Open Journal of Veterinary Medicine
Vol.3 No.2(2013), Article ID:32695,5 pages DOI:10.4236/ojvm.2013.32021
Penicillin-Resistant Aerococcus viridans Bacteremia Associated with Bovine Severe Respiratory Syndrome
1Department of Clinical Veterinary Science, Internal Medicine Section, Faculty of Veterinary Medicine, University of Naples “Federico II”, Naples, Italy
2Department of Pathology and Animal Health, Infectious Diseases Section, Faculty of Veterinary Medicine, University of Naples “Federico II”, Naples, Italy
Email: *luisa.demartino@unina.it
Copyright © 2013 Jacopo Guccione et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 11, 2013; revised February 11, 2013; accepted March 11, 2013
Keywords: Respiratory Syndrome; Aerococcus viridians; Antibiotic-Resistance
ABSTRACT
Aerococcus viridans, a less frequently isolated bacteria, is a gram-positive, catalase-negative coccus, found singly or in tetrads, with biochemical and growth characteristics of streptococci and enterococci. This microorganism, usually susceptible to penicillin, is often found in the environment and is infrequently associated with human/veterinary infections. We described a case of Holstein Friesian female calf, 150-day-old, affected by respiratory emergencies. Following the clinical signs, radiographic analysis and bacteriological/molecular examinations carried out on blood culture, a diagnosis of severe broncho-pulmonary disease associate with a multidrug-resistant A. viridans bacteremia was done. The present case highlights the invasive nature of a saprophytic bacterium showing a broad profile of antibiotic-resistance including β-lactams. Furthermore, this report confirms that the effectiveness of an antibiotic therapy is based primarily on a sure diagnosis including susceptibility testing.
1. Introduction
Aerococcus viridans is an unusual microorganism and it was first described as a potential human pathogen in 1967 [1], later it has been associated with some human infections [2-4], it is known as a pathogen of lobsters, fishes and sea turtle [5]. Furthermore, it has also been isolated from the milk of cows with subclinical mastitis [6] and, it was, recently, detached in different clinical specimens of normally sterile body sites of pigs [7].
This bacterium is generally considered a saprophytic microorganism which can be found as an indigenous inhabitant on the upper airways and skin of healthy individuals. There are limited data in the literature on the antimicrobial susceptibility of A. viridans because this microorganism has been infrequently associated with human and/or animal infections. Many different bacteria now exhibit multidrug-resistant and they represent serious public health problems. Antibiotic sensitivity patterns may change from time to time and place to place, so it is very important to follow the change of bacterial susceptibility pattern to antibiotics. Initially A. viridans has been described as a bacterial strain naturally susceptible to penicillins, macrolides and related drugs, tetracyclines, and chloramphenicol and intrinsically resistant at a low level to aminoglycosides [8]. However, to date the pathogenicity of A. viridans is not clearly understood but a profile of multidrug-resistance was demonstrated. In 1994 some authors reported a case of endocarditis caused by multidrug-resistant A. viridans (penicillin, ampicillin, cefotaxime, gentamicin, and intermediate resistant to ciprofloxacin) [9]. Susceptibility patterns with resistance not only to penicillin but also to chloramphenicol and quinolones have been also reported [10]. More recently, it has been demonstrated in A. viridans isolates from subclinical cases of bovine mastitis high level resistance to beta lactam antibiotics and sporadic resistance to streptomycin and erythromycin [11].
2. Materials and Methods
2.1. Case Report
In this paper, we described a case of Holstein Friesian female calf, 150-day-old, hospitalized at Internal Medicine Section, Department of Clinical Veterinary Science, University of Naples “Federico II”, for a severe respiretory distress started three weeks before. At the time of admission, the animal had been taking fluoroquinolones and penicillin for 20 days as prescribed by veterinary practitioner showing no improvement. The animal was depressed and an increase in body temperature (39.9˚C), heart and respiratory rates (96 heart beats/minute, 82 breaths/minute) were observed. Cyanosis of ocular mucosae, disorexia, weight loss and growth failure were also revealed. Breathing pattern showed severe dyspnea, cough and inspiratory stridors caused by upper airways; abnormal lung sounds, including wheezes, creckles and bronchial tones were identified mainly in cranial lobes. Lateral thoracic radiographs showed widespread nodular lung consolidation and atelectasis zones.
2.2. Haematological and Biochemical Profile
K3EDTA sample of blood and serum, obtained by centrifugation at 2000 × g for 15 min, were used to assay haematological and biochemical profile, respectively. For this purpose we employed automatic analyzers, SEACGenius/S/VET-Hemat8 and SABA18, respectively.
2.3. Microbiological Assay
10 mL of venous blood (with K3EDTA) was inoculated into a blood-culture-bottle (Liofilchem diagnostics). Once a day, for three days, from culture medium 1 - 2 drops were taken and inoculated on each selective plate for conventional subculture. API kit systems (bioMerieux SA, Marcy l’Etoile, France) were used for the bacterial identification. Blood culture was repeated after 15 days.
2.4. Antibiotic-Susceptibility Test
The isolate was tested for susceptibility to sixteen antimicrobial agents by the Kirby-Bauer disk diffusion method [12-14] (Table 1).
2.5. Polymerase Chain Reaction (PCR)
To obtain further confirmation, the isolated was characterized by molecular genetic identification, precisely, a 540 bp 16S rRNA gene fragment was amplified by PCR using the A. viridians species-specific primers AC2 (5’- GTGCTTGCACTTCTGACGTTAGC-3’) and AC4 (5’- TGAGCCGTGGGCTTTCACAT-3’). PCR contained 50 ng DNA, 0.4 µM each primer, 200 µM each dNTP, 2 mM MgCl2 and 2 U Taq DNA polymerase (TaKaRa Biotechnology), in the buffer supplied by the manufacturer. Amplifications were performed using a Mastercycle Eppendorf and cycling parameters included an initial denaturation at 94˚C for 3 min, followed by 35 cycles of 94˚C for 45 s, 58˚C for 1 min, 72˚C for 2 min and final extension at 72˚C for 7 min. The resultant PCR products were visualized in a 1% w/v agarose gel stained with ethidium bromide, sequenced and compared to sequence in GenBank database by using BLAST program, as described elsewhere [15,16].
3. Results
3.1. Clinical Findings
An increase in white-blood-cell (WBC) count (59.7 × 109/L) and neutrophils (NNS 27%) associated with a slight hypoproteinemia and an increase of alpha-globulins indicative of an inflammatory process were observed (Table 2).

Table 1. Susceptibility of the A. viridans strain to sixteen antibiotics.
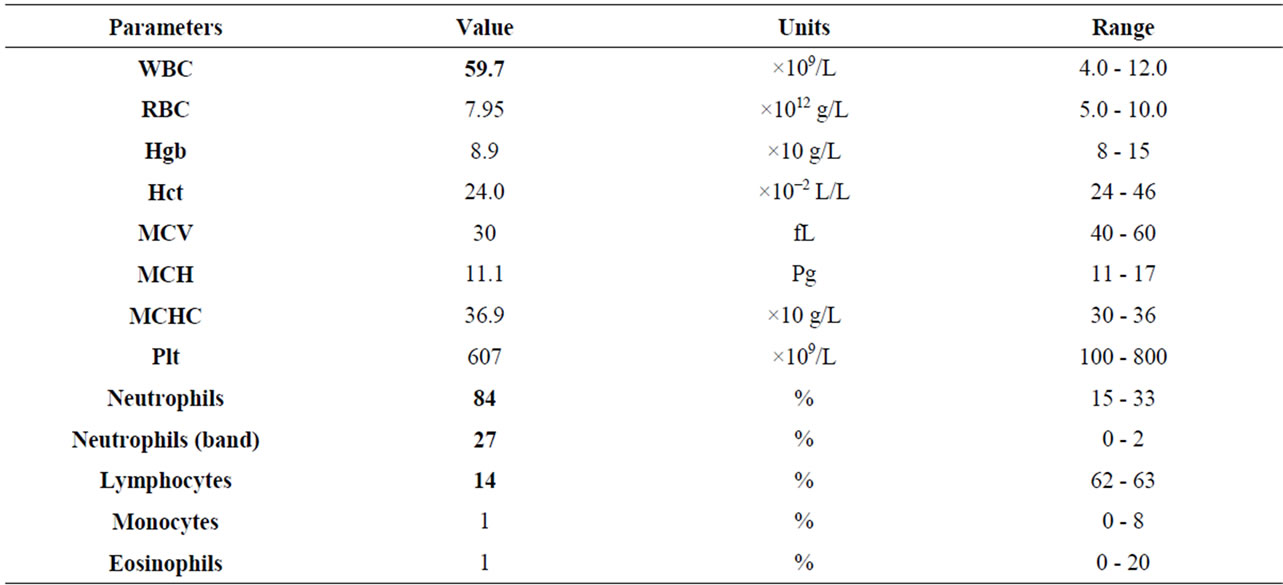
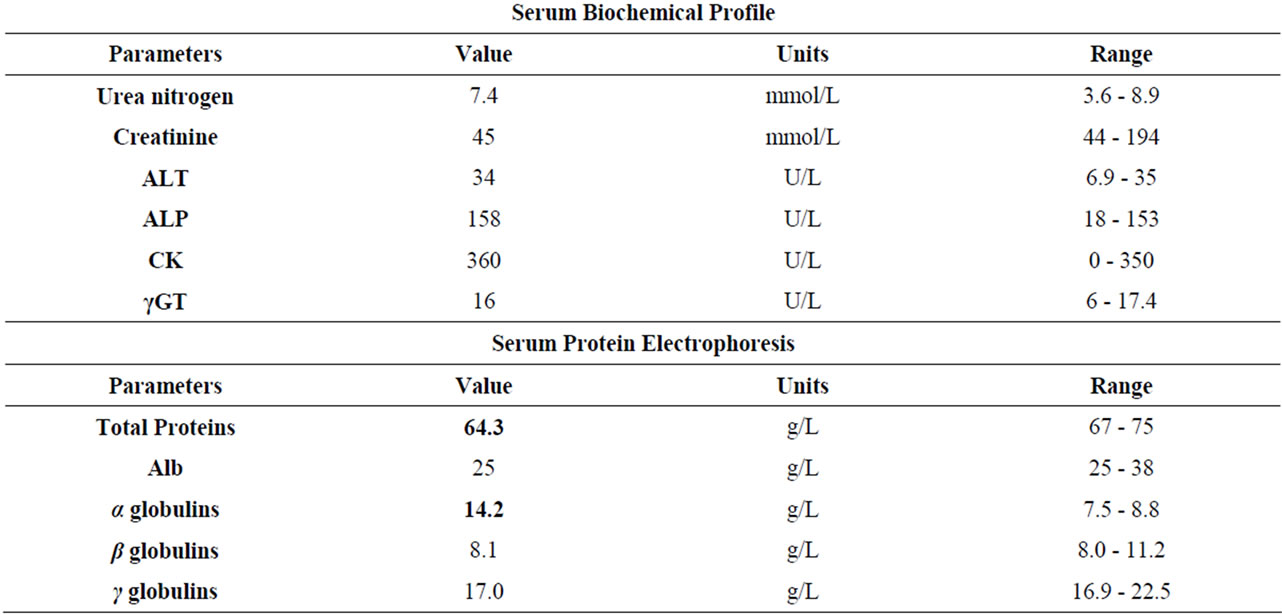
Table 2. Haematological and biochemical results.
3.2. Microbiological and Molecular Findings
From blood culture, only a pure bacterial culture was observed on Columbia agar containing 5% of defibrinated sheep blood (bioMerieux SA, Marcy l’Etoile, France) after 48 h of incubation at 37˚C under aerobic conditions. The colonies were gram-positive catalase-negative cocci arranged in single cells, in pairs, in tetrads, or in small groups. No other microorganism was isolated. The phenotypic reaction profile of this isolate (excellent identifycation 99.7%, biochemical profile number 6302711) was obtained using the API 20 Strep system (bioMérieux, Marcy l’Etoile, France) and was in accordance with the identification of Aerococcus viridans. To obtain further confirmation, the isolated was characterized by molecular genetic identification, precisely, a 540 bp 16 S rRNA gene fragment was amplified by PCR and the sequencing resulted in 99.8% similarity with Aerococcus viridans strain ATCC 11563 16S ribosomal RNA gene (accession number M58797).
Antimicrobial susceptibility test results showed that the isolate was susceptible to doxycycline, tetracycline, tilmicosin and erythromycin, intermediate-resistant to kanamycin, and resistant to all other antibiotics tested. The resistance to β-lactam antibiotics and fluoroquinolones confirmed the improper use of the initial choice for therapy treatment of the calf. Based on clinical signs, radiographic analysis and blood culture, a diagnosis of severe broncho-pulmonary disease associate with A. viridans bacteremia was done.
A treatment with tilmicosin was chosen at a dose of 10 mg/kg i.m. S.I.D. A nonsteroidal anti-infiammatory teraphy (Flunixin Meglumine 2.2 mg/kg i.v. S.I.D.), mucolytics (Acetylcysteine 10 ml/100kg s.c. B.I.D.) and bronchodilators (Aminophyllin 5 mg/kg i.m. T.I.D.) were also administered. An evident improvement was observed after 15 days of therapy so the animal was dismissed. The therapy was continued for other 7 days. The calf remained free of clinical illness at completion of therapy.
4. Discussion
The causes of bovine respiratory disease are multiple and complex, but three factors, stress, viral infection and bacterial infection, are almost always involved in cases of severe disease.
A. viridans, first described in 1953 [17], are the predominant components of oropharyngeal mucous membranes bacterial flora, and are therefore a common cause of bacterial infections of endogenous origin of upper respiratory tract. The pathogenicity and virulence of A. viridans is not well established; however infections due to this microorganism presumably seem to occur in immunecompromised patients, indicating that major defects in the immune system are necessary for the development of clinical disease. A very few data are presented in the literature on the antimicrobial susceptibility of A. viridans, because, until now, this microorganism has been infrequently associated with human/veterinary infections. In addition, standardized susceptibility testing methods and interpretative criteria are not available for aerococci. Thus, the antimicrobial susceptibility of A. viridans to different commonly used antimicrobials was determined by the CLSI for testing veterinary gram-positive microorganisms [14]. In gram-positive bacteria, β-lactam resistance most commonly results from expression of intrinsic low-affinity penicillin-binding proteins, and in recent years penicillin-resistance among strains of A. viridans has been reported [4,18]. Herein, A. viridans showed resistance to different category of antibiotics: β-lactam antibiotics, aminoglycosides, cephalosporins, fluoroquinolones and sulfonamides + diaminopyrimidines.
Clinically the limit of this work has been the decision to not perform the tracheobronchial washing because it could worsen the severity of dyspnea and distress [19]. However, the tilmicosin treatment showed an evident improvement of respiratory distress and, later, a complete recovery. Tilmicosin is a broad-spectrum semi-synthetic bactericidal macrolide antibiotic synthesized from tylosin. It has an antibacterial spectrum that is predominantly effective against Mycoplasma, Pasteurella and Haemophilus spp., and various gram-positive microorganisms such as Corynebacterium spp.
In conclusions, results of this study indicate that bovine respiratory bacterial pathogens can’t be susceptible at all to the most commonly prescribed antibiotics in veterinary medicine. Furthermore, this is, to our knowledge, the first case of a multidrug-resistant Aerococcus viridans isolated from the blood of a calf with a severe respiratory syndrome history that had been treated unsuccessfully with fluoroquinolones and penicillin combination. Generally, the drug resistance of many bacteria is induced by selective pressure by prolonged antibiotic use, and it is probable that, in this case, A. viridans became a potential causative agent of bacteremia in an animal already unhealthy. It is possible to hypothesize that this calf was immunocompromised and became bacteraemic following invasion of this saprophytic and rarely pathogen bacterium, according to the literature, that describes A. viridans infection overall in hosts with vulnerable conditions [7,20].
REFERENCES
- G. Colman, “Aerococcus-Like Microorganisms Isolated from Human Infections,” Journal of Clinical Pathology, Vol. 20, No. 3, 1967, pp. 294-297. doi:10.1136/jcp.20.3.294
- W. Kern and E. Vanek, “Aerococcus Bacteremia Associated with Granulocytopenia,” European Journal of Clinical Microbiology, Vol. 6, No. 6, 1987, pp. 670-673. doi:10.1007/BF02013068
- A. Leite, A. Vinhas-Da-Silva, L. Felicio, A. C. Vilarinho and G. Ferreira, “Aerococcus viridans Urinary Tract Infection in a Pediatric Patient with Secondary Pseudohypoaldosteronism,” Revista Argentina de Microbiologia, Vol. 42, No. 4, 2010, pp. 269-270.
- Y. Uh, J. S. Son, I. H. Jang, K. J. Yoon and S. I. Hong, “Penicillin-Resistant Aerococcus viridans Bacteremia Associated with Granulocytopenia,” Journal of Korean Medical Science, Vol. 17, No. 1, 2002, pp. 113-115.
- A. Torrent, S. Deniz, A. Ruiz, P. Calabuig, J. Sicilia and J. Oros, “Esophageal Diverticulum Associated with Aerococcus viridans Infection in a Loggerhead Sea Turtle (Caretta Caretta),” Journal of Wildlife Diseases, Vol. 38, No. 1, 2002, pp. 221-223.
- L. A. Devriese, J. Hommez, H. Laevens, B. Pot, P. Vandamme and F. Haesebrouck, “Identification of AesculinHydrolyzing Streptococci, Lactococci, Aerococci and Enterococci from Subclinical Intramammary Infections in Dairy Cows,” Veterinary Microbiology, Vol. 70, No. 1-2, 1999, pp. 87-94. doi:10.1016/S0378-1135(99)00124-8
- V. Martin, A. I. Vela, M. Gilbert, J. Cebolla, J. Goyache, L. Dominguez and J. F. Fernandez-Garayzabal, “Characterization of A. viridans Isolates from Swine Clinical Specimens,” Journal of Clinical Microbiology, Vol. 45, No. 9, 2007, pp. 3053-3057. doi:10.1128/JCM.00156-07
- T. Horaud and F. Delbos, “Streptococcaceae and Antibiotics,” Lett Infectiol, Vol. 19, 1987, pp. 595-604.
- T. A. Thirunavukkarasu, B. V. Bhat and B. D. Bhatia, “Aerococcus viridans Endocarditis. Case Report,” Indian Pediatrics, Vol. 31, No. 5, 1994, pp. 599-601.
- A. Gopalachar, R. L. Akins, W. R. Davis and A. A. Siddiqui, “Urinary Tract Infection Caused by Aerococcus viridans, a Case Report,” Medical Science Monitor, Vol. 10, No. 11, 2004, Article ID: CS73-5.
- T. Špaková, J. Elečko, M. Vasil, J. Legáth, P. Pristaš and P. Javorský, “Limited Genetic Diversity of Aerococcus viridians Strains Isolated from Clinical and Subclinical Cases of Bovine Mastitis in Slovakia,” Polish Journal of Veterinary Sciences, Vol. 15, No. 2, 2012, pp. 329-335. doi:10.2478/v10181-012-0051-1
- CLSI—National Committee for Clinical Laboratory Standards, “Zone Diameter Interpretive Standards and Minimal Inhibitory Concentration (MIC) Breakpoints for Veterinary Pathogens,” NCCLS Document 2002, M31-A2, NCCLS, Wayne.
- CLSI—National Committee for Clinical Laboratory Standards, “Performance Standards for Antimicrobial Susceptibility Testing, Eighth Informational Supplement,” NCCLS Document 1998, M100-S8, NCCLS, Wayne.
- CLSI—National Committee for Clinical Laboratory Standards, “Performance Standards for Antimicrobial Disk and Dilution Susceptibility Test for Bacteria Isolated from Animals: Informational Supplement,” NCCLS Document 2004, M31-S1, NCCLS, Wayne.
- K. A. Grant, J. H. Dickinson, M. D. Collins and R. G. Kroll, “Rapid Identification of Aerococcus viridans Using the Polymerase Chain Reaction and an Oligonucleotide Probe,” FEMS Microbiology Letters, Vol. 95, No. 1, 1992, pp. 63-69. doi:10.1111/j.1574-6968.1992.tb05343.x
- A. I. Vela, N. Garcia, M. V. Latre, A. Casamayor, C. Sanchez-Porro, V. Briones, A. Ventosa, L. Dominguez and J. F. Fernandez-Garayzabal, “Aerococcus suis sp. nov., Isolated from Clinical Specimens from Swine,” International Journal of Systematic and Evolutionary Micryobiology, Vol. 57, No. 6, 2007, pp. 1291-1294. doi:10.1099/ijs.0.64537-0
- R. E. O. William, A. Hirch and S. T. Cowan, “Aerococcus, a New Bacterial Genus,” Journal of General Microbiology, Vol. 8, No. 3, 1953, pp. 475-480. doi:10.1099/00221287-8-3-475
- H. Swanson, E. Cutts and M. Lepow, “Penicillin-Resistant Aerococcus viridans Bacteremia in a Child Receiving Prophylaxis for Sickle-Cell Disease,” Clinical Infectious Diseases, Vol. 22, No. 2, 1996, pp. 387-388. doi:10.1093/clinids/22.2.387
- S. F. Peek, “Respiratory Emergency in Cattle,” Veterinary Clinics Food Animal, Vol. 21, No. 3, 2005, pp. 697- 710. doi:10.1016/j.cvfa.2005.07.001
- F. Dagnaes-Hansen, M. Kilian and K. Fuursted, “Septicaemia Associated with an Aerococcus viridians Infection in Immunodeficient Mice,” Laboratory Animals, Vol. 38, No. 3, 2004, pp. 321-325. doi:10.1258/002367704323133718
NOTES
*Corresponding author.

