Open Journal of Veterinary Medicine
Vol. 2 No. 4 (2012) , Article ID: 25397 , 6 pages DOI:10.4236/ojvm.2012.24032
Molecular Characterization of Echinococcus granulosus in South of Iran
1Department of Food Hygiene, School of Veterinary Medicine, Shiraz University, Shiraz, Iran
2Department of Basic Sciences, School of Veterinary Medicine, Shiraz University, Shiraz, Iran
3Department of Pathobiology, School of Veterinary Medicine, Shiraz University, Shiraz, Iran
Email: *hosseinzadeh@shirazu.ac.ir
Received June 27, 2012; revised August 21, 2012; accepted September 11, 2012
Keywords: Hydatid Cyst; PCR; Echinococcus; Epidemiology
ABSTRACT
The focus of present study was to determine the epidemiological and molecular aspects of different strains of cystic echinococcosis in Fars province, Iran. Liver and lung samples from 410 sheep, 206 goats and 315 cattle were collected. In cattle, the infestation rate was 18.1% (57/315), with 11.1% hepatic cysts and 7.0% pulmonary cysts. Out of all identified cysts, 31.4% of the hepatic and 31.8% of the pulmonary cysts were found fertile. Incidence rate of hydatid cyst infection in sheep was 15.5% (64/410) with 11.9% hepatic cysts and 3.6% pulmonary cysts, of which 24.5% and 20% of hepatic and pulmonary cysts were respectively identified as fertile. The infestation rate was 16.0% (33/206) in goat, in which 10.2% and 5.8% cysts were collected from liver and lung, correspondingly. The prevalence of fertile hepatic and pulmonary cysts was recorded as 23.8% and 16.7%, respectively. Genotyping the cystic materials using PCR showed that the most prominent strains responsible for cystic echinococcosis in the Fars province are G1 and G6/7, while no evidence of E. multilocularis was recorded. This information may give us some clues to find out more about strains distribution in different regions in Iran, which may finally use to find tools in the eradication program of the disease, here and elsewhere.
1. Introduction
Hydatid cyst is medically and economically considered as one of the most serious zoonotic diseases caused by different species of tape worm echinococcus with the worldwide importance [1-3]. However, species of Echinococcosis have been commonly identified in sheep, cattle, goats and camels in different regions of Iran [4-10]. Moreover, human hydatidosis has been reported throughout the country [11,12]. The presence of two distinct strains of the parasite was genotypically confirmed in different animal species, in Iran [13].
The noticeable health issue is due to the ingestion of eggs from canine feces, causing the infestation of various tissues [14]. There are some growing evidences imply to five species of echinococcus which include a number of genotypes or strains. Identification of these genotypes has a big implication on the surveillance and control of the disease [15]. Currently, ten main genotypes of E. granulose (G1-G10) have been identified considering the biological and molecular aspects [16-18]. The strains distribution of the parasite in different regions, have been previously well documented. For instance in the Mediterranean area, G1 (sheep strain), G2, G3 (the buffalo strain) and G4 (equine strain) have been reported from Spain, Italy, Lebanon and Syria. Moreover, G6 (the camel strain) is dominant strain in North Africa and in the Middle East. The transmission of the camel strain to human was firstly documented, in Iran [19]. G7 has been documented in Spain, Italy, Slovak Republic and Poland [20,21]. Even though different approaches, for instance, clinical, radiological, serological and histological techniques, have been introduced as diagnostic procedures of hydatid to distinguish the strains is feasible by molecular techniques [22-24].
The combination of morphological taxonomy, molecular genetics and evolutionary ecology is required to better understand the biodiversity of parasitic organisms. Fundamental information on their phenotypic and genotypic characteristics is especially important to control human and animal parasitic diseases. The amplification of DNA fragments by polymerase chain reaction (PCR) has greatly accelerated taxonomic studies on parasites, which make possible the identification parasites by nonmorphologists [25].
The clinical diagnosis of echinococcosis in humans can be carried out based on the morphological characteristics of macro and microstructures, whereas the pathological identification of the causative species is difficult in the cases of aberrant forms [26]. Such lesions should be subjected to molecular diagnosis for species identification. At present time, clinical samples taken at biopsy are subjected to PCR, and the amplified fragments of mitochondrial and nuclear DNA are subsequently sequenced [25].
The application of PCR to identify E. granulosus eggs in dog faeces has also been reported by different research groups [27-29]. To identifying species, exploring intraspecific variations has become a scientific imperative to characterize the local populations of parasites [25].
Although hydatid cysts have been recognized public heath problem in Iran, there are a few studies suggesting the presence of G1 and G6 strains in both Lorestan and Mazandaran provinces in south west and north of Iran, respectively [30]. However, little is known about the strain distribution in south of Iran with a relatively high population of animals and so high incidence of the disease in both human and other intermediate hosts. Based on the different molecular techniques targeting the 12S rRNA for the detection of distinct strains of the parasite, we approved the PCR technique as a reliable tool to differentiate various strains and distinguish between different stages of the cyst. Thus, the focus was on the prevalence of the disease with a special concern about strain distribution in Fars province, south of Iran.
2. Materials and Methods
2.1. Sample Collection
During September 2010 to June 2011, total samples of 315 cattle (24 - 48 months), 410 sheep (10 - 40 months) and 206 goats (12 - 38 months) were inspected for the presence of hydatid disease infestation in Shiraz abattoir, southern region of Iran. The samples were transferred on ice to the laboratory to be inspected for fertility of the cysts before being stored at –20˚C for further analysis.
2.2. Characterizations of the Cysts
To find out whether the cysts are fertile or not, the contents of each cyst was investigated using a vital dye (0.1% eosin), followed by checking under the light microscope. The presence of protoscolex and hydatid sands in the cysts was considered as the major criteria.
2.3. Genotyping the Cysts Using PCR Technique
2.3.1. DNA Extraction
Total genomic DNA was extracted from laminated membrane/protoscoleces and hydatid fluid (for purification of DNA from any existed scolices suspended in hydatid fluid of the fertile cysts). The laminated membrane/protoscoleces were cut and chopped followed by boiling in 300 µl lysis buffer (Tris-HCl 0.1 M, EDTA 0.01 M, SDS 1% and NaCl 0.1 M) [31]. The hydatid fluid was centrifuged at high speed (16,500 g) for 15 minutes, the supernatant was discarded and the bottom precipitate was boiled in the same manner as described. The heated specimens were then treated with 15 µl proteinase K (2 µg/µl) and precipitated using conventional phenol/chloroform/ethanol method [32]. The aliquots were subsequently stored at –20˚C for further analysis.
2.3.2. PCR Assay
Three species/strain specific sets of primers, that amplify the partial region of mitochondrial ND1 (NADH dehydrogenase subunit 1) gene, were used to detect the E. granulosus G1 (shPCR), E. granulosus G6/7 (G6/7PCR) and E. multilocularis (emPCR) [24] (Table 1).
The predicted size of amplicons was 252 bp for E. multilocularis, 295 bp for G1 and 234 bp for G6/7 strains of the E. granulosus.
PCR was carried out on 3 μl of single strand cDNA in a final reaction mixture of 25 μl containing 2.5 μl of 10x PCR buffer, 3 mM MgCl2, 200 mM of each of dNTPs, 400 mM of each forward and reverse primer, 2 units of Taq DNA polymerase (Cinagene, Iran). The PCR cycling
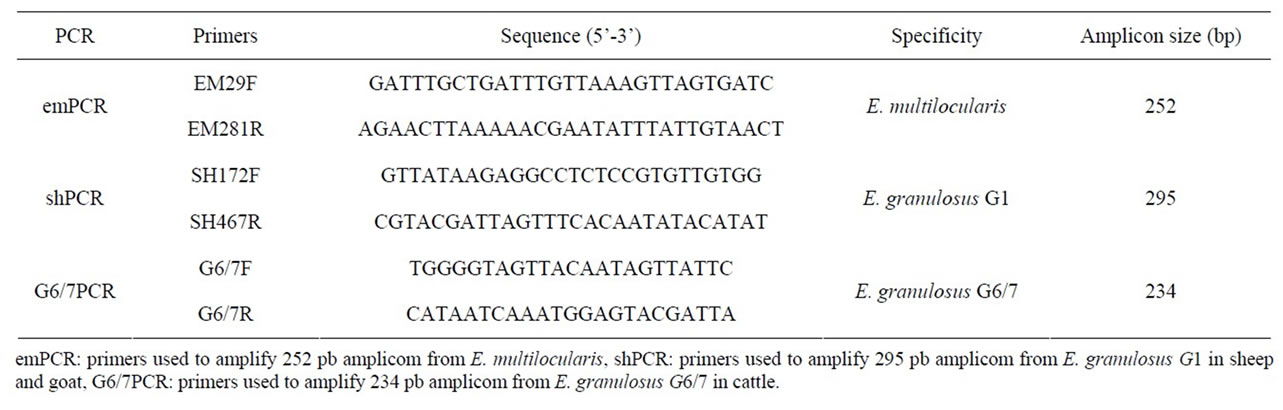
Table 1. Primers used for specification of hydatid cyst samples.
was performed in a gradient thermocycler (Eppendorf, Germany) with an initial denaturation step of 94˚C for 10 min., followed by 45 cycles of 94˚C for 30 sec, 53˚C (G6/7PCR), 56˚C (emPCR), and 57˚C (shPCR) for 45 sec and 72˚C for 1 min. The PCR was terminated with a final extension step at 72˚C for 5 min. PCR products were electrophoresed on a 1.5% ethidium bromidestained agarose gel and the specific DNA fragments with desired size of 5 samples of shPCR were purified and sequenced (Macrogene, South Korea). To analyse the sequencing data, BLASTn comparison was performed with the NCBI/GeneBank database.
3. Results
3.1. Cattle
A total of 57 cases (1 - 10 hydatid cysts per case) were found to be positive from 315 inspected cattles (24 - 48 months age) slaughtered in Shiraz abattoir, south of Iran, giving a 18.1% prevalence rate of the infection. The rate of infection in different organs was 11.1% for liver and 7.0% for lung. Regarding morphotype, 31.4% and 31.8% of the hepatic and pulmonary cysts were correspondingly fertile. Moreover, 54.3% were calcified in the livers and 54.6% in the lungs. In the case of secondary bacterial infected cysts, 14.3% and 13.6% in the livers and the lungs were positive, respectively. The average volume of hydatid fluid collected in the livers and the lungs were 3.5 ± 2.1 ml and 1.75 ± 0.35 ml (per cyst), respectively (Table 2).
3.2. Sheep
From 410 (6 - 40 months age) inspected sheep slaughtered in Shiraz abattoir, 64 positive cases (1 - 15 hydatid cysts per case) were found, indicating the prevalence rate of 15.5 percent. The rate of infection in different organs was 11.9% for liver and 3.6% for lung. Phenotypically, 24.5% were fertile in the livers, and 20.0% were determined fertile in the lungs, 57.1% were calcified in the livers and 46.7% in the lungs. Moreover, 18.4% and 33.3% in the livers and the lungs were respectively positive for secondary bacterial infection. The average volume of hydatid fluid collected in the livers and the lungs were 1 ± 0.5 ml and 0.88 ± 0.25 ml (per cyst), respectively (Table 2).
3.3. Goat
Out of 206 (8 - 32 months age) inspected slaughtered goats, 33 positive cases (1 - 15 hydatid cysts per case) were recorded, indicating the prevalence rate of 16.0 percent. However, the rate of infection in different organs was 10.2% for liver and 5.8% for lung. Phenotypically, 23.8% and 16.7% were respectively determined fertile in the livers and lungs while, 47.6% were calcified in the livers and 50.0% in the lungs. Moreover, 28.6% and 33.3% of the cysts in the livers and the lungs were respectively positive for secondary bacterial infection. The average volume of hydatid fluid collected in the livers and the lungs were 0.8 ± 0.3 ml and 0.68 ± 0.25 ml (per cyst), respectively (Table 2).
3.4. PCR and Sequencing
To determine the responsible genotypes, PCR was employed using three pairs of primers for emPCR, shPCR and G6/7PCR (Table 1) which could amplify 252 bp, 295 bp and 234 bp genes for E. multilocularis, E. granulosus G1 and E. granulosus G6/7, accordingly. PCR yielded desired products from germinal layer, while no DNA was produced from hydatid fluid. From PCR results, it could be speculated that the strain distribution in sheep and goats was G1, this was G6/7 for cattle and, not surprisingly, no evidence of E. multilocularis was found (Figure 1).
BLASTn comparison of the sequences of 5 samples from shPCR and one sample from G6/7PCR, against the
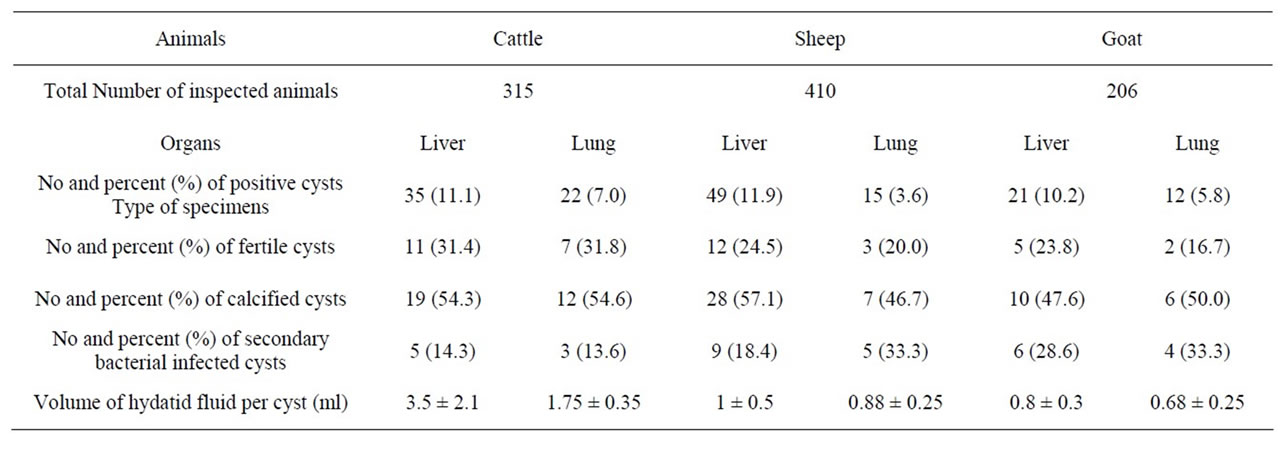
Table 2. Frequency of different organs infested with hydatid cysts in a meat inspection program.

Lane 1: Negative control (no template), Lane 2-6: PCR with G1 primers (295 bp in sheep and goat), Lane 7: PCR with G6/7 primers (234 bp in cattle), Lane 8: PCR with E. multilocularis primers (not detected), Lane M: 100 bp ladder (Fermentas).
Figure 1. Gel electrophoresis of products of PCR with 3 pairs of primers on genomic DNA of some isolates.
nucleotide database, did return a significant result to E. granulosus. The closest identity were seen between the sequences of samples and those of echinococcus isolates submitted at NCBI with accession numbers GQ168810 and GQ357999-GQ358013 for shPCR and G6/7PCR respectively.
4. Discussion
Different molecular approaches have shown the advantage of conclusive species determination and also strain differentiation within the genus echinococcus which is responsible for important parasitic zoonoses worldwide. Numerous studies have been carried out on the genetic characterization of hydatid cyst, using hydatid fluid, cystic membrane and scolices [22]. Various PCR techniques have been introduced to detect E. granulosus genome in biological specimens [33]. PCR is currently developed as a complementary diagnostic tool for species differentiation for echinococcosis, using biopsy samples and tissue specimens [34,35]. The usefulness of the technique was also confirmed by Rostami-Nejad [30], with molecular detection of different strains of E. granulosus from West and North of Iran. However, performing such techniques often need a careful attention in designing primers and preparation of DNA in adequate quantities [36]. A relatively high prevalence of E. granulosus with the percentages of 44.83% and 25.53% respectively in male and female stray dogs checked in the Fars province [8], led us to perform an epidemiological study in the region. Even though, the rate of infestation in our study, was in accordance with the previous studies, where the rate of cystic hydatidosis in liver and lung of different animals were reported respectively, as follows: sheep 2.09% and 2.68%, goats 2.17% and 2.36%, cattle 4.49% and 6.48% and buffaloes 4% and 8% [8]. However, different rates of the infection have been previously reported from different regions of the country, for instance, rate of 27.5% in sheep, 16.1% in goats and 25.9% in cattles, in Hamadan [37]. Results of the present study indicated a predominent rate of the infestation with G1 strain in the south region of Iran. This was in accordance with results of the work conducted by Sharbatkhori and colleagues [31], in which using ITS1-RFLP was confirmed G1 and G6 strains, in north of Iran with a higher prevalence of G1 strain in livestock. It is concluded that cystic hydatidosis needs more profound investigations because of its public heath and economic issues here and elsewhere.
REFERENCES
- J. Euzeby, “The Epidemiology of Hydatidosis with Special Reference to the Mediterranean Area,” Parassitologia, Vol. 33, No. 1, 1991, pp. 25-39.
- P. Schantz, “Echinococcus granulosus, E. multilocularis and E. vogli (Agents of Cystic, Alveolar, Polycystic Echinococcosis),” In: S. S. Long and C. G. Prober, Eds., Pediatrics Infectious Diseases, Churchill Livingstone, New York, 1997, pp. 1488-1492.
- R. C. A. Thompson, “Echinococcosis,” In: S. H. Gillespie and R. Pearson, Eds., Principles and Practice of Clinical Parasitolog, Wiley, Sussex, 2001, pp. 595-612.
- I. Mobedi, H. Madadi and F. Arfaa, “Camel ‘Camelus dromedarius’ Intermediate Host of Echinococcus granulosus in Iran,” The Journal of Parasitology, Vol. 56, No. 6, 1970, pp. 1255-1259. doi:10.2307/3277581
- N. Moghaddar, A. Oryan and M. R. Hanifpour, “Helminthes Recovered from the Liver and Lungs Camel with Special Reference to Their Incidence and Pathogenesis in Shiraz, Islamic Republic of Iran,” The Indian Journal of Animal, Vol. 62, No. 11, 1992, pp. 1018-1023.
- A. Oryan, N. Moghadar and S. N. S. Gaur, “Metacestodes of Sheep with Special Reference to Their Epidemiological Status, Pathogenesis and Economic Implications in Fars Province, Iran,” Veterinary Parasitology, Vol. 51, No. 3, 1994, pp. 231-240. doi:10.1016/0304-4017(94)90160-0
- Hosseini S.H. and A. Eslami, “Morphological and Developmental Characteristics of Echinococcus granulosus Derived from Sheep, Cattle and Camels in Iran,” Journal of Helminthology, Vol. 72, No. 4, 1998, pp. 337-341. doi:10.1017/S0022149X00016709
- D. Mehrabani, A. Oryan and S. M. Sadjjadi, “Prevalence of Echinococcus granulosus Infection in Stray Dogs and Herbivores in Shiraz, Iran,” Veterinary Parasitology, Vol. 86, No. 3, 1999, pp. 217-220. doi:10.1016/S0304-4017(99)00151-X
- A. Dalimi, G. H. Motamedi, M. Hosseini, B. Mohammadian, H. Malaki, Z. Ghamari and F. Ghaffari-far, “Echinococcosis/Hydatidosis in Western Iran,” Veterinary Parasitology, Vol. 105, No. 2, 2002, pp. 161-171. doi:10.1016/S0304-4017(02)00005-5
- N. A. Ahmadi, “Hydatidosis in Camels (Camelus dromedarius) and Their Potential Role in the Epidemiology of Echinococcus granulosus in Iran,” Journal of Helminthology, Vol. 79, No. 2, 2005, pp. 119-125. doi:10.1079/JOH2005279
- B. Bastani and F. Dehdashti, “Hepatic Hydatid Disease in Iran, with Review of the Literature,” Mount Sinai Journal, Vol. 62, No. 2, 1995, pp. 62-69.
- N. A. Ahmadi and A. Dalimi, “Characterization of Echinococcus granulosus Isolates from Human, Sheep and Camel in Iran,” Infection, Genetics and Evolution, Vol. 6, No. 2, 2006, pp. 85-90. doi:10.1016/j.meegid.2005.01.005
- L. A. E. Zhang, S. H. Hosseini and D. P. McManus, “Indication of the Presence of Two Distinct Strains of Ecchinococcus granulosus in Iran by Mitochondrial DNA Markers,” The American Journal of Tropical Medicine and Hygiene, Vol. 59, No. 1, 1998, pp. 171-174.
- H. Per, S. Kumandas, H. Gumus and A. Kurtsov, “Primary Soliter and Multiple Intracranial Cyst Hydatid Disease: Report of Five Cases,” Brain and Development, Vol. 31, No. 3, 2009, pp. 228-233. doi:10.1016/j.braindev.2008.03.009
- P. R. Torgerson and D. D. Heath, “Transmission Dynamics and Control Options for Echinococcus granulosus,” Parasitology, Vol. 127, Suppl. S1, 2003, pp. S143-S158. doi:10.1017/S0031182003003810
- J. Bowles, D. Blair and D. P. McManus, “Genetic Variants within the Genus Echinococcus Identified by Mitochondrial DNA Sequencing,” Molecular and Biochemical Parasitology, Vol. 54, No. 2, 1992, pp. 169-174. doi:10.1016/0166-6851(92)90109-W
- M. Pearson, T. H. Le, L. H. Zhang, D. Blair, T. H. N. Dai and D. P. McManus, “Molecular Taxonomy and Strain Analysis in Echinococcus,” In: P. Craig and Z. Pawlowski, Eds., Cestode Zoonoses: Echinococcus and Cysticercosis. An Emergent and Global Problem, Nato Science Series, Vol. 341, IOS Press, Amsterdam, 2002, pp. pp. 205-219.
- E. Sanchez, O. Caceres, C. Naquirel, D. Garcia, G. Patino, H. Silvia, A. C. Volotao and O. Fernandez, “Molecular Characterization of Echinococcus granulosus from Peru by Equencing of the Mitochondrial Cytochrome c Oxidase Subunit 1 Gene,” Memórias do Instituto Oswaldo Cruz, Vol. 105, No. 6, 2010, pp. 806-810.
- M. F. Harandi, R. P. Hobbs, P. J. Adams, I. Mobedi, U. M. Morgan-Ryan and R. C. Thompson, “Molecular and Morphological Characterization of Echinococcus granulosus of Human and Animal Origin in Iran,” Parasitology, Vol. 125, No. 4, 2002, pp. 367-373.
- R. C. Thompson and D. P. McManus, “Towards a Taxonomic Revision of the Genus Echinococcus,” Trends in Parasitology, Vol. 18, No. 10, 2002, pp. 452-457. doi:10.1016/S1471-4922(02)02358-9
- A. Varcasia, S. Canu, M. W. Lightowlers, A. Scala and G. Garippa, “Molecular Characterization of Echinococcus granulosus Strains in Sardinia,” Parasitology Research, Vol. 98, No. 3, 2006, pp. 273-277. doi:10.1007/s00436-005-0059-x
- A. Dinkel, E. M. Njoroge, A. Zimmermann, M. Wälz, E. Zeyhle, I. E. Elmahdi, U. Mackenstedt and T. Romig, “A PCR System for Detection of Species and Genotypes of the Echinococcus granulosus-Complex, with Reference to the Epidemiological Situation in Eastern Africa,” International Journal for Parasitology, Vol. 34, No. 5, 2004, pp. 645-653. doi:10.1016/j.ijpara.2003.12.013
- A. Obwaller, R. Schneider, J. Walochnik, B. Gollackner, A. Deutz, K. Janitschke, H. Aspöck and H. Auer, “Echinococcus granulosus Strain Differentiation Based on Sequence Heterogeneity in Mitochondrial Genes of Cytochrome c Oxidase-1 and NADH Dehydrogenase-1,” Parasitology, Vol. 128, No. 5, 2004, pp. 569-575. doi:10.1017/S0031182004004871
- R. Schneider, B. Gollackner, B. Edel, K. Schmid, F. Wrba, G. Tucek, J. Walochnik and H. Auer, “Development of a New PCR Protocol for the Detection of Species and Genotypes (Strains) of Echinococcus in formalinFixed, Paraffin-Embedded Tissues,” International Journal for Parasitology, Vol. 38, No. 8-9, 2008, pp. 1065- 1071. doi:10.1016/j.ijpara.2007.11.008
- M. Nakao, Y. A. Tetsuya, O. B. Munehiro, K. A. Jenny, N. A. Agathe, S. A. Yasuhito and I. A. Akira, “State-ofthe-Art Echinococcus and Taenia: Phylogenetic Taxonomy of Human-Pathogenic Tapeworms and Its Application to Molecular Diagnosis,” Infection, Genetics and Evolution, Vol. 10, No. 4, 2010, pp. 444-452. doi:10.1016/j.meegid.2010.01.011
- J. Eckert and P. Deplazes, “Biological, Epidemiological, and Clinical Spects of Echinococcosis, a Zoonosis of Increasing Concern,” Clinical Microbiology Reviews, Vol. 17, No. 1, 2004, pp. 107-135. doi:10.1128/CMR.17.1.107-135.2004
- I. Abbasi, A. Branzburg, M. Campos-Ponce, S. K. Abdel Hafez, F. Raoul, P. S. Craig and J. Hamburger, “CoproDiagnosis of Echinococcus granulosus Infection in Dogs by Amplification of a Newly Identified Repeated DNA Sequence,” The American Journal of Tropical Medicine and Hygiene, Vol. 69, No. 3, 2003, pp. 324-230.
- S. Stefanić, B. S. Shaikenov, P. Deplazes, A. Dinkel, P. R. Torgerson and A. Mathis, “Polymerase Chain Reaction for Detection of Patent Infections of Echinococcus granulosus (‘Sheep Strain’) in Naturally Infected Dogs,” Parasitology Research, Vol. 92, No. 4, 2004, pp. 347-351. doi:10.1007/s00436-003-1043-y
- A. Naidich, D. P. McManus, S. G. Canova, A. M. Gutierrez, W. Zhang, E. A. Guarnera and M. C. Rosenzvit, “Patent and Pre-Patent Detection of Echinococcus granulosus Genotypes in the Definitive Host,” Molecular and Cellular Probes, Vol. 20, No. 1, 2006, pp. 5-10. doi:10.1016/j.mcp.2005.08.001
- M. Rostami-Nejad, M. Nazemalhosseini, Z. Nochi, M. F. Harandi, K. Cheraghipour, G. R. Mowlavi and M. R. Zali, “Echinococcus granulosus Strain Differentiation in Iran Based on Sequence Heterogenecity in the Mitochondrial 12S rRNA Gene,” Journal of Helminthology, Vol. 82, No. 4, 2008, pp. 343-347. doi:10.1017/S0022149X0804594X
- M. Sharbatkhori, H. Mirhendi, M. F. Harandi, M. Rezaeian, M. Mohebali, M. Eshraghian, H. Rahimi and E. B. Kia, “Echinococcus granulosus Genotypes in Livestock of Iran Indicating High Frequency of G1 Genotype in Camels,” Experimental Parasitology, Vol. 124, No. 4, 2010, pp. 373-379. doi:10.1016/j.exppara.2009.11.020
- J. Sambrook, E. F. Fritsch and T. Maniatis, “Molecular Cloning, a Laboratory Manual,” 2nd Edition, Cold Spring Harbor Laboratory Press, New York, 1989.
- M. M. Siles-Lucas and B. B. Gottstein, “Molecular Tools for the Diagnosis of Cystic and Alveolar Echinococcosis,” Tropical Medicine & International Health, Vol. 6, No. 6, 2001, pp. 463-475. doi:10.1046/j.1365-3156.2001.00732.x
- P. Kern, P. Frosch, M. Helbig, J. G. Wechsler, S. Usadel, K. Beckh, R. Kunz, R. lucius and M. Frosch, “Diagnosis of Echinococcus multilocularis Infection by ReverseTranscription Polymerase Chain Reaction,” Gastroenterology, Vol. 109, No. 2, 1995, pp. 596-600. doi:10.1016/0016-5085(95)90350-X
- P. Myjak, W. Nahorski, H. Pietkiewicz, M. von Nickisch-Rosenegk, J. Stolarczyk, E. Kacprzak, I. FelczakKorzybska, B. Szostakowska and R. Lucius, “Molecular Confirmation of Human Alveolar Echinococcosis in Poland,” Clinical Infectious Diseases, Vol. 37, No. 8, 2003, pp. 121-125. doi:10.1086/378296
- D. P. McManus and R. C. A. Thompson, “Molecular Epidemiology of Cystic Echinococcosis,” Parasitology, Vol. 127, Suppl. S1, 2003, pp. S37-S51. doi:10.1086/378296
- M. Arbabi, J. Massoud, A. Dalimi-Asl and S. M. Sadjjadi, “Prevalence of Hydatidosis in Slaughtered Animals in Hamedan,” Journal of Shahed Universty, Vol. 5, 1998, pp. 57-61.
NOTES
*Corresponding author.

