Smith, S. C. Waring, R. J. Ivnik, E. F. Tangalos and E. Kokmen, “Mild Cognitive Impairment; Clinical Characterization and Outcome,” Archives of Neurology, Vol. 56, No. 3, March 1999, pp. 303-308.
- R. C. Petersen, R. Doody, A. Kurz, R. C. Mohs, J. C. Morris, P. V. Rabins, K. Ritchie, M. Rossor, L. Thal and B. Winblad, “Current Concepts in Mild Cognitive Impairment,” Archives of Neurology, Vol. 58, No. 12, December 2001, pp. 1985-1992.
- M. Zaudig, “Mild Cognitive Impairment in the Elderly,” Current Opinion in Psychiatry, Vol. 15, No. 4, 2002, pp. 387-393.
- H. Wolf, M. Grunwald, G. M. Ecke, D. Zedlick, S. Bettin, C. Dannenberg, J. Dietrich, K. Eschrich, T. Arendt and H. J. Gertz, “The Prognosis of Mild Cognitive Impairment in the Elderly,” Journal of Neural Transmission Supplements, Vol. 54, 1998, pp. 31-50.
- J. C. Morris, M. Storandt, P. Miller, D. W. McKeel, J. L. Price, E. H. Rubin and L. Berg, “Mild Cognitive Impairment Represents Early-Stage Alzheimer Disease,” Archives of Neurology, Vol. 58, No. 3, March 2001, pp. 397-405.
- S. Larrieu, L. Letenneur, J. M. Orgogozo, C. Fabrigoule, H. Amieva, N. Le Carret, P. Barberger-Gateau and J. F. Dartigues, “Incidence and Outcome of Mild Cognitive Impairment in a Population-Based Prospec -tive Cohort,” Neurology, Vol. 59, No. 10, November 2002, pp. 1594-1599.
- G. Smith, “Is Mild Cognitive Impairment Bridging the Gap between Normal Aging and Alzheimer’s Disease?” Journal of Neural Transmission Supplements, Vol. 62, No. 2, 2002, pp. 97-104.
- M. F. Elias, A. Beiser, P. A. Wolf, R. Au, R. F. White and R. B. D’Agostino, “The Preclinical Phase of Alzheimer Disease; A 22-Year Prospective Study of the Framingham Cohort,” Archives of Neurology, Vol. 57, No. 6, June 2000, pp. 808-813.
- A. Collie, P. Maruff, R. Shafiq-Antonacci, M. Smith, M. Hallup, P. R. Schofield, C. L. Masters and J. Currie, “Memory Decline in Healthy Older People; Implications for Identifying Mild Cognitive Impairment,” Neurology, Vol. 56, No. 11, June 2001, pp. 1533-1538.
- C. H. Kawas, M. M. Corrada, R. Brookmeyer, A. Morrison, S. M. Resnick, A. B. Zonderman and D. Arenberg, “Visual Memory Predicts Alzheimer’s Disease More than a Decade before Diagnosis,” Neuorology, Vol. 60, No. 7, April 2003, pp. 1089-1093.
- T. Dwolatzky, V. Whitehead, G. M. Doniger, E. S. Simon, A. Schweiger, D. Jaffe and H. Chertkow, “Validity of a Novel Computerized Cognitive Battery for Mild Cognitive Impairment,” BMC Geriatrics, Vol. 3, No. 1, November 2003, p. 4. http://www.biomedcentral.com/1471-2318/3/4
- B. Schmand, C. Jonker, M. I. Geerlings and J. Linde -boom, “Subjective Memory Complaints in the Elderly: Depressive Symptoms and Future Dementia,” British Journal of Psychiatry, Vol. 171, October 1997, pp. 373- 376.
- P. W. Schofield, K. Marder, G. Dooneief, D. Jacobs, M. Sano and Y. Stern, “Association of Subjective Memory Complaints with Subsequent Cognitive Decline in Community-Dwelling Elderly Individuals with Baseline Cognitive Impairment,” American Journal of Psychiatry, Vol. 154, No. 5, May 1997, pp. 609-615.
- P. W. Schofield, D. Jacobs, K. Marder, M. Sano and Y. Stern, “The Validity of New Memory Complaints in the Elderly,” Archives of Neurology, Vol. 54, No. 6, June 1997, pp. 756-759.
- M. I. Geerlings, C. Jonker, L. M. Bouter, H. J. Adèr and B. Schmand, “Association between Memory ComPlaints and Incident Alzheimer’s Disease in Elderly People with Normal Baseline Cognition,” American Journal of Psychiatry, Vol. 156, No. 4, April 1999, pp. 531-537. http://ajp.psychiatryonline.org/cgi/content/full/156/4/531
- A. K. Berger, L. Fratiglioni, Y. Forsell, B. Winblad and L. Bäckman, “The Occurrence of Depressive Symptoms in the Preclinical Phase of AD; A PopulationBased Study,” Neurology, Vol. 53, No. 9, December 1999, pp. 1998-2002.
- N. Rasgon and L. Jarvik, “Insulin Resistance May Link Affective Disorders and Alzheimer’s Disease: A Missing Link Hypothesis,” International Psychogeriatrics, Vol. 15, No. S2, August 2003, p. 153 (Abstract S071- 005).
- C. G. Lyketsos, O. Lopez, B. Jones, A. L. Fitzpatrick, J. Breitner and S. T. DeKosky, “Prevalence of NeuroPsychiatric Symptoms in Dementia and Mild Cognitive Impairment; Results from the Cardiovascular Health Study,” Journal of the American Medical Association, Vol. 288, No. 12, September 2002, pp. 1475-1483. http://jama.ama-assn.org/cgi/content/full/288/12/1475
- D. C. Chan, J. D. Kasper, B. S. Black and P. V. Rabins, “Prevalence and Correlates of Behavioral and Psychiat -ric Symptoms in Community-Dwelling Elders with De-Mentia or Mild Cognitive Impairment: The Memory and Medical Care Study,” International Journal of Geriatric Psychiatry, Vol. 18, No. 2, February 2003, pp. 174-182.
- Y. Forsell, K. Palmer and L. Fratiglioni, “Psychiatric Symptoms/Syndromes in Elderly Persons with Mild Cognitive Impairment; Data from a Cross-Sectional Study,” Acta Neurologica Scandinavica, Vol. 107, No. S179, 2003, pp. 25-28.
- D. Wechsler, “WMS-III Administration and Scoring Manual,” The Psychological Corporation, San Antonio, 1997.
- W. Neuwirth and M. Benesch, “Arbeitsleistungsserie Version 25.00; Handanweisung,” Schuhfried, Mödling, 2004.
- W. Neuwirth and M. Benesch, “Wiener Determina -tionstest Version 31.00; Handanweisung,” Schuhfried, Mödling, 2005.
- D. von Zerssen, “Clinical Self-Rating Scales (CSRS) of the Munich Psychiatric Information System (PSYCHIS München),” In: N. Sartorius and T. A. Ban, Eds., Assessment of Depression, Springer, Berlin, 1986, pp. 270-303.
- J. E. Ware, K. K. Snow, M. Kosinski and B. Gandek, “SF-36 Health Survey Manual and Interpretation Guide.” New England Medical Center, the Health Institute, Boston, 1993.
- A. E. Zehnder, S. Bläsi, M. Berres, R. Spiegel and A. U. Monsch, “Lack of Practice Effects on Neuropsychological Tests as Early Cognitive Markers of Alzheimer Disease?” American Journal of Alzheimer’s Disease & Other Dementias, Vol. 22, No. 5, October-November 2007, pp. 416-426.
- H. Schubert and P. Halama, “Depressive Episode Primarily Unresponsive to Therapy in Elderly Patients: Efficacy of Ginkgo Biloba Extract EGb 761® in Combination with Antidepressants [Primär therapieresistente depressive Verstimmung älterer Patienten mit Hirnleistungsstörungen: Wirksamkeit der Kombination von Ginkgo-biloba-Extrakt EGb 761® mit Antidepressiva],” Geriatrie Forschung, Vol. 3, No. 1, 1993, pp. 45-53.
- U. Stocksmeier and M. Eberlein, “Depression Associated with Impairment of Cerebral Function; Effects of a Ginkgo Biloba Extract Investigated in Double-Blind Trial [Depressive Verstimmung bei Hirnleistungsstörungen; Wirkung eines Ginkgo-biloba-Extraktes in Doppelblind-Studieüberprüft],” TW Neurologie Psychiatrie, Vol. 6, January-February 1992, pp. 74-76.
NOTES
*Sponsor: Dr. Willmar Schwabe GmbH & Co. KG, Karlsurhe, Germany.
1EGb 761® is a registered trademark of Dr. Willmar Schwabe GmbH & Co. KG Pharmaceuticals, Karlsruhe, Germany.

