International Journal of Clinical Medicine
Vol. 3 No. 6 (2012) , Article ID: 24544 , 13 pages DOI:10.4236/ijcm.2012.36088
Cellular Profiles in Peripheral Blood Accompanying Particular Asthmatic Response Types
![]()
Allergy Research Foundation, Breda, The Netherlands.
Email: zpelikan@casema.nl
Received July 23rd, 2012; revised August 26th, 2012; accepted September 13th, 2012
Keywords: Blood Cell Counts; Immediate/Early Asthmatic Response; Late Asthmatic Response; Delayed Asthmatic Response
ABSTRACT
Background: Patients with allergic bronchial asthma develop various asthmatic response types to bronchial challenge with allergen, such as immediate (IAR), late (LAR), dual late (DLAR) or delayed (DYAR), displaying different clinical, immunologic and pharmacologic features. This study deals with count changes of particular blood cells accompanying the IAR, LAR and DYAR. Methods: In 63 patients developing 22 IAR, 26 LAR and 15 DYAR, the repeated allergen challenges were supplemented with recording of blood cell counts, Th1/Th2 ratio, leukotrines B4 (LTB4) and C4 (LTC4), eosinophil cationic protein (ECP), myeloperoxidase (MPO), and histamine in blood, and intracellular IFN-γ and IL-4 in peripheral blood mononuclear cells. Results: The IAR was accompanied by increased eosinophil and basophil counts, increased serum concentrations of histamine, LTC4 and ECP, decreased Th1/Th2 ratio in favour of Th2 cells, and increased intracellular IL-4. The LAR was associated with increased eosinophil and neutrophil counts, increased serum concentrations of LTC4 and LTB4, unchanged Th1/Th2 ratio, and increased intracellular IL-4. The DYAR was accompanied by increased total leukocyte, neutrophil, monocyte, lymphocyte and thrombocyte counts, increased serum concentrations of LTB4 and MPO, increased Th1/Th2 ratio in favour of Th1 cells, and increased intracellular IFN-γ. Conclusions: These results provide evidence for different involvement of particular blood cell types and different hypersensitivity mechanisms in IAR, LAR and DYAR. The monitoring of peripheral blood cell counts seems to be an useful supplementary parameter to the bronchial challenge with allergen.
1. Introduction
Allergic bronchial asthma (BA) is a multifaceted disorder in which various immunologic mechanisms can be involved [1,2]. The role of immediate (IgE-mediated) hypersensitivity mechanism in bronchial asthma has already been established [1-3]. However, the possible involvement of the non-immediate hypersensitivity mechanisms in this disorder is still under investigation [1-9]. Different hypersensitivity mechanisms can result in different types of asthmatic response, such as immediate (IAR) [5,9,10-12], late (LAR) [2,9,11-13], dual late (DLAR; a combination of an immediate and a late) [2,9, 11,13]. The IAR, LAR and DLAR have been studied extensively from various points of view [1-3,9,10-13]. Recently, we have reported the existence of a new phenotype of asthmatic response, delayed asthmatic response (DYAR), appearing 26 - 56 hours after the bronchial challenge and displaying clinical and immunologic features different from those of the IAR and LAR [14,15].
The purpose of this study, being a continuation of our previous pilot studies [16,17] was: 1) To investigate the changes in cellular counts in peripheral blood associated with particular types of asthmatic response to allergen challenge (BPT); 2) To assess the possible involvement of the individual circulating cell types in the hypersensitivity mechanisms underlying the particular types of asthmatic response.
2. Patients and Methods
2.1. Patients
Sixty-three asthmatics examined at our Department of Allergology & Immunology, Institute Medical Sciences “De Klokkenberg”, Breda, The Netherlands) and developing 22 IARs, 26 LARs and 15 DYARs to bronchial challenge with allergen (BPT) (Figures 1-3), volunteered to participate in this study.
These patients, 21 - 44 years of age, suffered from reversible bronchial obstruction, alternating with symptom-free periods, without any restrictive changes of their pulmonary function [18]. They had no airway infections and did not use oral corticosteroids or immunotherapy. They were examined by routine diagnostic procedure, serving also as an inclusion-exclsion criteria, including also 87 BPTs with inhalant allergens and 63 PBS (phosphate buffered saline) control challenges (Tables 1 and 2) All BPTs were performed in a period without manifest bronchial complaints, outside the allergen-relevant season and during hospitalization.
Inhalation glucocorticosteroids (n = 24) and long-acting β2-sympathomimetics (n = 23) were withdrawn 4 weeks, cromolyn (n = 7), nedocromil sodium (n = 9) 2 weeks, while other drugs 48 hours prior the BPTs. If the FEV1 values decreased after allergen challenge with 50% or more, with respect to the predicted values, the patients (n = 3) were treated with a single dose of 200 - 400 mcg aerosolized Salbutamol.
In these 63 patients the positive BPTs with the same allergens (Table 2) and the PBS controls were repeated 2 - 3 weeks later (Figures 1-3) and supplemented with recording of cell counts and other factors in venous blood before, and 1, 12, 24, 36, 48, 56 and 72 hours after the challenge (Tables 3-5). The local ethical committee (IBR-MCK) approved this study and an informed consent was obtained from all participants.
2.2. Control Subjects
In 17 healthy subjects volunteering to participate as control subjects 17 BPTs with PBS were supplemented with the blood cell counts up to 72 hours after the challenge (Table 1).
2.3. Allergens
Dialyzed and lyophilized allergen extracts (Allergopharma, Reinbek, Germany) diluted in PBS were used in concentrations of 100 - 500 BU/mL for skin tests and 1000 - 3000 BU/mL for BPTs (Table 2). The recommended concentrations by the manufacturer were 500 BU/mL for skin tests and 5000 BU/mL for the BPTs.
2.4. Skin Tests
Skin prick tests (SPT) with allergenic extracts in concentrations of 500 BU/mL were evaluated after 20 minutes. The intracutaneous tests (i.c.) in concentration of 100 BU/mL and then 500 BU/mL, performed in all patients, were evaluated 20 minutes, 6, 12, 24, 36, 48, 72 and 96 hours after the intradermal injection. Histamine diphosphate was used as a positive and PBS as a negative control [3,13-15].
2.5. Bronchial Provocation Tests (BPT)
The BPTs were performed by means of spirometry (Spirograph D-75 Lode, Groningen, The Netherlands) recording the FVC and FEV1 values. The aerosolized allergen extracts and PBS were inhaled using Wiebadener Doppel-Inhalator at an airflow of 10 L/min. The nebulizer output was 0.12 - 0.14 mL/min and the aerosol particles had a median mass diameter of 2.8 - 3.6 μ.
The BPTs, being a modification of the European standard [19,20], were performed as follows: 1) Initial (baseline) values recorded at 0, 5 and 10 minutes; 2) PBS control values recorded at 0, 5 and 10 minutes after a 10 minute PBS inhalation; 3) Inhalation of allergen aerosol for 2 × 5 minutes, with inserted spirometry measurement, followed by the recording of the FEV1 and FVC values at 0, 5, 10, 20, 30, 45, 60, 90 and 120 minutes and the every hour up to 12th hour, every second hour during the 22nd and 38th , the 46th and 62nd and at the 72nd hour interval. The PBS control challenge was performed by the same schedule as that of the BPTs with allergens. A 5-day interval has always been inserted between the consecutive tests.
2.6. Blood Cell Counts
The venous blood was collected at following time-intervals: 1) IAR: before, and 0, 10, 20, 30, 45, 60 and 120 minutes after the BPT; 2) LAR: before, and 1, 2, 4, 6, 8, 10, 12 and 24 hours after the BPT; 3) DYAR: before, and 1, 6, 12, 24, 36,48, 56 and 72 hours after the BPT. The total and differential blood cell counts were performed from the EDTA blood samples, by means of peroxidasefluorometric method using automated hematologic analyzer (Advia 120, Siemens, Germany), in duplicate.
The intra-assay as well as the inter-assay coefficients of variations were less than 5%.
2.7. Supplementary Parameters
1) Th1/Th2 ratio (%) in peripheral blood Th1/Th2 ratio (%) values in heparinised peripheral blood were determined by flowcytometry using the three-color FACS-Calibur flowcytometer (BD), equipped with a 15-mW argon ion laser and appropriate filters for FITC (530 nm), PE (585 nm), and PerCP (>650 nm), up to 72 hours after the challenge [14].
2) Intracellular cytokines from activated PBMC cultures The peripheral blood mononuclear cells (PBMC) were separated by Hypaque-Ficoll density-gradient centrifugation (Pharmacia, Sweden) and cultured at a concentration of 5 × 106 cells/mL in presence of 50 ng/mL Phorbol 12-myristate 13-acetate (PMA, Sigma) and 1 μg/mL ionomycin for 24 hours. After culture, the cells were centrifuged and viability was determined using trypan blue dye exclusion. Supernatants were stored at −80˚C. The concentrations of cytokines were measured in the centrifuged supernatants by means of enzyme-linked immunoassay (ELISA) kits (Quantikine, R & D Systems,
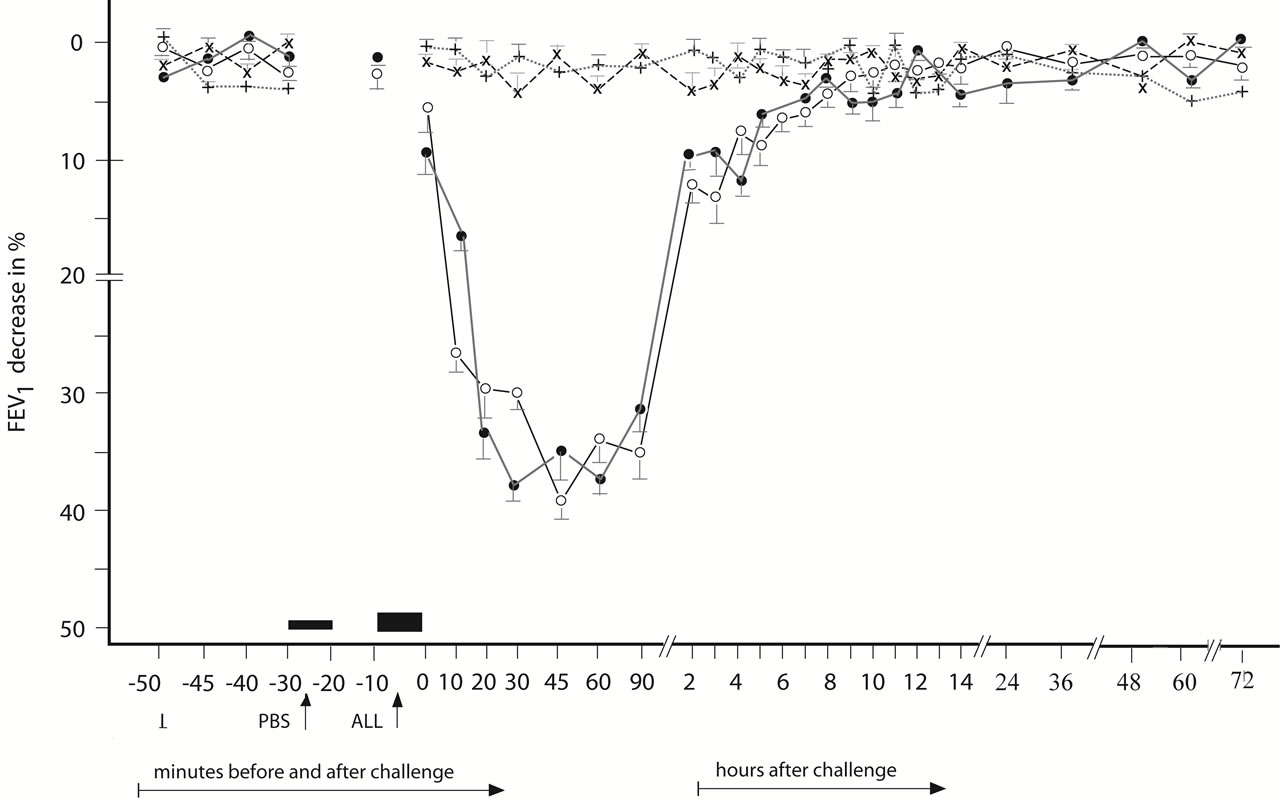
Figure 1. Immediate asthmatic response to allergen challenge (IAR) and phosphate buffered saline (PBS) control challenge. The mean percentage changes in the FEV1 values calculated from 22 IARs and 22 PBS control challenges; (○) = the initial IAR; (●) = the repeated IAR; (+) = the initial PBS; (x) = the repeated PBS; I = initial (baseline) values; ALL = allergen challenge; PBS = phosphate buffered saline; Bars = means ± SEM.
USA), in accordance with the manufacturer’s instructions. The minimal detectable limits (pg/mL) were: IFN-γ: 8.0 and IL-4: <10.0. The intra-assay coefficients of variations of these assays were <9% and the inter-assay coefficients of variations were <10% [14,15].
3) Histamine in the serum Histamine serum concentrations, so-called “blanks”, were measured by the Siraganian’s fluorometric method. The total histamine content was obtained by lysing the cells in whole blood with 2.0% perchloric acid, the so-called “completes”, and also measured by fluorometric assay. All measurements were duplicated. The spontaneously released histamine in “blanks” was expressed in ng/mL and as a percentage of the total histamine content in “completes” [14,15].
4) Cellular constituents in the serum/plasma Venous blood samples (5 mL) were collected into separator tubes (S-Monivette, Sarstedt, Germany), kept at room temperature for 1 hour and then centrifuged at 3000 × g for 10 minutes at 4˚C. The serum supernatants were removed, stored at 2˚C - 8˚C and processed within 1 hour. Other venous blood portions were collected in vacutainers containing EDTA, kept for 1 hour at room temperature and then centrifuged at 2500 × g for 10 minutes at 4˚C. The plasma supernatants were removed and processed within 1 hour. The resting aliquots were stored at −70˚C [15].
The serum/plasma levels of appropriate factors were measured by using commercially available kits, following manufacturer’s recommendations. ECP was estimated in the serum, whereas all other factors were determined in the plasma. All measurements were performed in duplicate by a double-blind schedule. The intra-assay as well as the inter-assay coefficients of variations for all the assay kits employed were <10%.
1) Leukotrienes B4 and C4 -EIA kits (Cayman Chemical Company, Ann Arbor/MI, USA). Detection limits (DL): LTB4 = 4.8 pg/mL; LTC4 = 2 pg/mL; 2) Eosinophil cationic protein was ImmunoCAP (Pharmacia Diagnostics, Uppsala, Sweden). DL: ECP = 2 μg/L; 3) Myeloperoxidas ELISA kit (Oxis International Inc, Portland/OR, USA). DL: MPO = 25 ng/mL [15].
2.8. Statistical Analysis
The asthmatic responses and the PBS controls were statistically analyzed by means of fitting polynomials to the mean curves over time; eight time points within 120 minutes and twenty-five time points up to 72 hours after
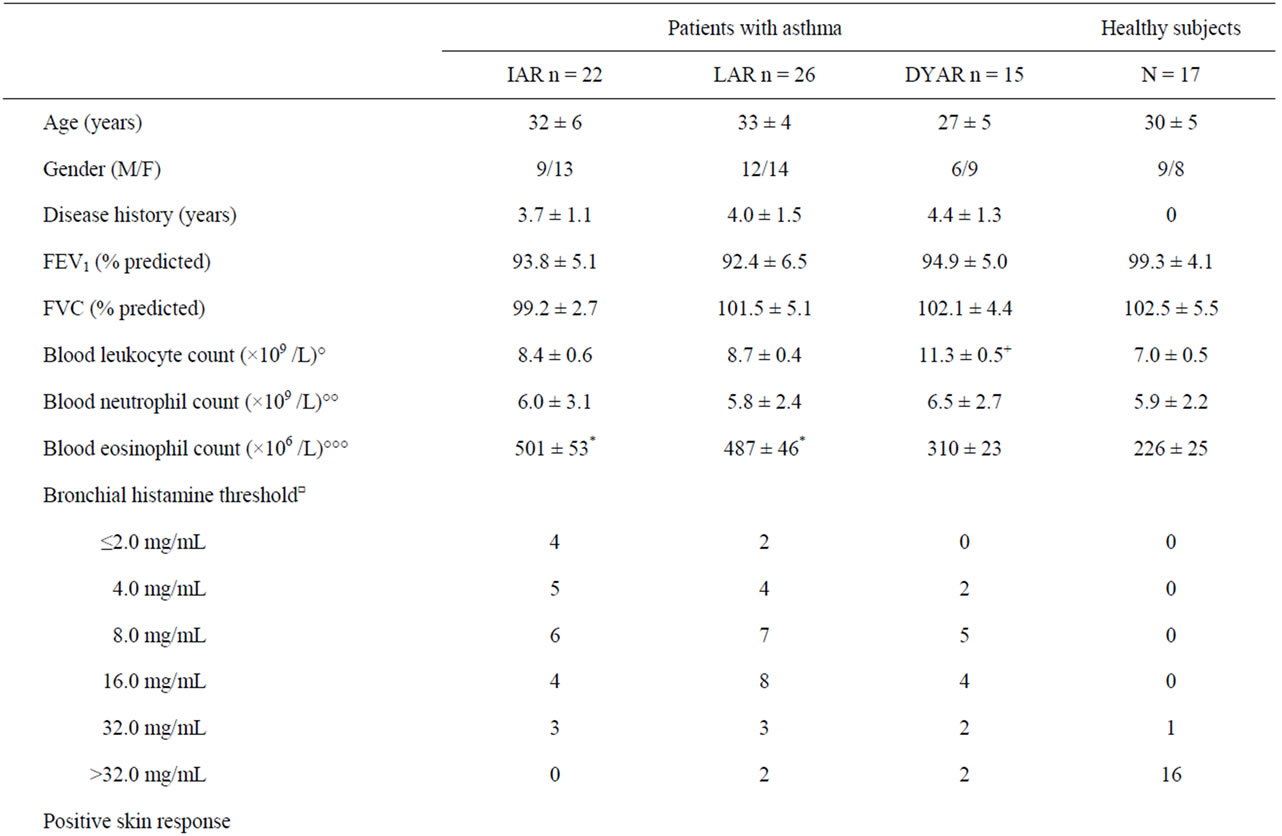

Table 1. Characteristics of the patients and control subjects.
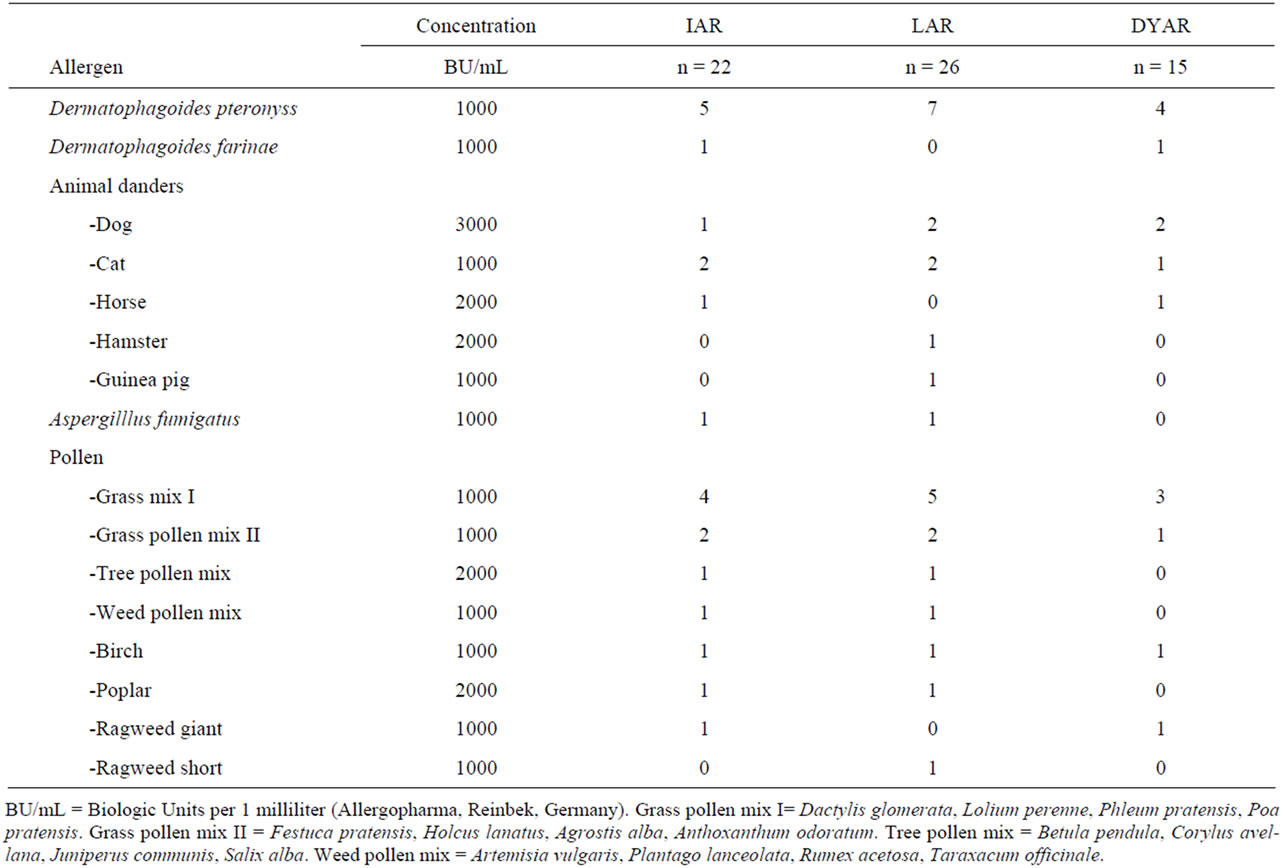
Table 2. Allergens caused particular types of asthmatic response.
the BPT The hypotheses were tested by generalized multivariate analysis of variance model (MANOVA) [21].
The post-challenge cell counts and factors recorded at each of the time points during the asthmatic responses and PBS controls in individual patients were compared with their pre-challenge values and statistically analyzed by Wilcoxon matched-pair signed rank test. The mean post-challenge cell counts and other factors measured at each time point during the asthmatic responses were compared with corresponding PBS values and evaluated by Mann-Whitney U test. A p value < 0.05 was considered to be statistically significant.
3. Results
The IAR (n = 22) appearing within 120 minutes after the BPT (Figure 1) was statistically significant, both in comparison of the post-challenge with the pre-challenge FEV1 values and as compared with the PBS controls (p < 0.01, p < 0.001, respectively).
The LAR (n = 26) occurring between 8 - 12 after the BPT (Figure 2) was statistically significant both in comparing the post-challenge with the pre-challenge FEV1 values and in comparison with the PBS controls (p < 0.001, p < 0.001, respectively).
The DYAR (n = 15) appearing between 26 - 56 hours after the BPT (Figure 3) was statistically significant, both in comparing the postwith the pre-challenge FEV1 values and in comparing with the PBS controls (p < 0.001, p < 0.01, respectively). No significant differences were found between the initial and the repeated IAR (p > 0.1), LAR (p > 0.2) or DYAR (p > 0.05).
The IAR was associated with immediate skin response in 73% and positive allergen-specific IgE in the serum in 41% (Table 1).
The LAR was associated with late skin response in 69%, positive specific IgE in the serum in 12%, increased total serum IgG in 38%, IgG3 in 12%, IgG4 in 19% (Table 1).
The DYAR was associated with delayed skin response in 60%, and increased total serum IgG in 13% (Table 1).
The IAR was accompanied by: 1) Changes in eosinophil and basophil counts, 2) Th1/Th2 ratio changes in favour of Th2, 3) Increased IL-4 in PBMC, 4) Increased concentrations of serum ECP, histamine and plasma LTC4 (Table 3). The increased basophil counts correlated with increased post-challenge concentrations of IL-4 at 10, 20 and 30 minutes (p < 0.01) and histamine at 10 and 20 minutes (p < 0.05), whereas the increased eosinophil counts correlated with increased concentrations of
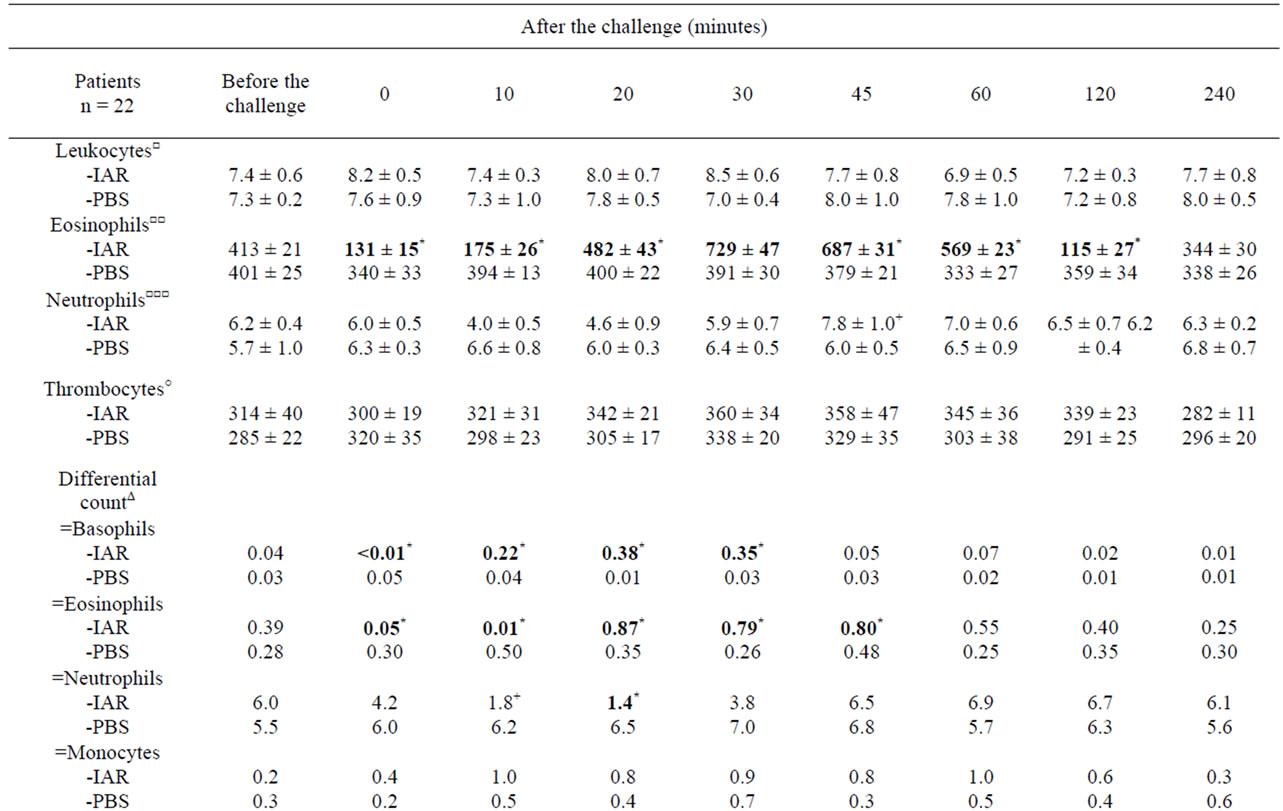
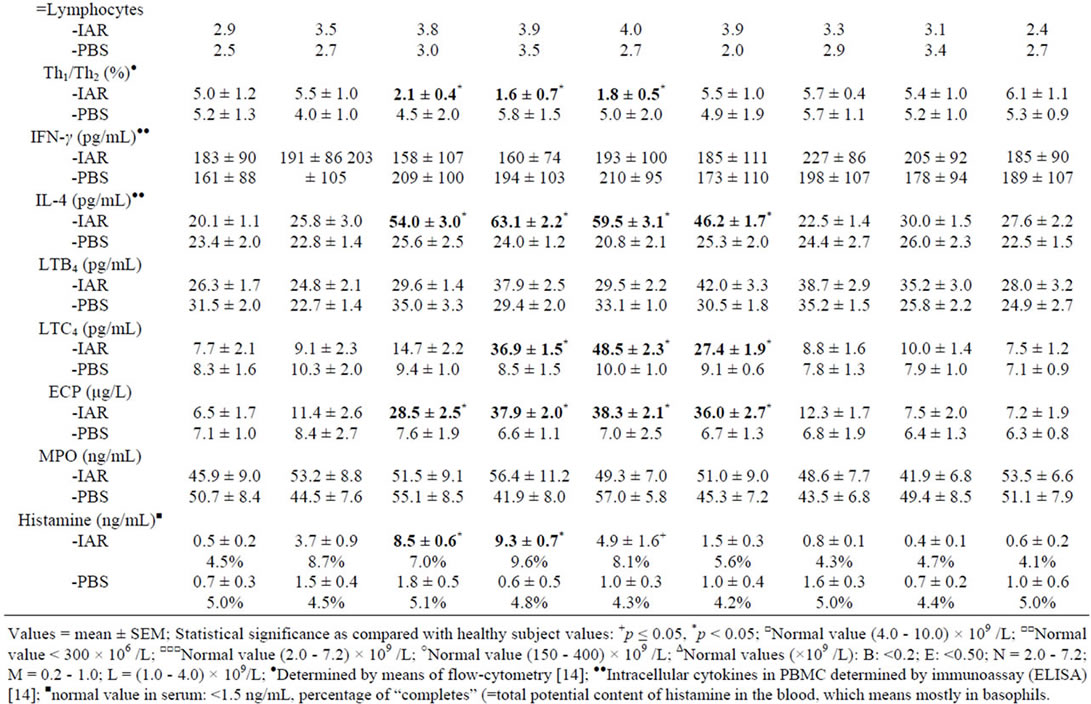
Table 3. Cellular counts and other factors in peripheral blood during the immediate asthmatic response (IAR) and PBS controls.
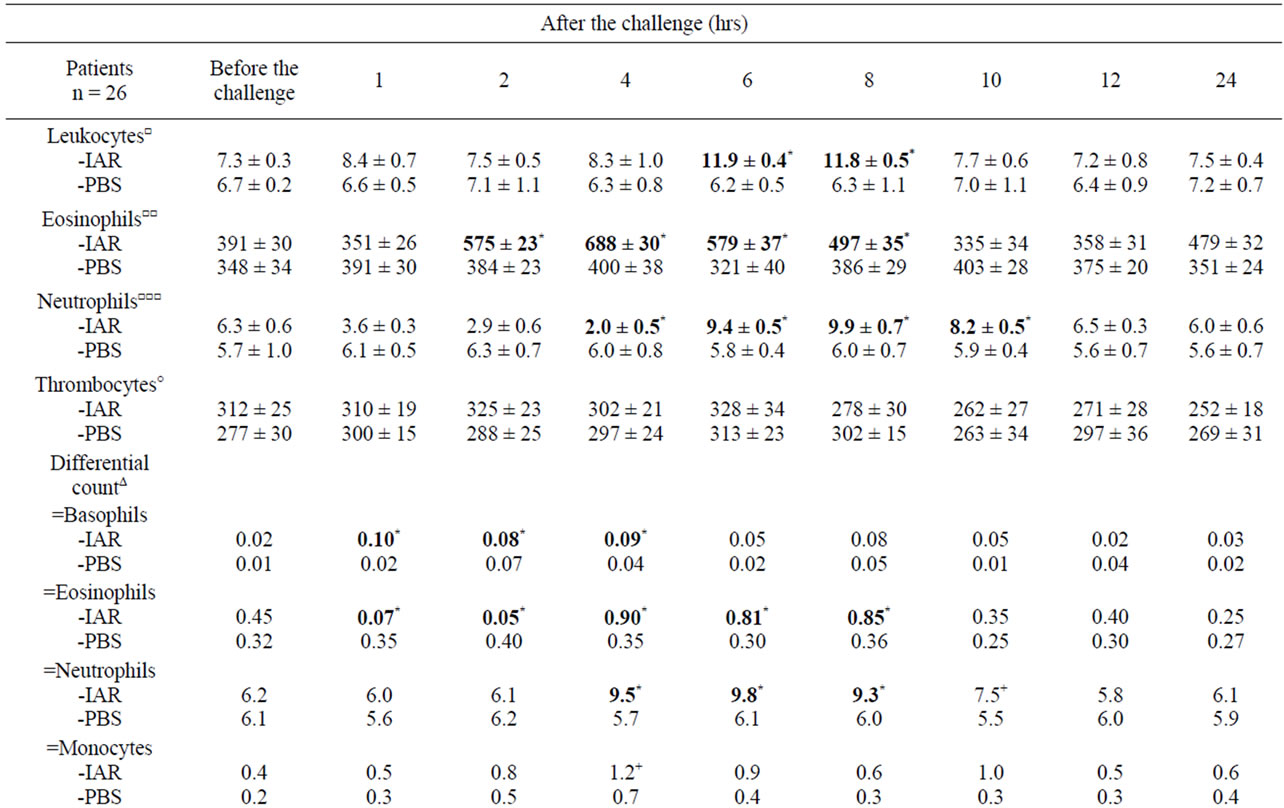
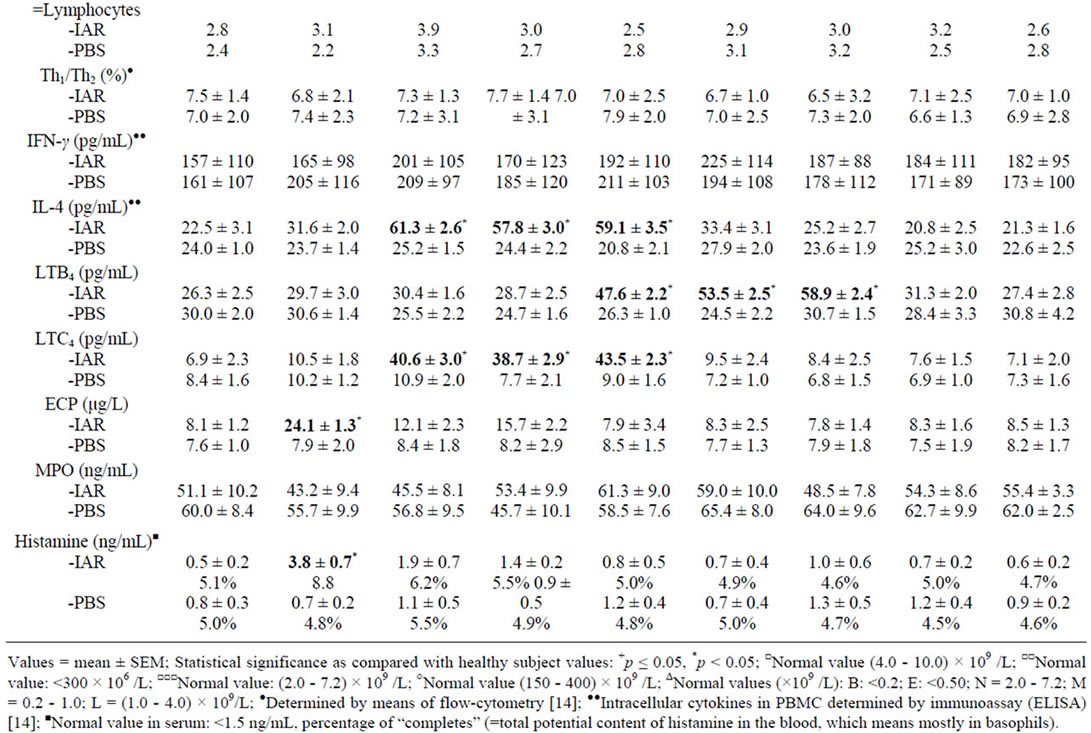
Table 4. Cellular counts and other factors in peripheral blood during the late asthmatic response (LAR) and PBS controls.
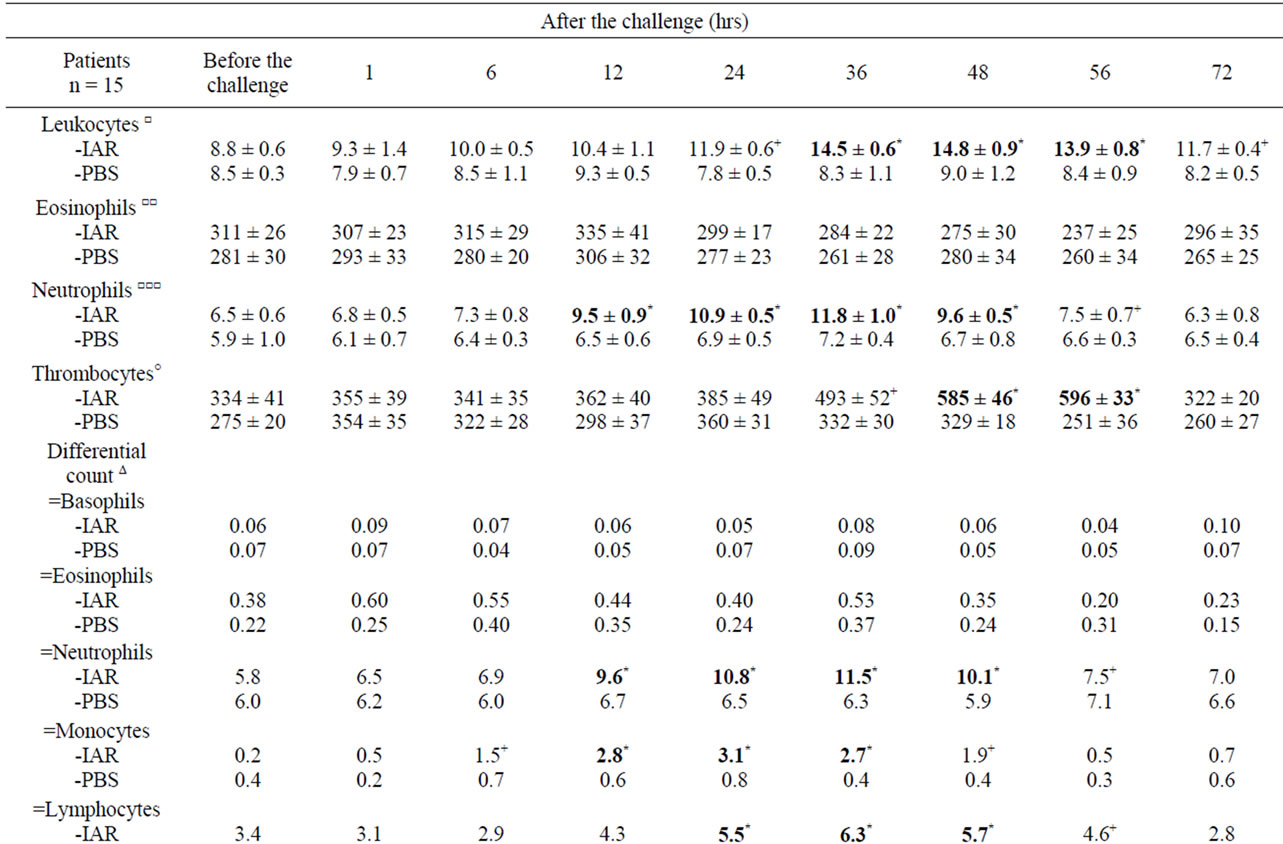
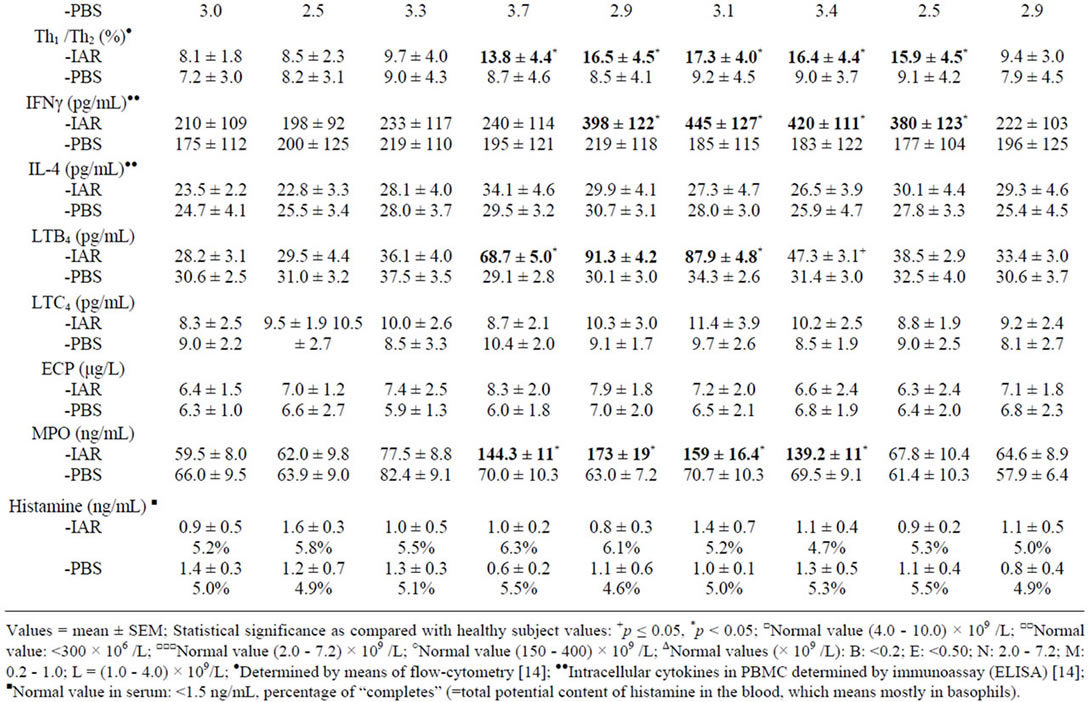
Table 5. Cellular counts and other factors in peripheral blood during the delayed asthmatic response (DYAR) and PBS controls.
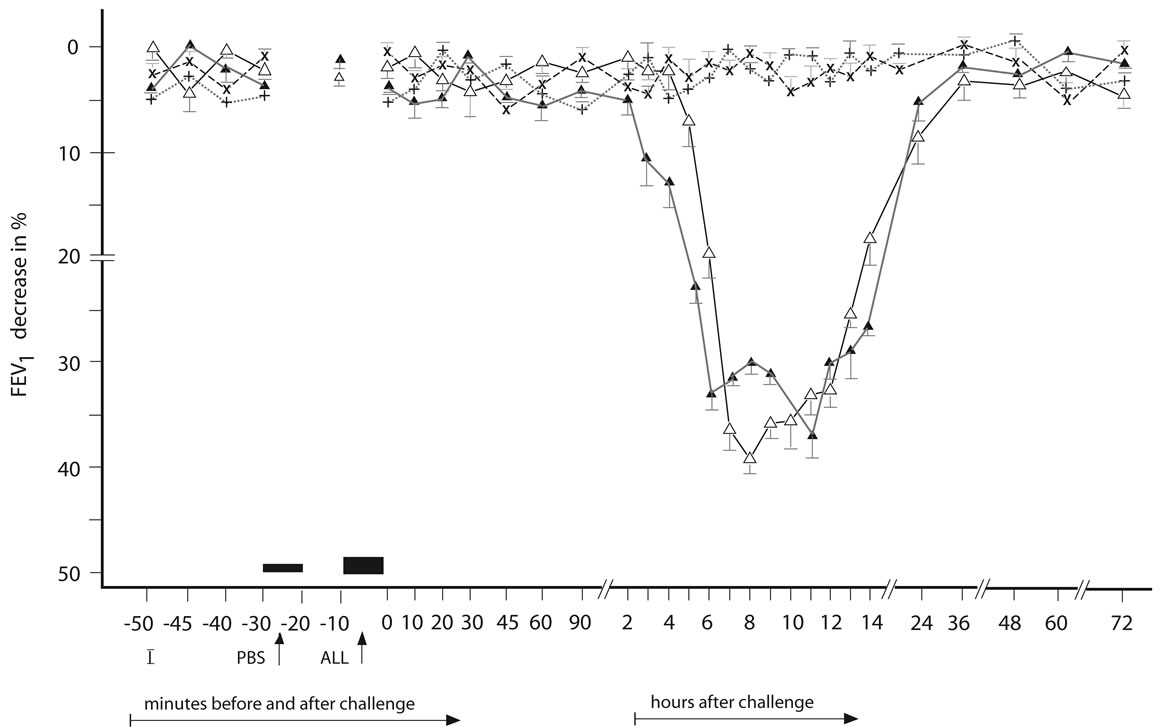
Figure 2. Late asthmatic response to allergen challenge (LAR) and phosphate buffered saline (PBS) control challenge. The mean percentage changes in the FEV1 values calculated from 26 LARs and 26 PBS control challenges; (∆) = the initial LAR; (▲) = the repeated LAR; (+) = the initial PBS; (x) = the repeated PBS; I = initial (baseline) values; ALL = allergen challenge; PBS = phosphate buffered saline; Bars = means ± SEM.
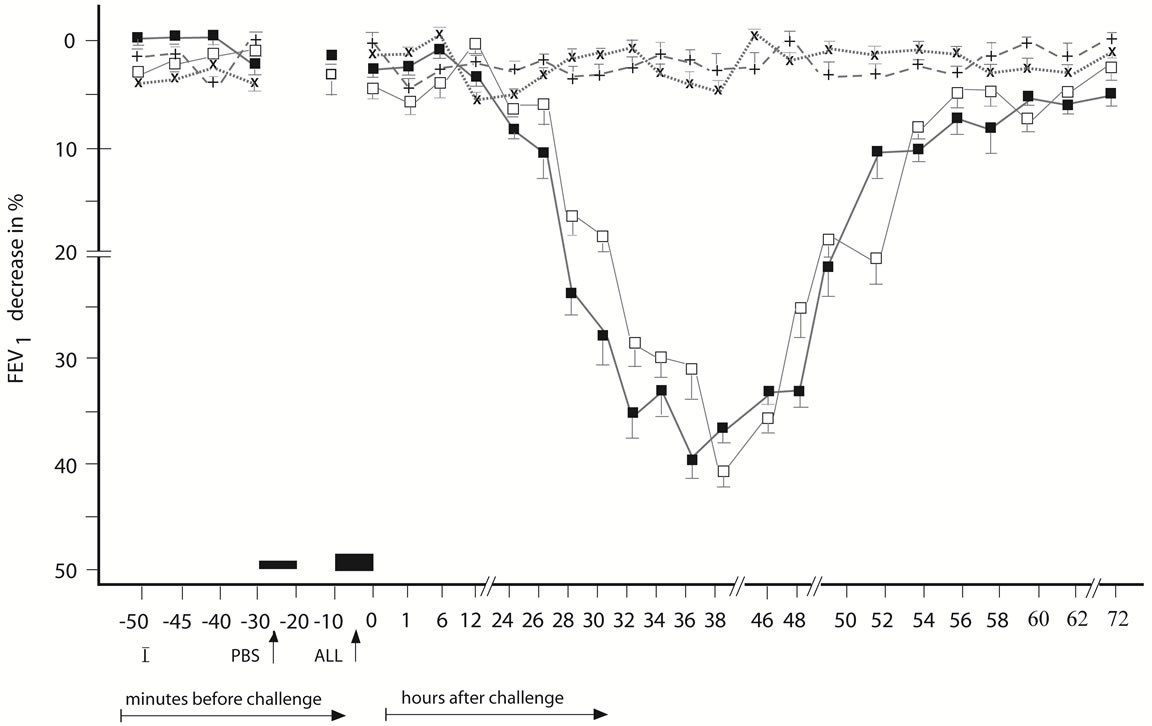
Figure 3. Delayed asthmatic response to allergen challenge (DYAR) and phosphate buffered saline (PBS) control challenge. The mean percentage changes in the FEV1 values calculated from 15 DYARs and 15 PBS control challenges; (□) = the initial DYAR; (■) = the repeated DYAR; (+) = the initial PBS; (x) = the repeated PBS; I = initial (baseline) values; ALL = allergen challenge; PBS = phosphate buffered saline; Bars = means ± SEM.
IL-4 at 30 and 45 minutes (p < 0.05), LTC4 at 30 and 45 minutes (p < 0.05) and ECP at 30 and 45 minutes (p < 0.01).
The LAR was accompanied by: 1) Increased of total leukocyte counts; 2) Changes in eosinophil and neutrophil counts; 3) Increased IL-4 in PBMC; 4) Increased plasma concentrations of LTB4 and LTC4 (Table 4). The increased eosinophil counts correlated with increased postchallenge concentrations of IL-4 at 2, 4, and 6 hours (p < 0.05) and LTC4 at 2, 4 and 6 hours (p < 0.01), whereas the increased neutrophil counts correlated with increased post-challenge concentrations of IL-4 at 6 hours (p < 0.05) and LTB4 at 6 and 8 hours (p < 0.05).
The DYAR was accompanied by: 1) Changes in total leukocyte counts; 2) Changes in neutrophil, monocytes, lymphocyte and thrombocyte counts; 3) Th1/Th2 ratio changes in favour of Th1; 4) Increased IFN-γ in PBMC; 5) Increased plasma LTB4 and MPO (Table 5). The increased neutrophil counts correlated with increased postchallenge serum concentrations of LTB4 at 12, 24 and 36 hours (p < 0.01) and MPO at 12, 24, 36 and 48 hours (p < 0.01), whereas the increased lymphocyte counts correlated with increased post-challenge concentrations of IFN-γ at 24, 36 and 48 hours (p < 0.01) and increased Th1/Th2 ratio in favour of Th1 cells at 24, 36 and 48 hours (p < 0.01).
No significant differences were found in the appearance of the individual asthmatic response types and their cellular profiles in peripheral blood with respect to the particular allergens (p > 0.1).
Control Subjects
Neither significant changes in the counts of any cell type nor significant changes in the concentrations of the other factors in blood were found in the control subjects after the PBS control challenges (p > 0.05).
4. Discussion
Patients with allergic BA when challenged with allergen, can develop various asthmatic response types, such as immediate/early response (IAR/EAR) [1-3,9-12], late response (LAR) [2,9,11-13,22,23], dual response (DLAR) [2,3,11-13], or as recently reported by us, a delayed response (DYAR) [14,15].
The role of particular circulating cell types in the IAR, LAR and DLAR, have been studied in the sputum, bronchoalveolar lavage (BAL) fluid and bronchial biopsies after the inhalational, segmental or intra-bronchial challenge with allergen [1-3,5-15,22-27].
The cellular changes in blood during the individual asthmatic response types have been investigated intensively in animals [28,29]. However, papers concerning repeated counting of the blood cell, especially in conjunction with their constituents in the blood, during the particular asthmatic response types in humans are not numerous [9,11,12,16,17,22-27]. Moreover, in most of these studies only some of the cell types were counted.
The studies concerning the cellular changes on the materials collected directly from the asthmatic lungs, such as (induced) sputum, BAL fluid or lung biopsies, provide important data related to the site of the immunologic processes, which is the bronchial tissue and bronchial lumen [6-9,30,31]. However, the bronchial tissue also has relationships with other organs, especially with the vascular system and blood, and immunologic events occurring in the bronchial tree may display reciprocal influence with the capillary network and circulating blood cells [1,5,6,10,13,26,30-33]. The inflammatory cells participating in the immunologic processes in the bronchial tissue are either cells recruited from the circulation into the airway tissues or the cells already resident in those tissues. Vice versa, some of the inflammatory cells after participating in the bronchial immunologic processes can re-migrate into the circulation, and various factors released in the bronchial tissue can penetrate into the circulation and affect there the circulating cells [1-3,5,6,9,10,13,22-27].
The role of a particular blood cell type in a certain immunologic process may be defined by a its morphologic and functional properties and by its temporary stage and location [6,8,9,26,30,34-36]. The cells can be involved in an immunologic process either as its participant or as its target. This role can be evaluated by various criteria: 1) The cell count (density) and its changes in time, related to a certain event, e.g. allergen exposure/challenge, representing the dynamic aspect of such involvement; 2) The activation degree of the cells, associated often with intracellular granule changes; 3) The ability of the cells to generate and release typical constituents during a certain period of the immunologic process; 4) the localization and kinetics of that cell type [1-3,7-10,13,23,26,27,30]. The satisfactory evaluation of the role of a certain cell type requires measurement of the representative parameters before and repeatedly after a well-defined event, such as bronchial challenge with an allergen [3,10-17].
The blood cell counting cannot be fully compared with other techniques, such as measurements of cells in the (induced) sputum, BAL fluid or bronchial biopsies, having also their advantages and disadvantages [10,24,31-36]. Their important advantage is the generation of highly specific data related directly to the immunologic event in the bronchial tree and mucosa. Their disadvantage includes relatively high variations in the cell counts and in amounts of the recovered fluid (e.g. BAL), the necessity of special facilities and personal skill, and some standardization problems [30-32,35]. Moreover, the BAL and lung biopsies represent also a certain burden and risk for the patient, and cannot be therefore repeated frequently within a short period of time [32-35].
Conversely, the blood cell counts can also be influenced by various extra-pulmonary factors and these data may therefore be less specific for the bronchial events than the cell counts in the sputum or BAL fluid. On the other hand, the blood cell counting is an easy, non-burdening, method, requiring no special facilities, which can be repeated without any limitation.
Our results demonstrating different kinetics of the individual cell types in peripheral blood during the particular asthmatic response types suggest involvement of different immunologic mechanisms in these types. Our results confirm also the existence of the so-called noneosinophilic or neutrophilic asthma phenotypes [4,6-8,27]. They also suggest the need to refine the classical interpretation of the blood eosinophilia as one of the most important indicator for the allergic bronchial asthma.
During the IAR, the eosinophil counts decreased after the BPT, followed by their increase, together with increased serum concentrations of ECP and LTC4. The LAR was accompanied by increased eosinophil counts and increased concentrations of LTC4, but not by changes of ECP. These findings suggest different involvement of eosinophils in the IAR and in the LAR. In contrast, no changes in eosinophil count were recorded during the DYAR [14-16,37,38].
Another interesting finding were increased neutrophil counts accompanied by concentration changes of LTB4 but not of MPO during the LAR, whereas increased neutrophil counts during the DYAR were associated with concentration changes both of LTB4 and MPO [13-17,37,38].
The changes in Th1/Th2 ratio in favour of Th2 and increased intracellular concentrations of IL-4, but not that of IFN-γ, during the IAR would suggest an active involvement of Th2-cells in immunologic mechanisms underlying this asthmatic response type.
The changes in Th1/Th2 ratio in favour of Th1 and increased intracellular concentrations of IFN-γ, but not that of IL-4, during the DYAR would indicate an important role of Th1-cells in the immunologic processes leading to the DYAR. The balanced Th1/Th2 ratio, upon increased IL-4 concentration, would point to an almost simultaneous involvement of Th1 and Th2 cells in mechanisms underlying the LAR [13-17,37,38].
Results this study would allow the following conclusions: 1) The IAR associated with count changes of the activated eosinophils, basophils and Th2-lymphocytes in the blood, within 120 minutes after the BPT, can be classified as a rapid and fully reversible functional event due to the classical IgE-mediated hypersensitivity mechanism [3,9-12,16,24,25-27,30]; 2) The LAR associated with count changes of the activated neutrophils, Th2-cells and eosinophils in the blood within 2 - 8 hours after the BPT, may be classified as a combination of a functional and a inflammatory event, suggesting involvement of mechanism(s) different from the classical IgE-mediated, but not yet sufficiently clarified, mechanism [2,10-13,17,22-27,36]; 3) The DYAR associated with changes in the blood counts of the activated neutrophils, monocytes, Th1-cells and thrombocytes within 24 - 56 hours after the BPT, may be considered an inflammatory even, suggesting involvement of the cell-mediated hypersensitivity mechanism(s) [14,15,26,28].
The serial monitoring of the blood cell counts during the BPTs can be helpful to discriminate the particular asthmatic response types and can act as an additional confirmation of the recorded asthmatic response type. This technique can also be combined with other techniques, if necessary.
REFERENCES
- S. T. Holgate, R. F. Lemanske, P. M. O’Byrne, et al., “Asthma Pathogenesis,” In: N. F. Adkinson, B. S. Bochner, W. W. Busse, S. T. Holgate, R. F. Lemanske, F. E. Simons, Eds., Middleton’s Allergy, Principles & Practice (7th ed.), Elsevier Inc., Philadelphia, 2009, pp. 893-919. doi:10.1016/B978-0-323-05659-5.00051-6
- A. M. Bentley, A. B. Kay and S.R. Durham, “Human Late Asthmatic Responses,” In: A. B. Kay, Ed., Allergy and Allergic Diseases, Blackwell Sci Ltd., Hoboken, 1997, pp. 1113-1129.
- Z. Pelikan, M. Pelikan-Filipek, M. Kruis and M. P. F. Berger, “The Immediate Asthmatic Response to Allergen Challenge,” Ann Allergy, Vol. 56, 1986, pp. 252-260.
- J. Douwes, P. Gibson, J. Pekkanen and N. Pearse, “NonEosinophilic Asthma: Importance and Possible Mechanisms,” Thorax, Vol. 57, No. 7, 2002, pp. 643-648. doi:10.1136/thorax.57.7.643
- T. J. Huang, P. A. MacAry, P. Eynott, A. Mousavi, K. C. Daniel, P. W. Askenase, D. M. Kemeny and K. F. Chung, “Allergen-Specific Th1 Cells Counteract Efferent Th2 Cell-Dependent Bronchial Hyperresponsiveness and Eosinophilic Inflammation Partly via IFN-γ,” Journal of Immunology, Vol. 166, No. 1, 2001, pp. 207-217.
- D. E. Shaw, M. A. Berry, B. Hargadon, et al., “Association between Neutrophilic Airway Inflammation and Airflow Limitation in Adults with Asthma,” Chest, Vol. 132, No. 6, 2007, pp. 1871-1875. doi:10.1378/chest.07-1047
- P. Haldar and I. D. Pavord, “Noneosinophilic Asthma: A Distinct Clinical and Pathologic Phenotype,” Journal of Allergy and Clinical Immunology, Vol. 119, No. 5, 2007, pp. 1043-1052. doi:10.1016/j.jaci.2007.02.042
- J. V. Fahy, “Eosinophilic and Neutrophilic Inflammation in Asthma,” Proceedings of the American Thoracic Society, Vol. 6, No. 3, 2009, pp. 256-259. doi:10.1513/pats.200808-087RM
- M. Yoshida, R. M. Watson, T. Rerecich and P. M. O’Byrne, “Different Profiles of T-Cell IFN-γ and IL-12 in Allergen-Induced Early and Dual Responders with Asthma,” Journal of Allergy and Clinical Immunology, Vol. 115, No. 5, 2005, pp. 1004-1009. doi:10.1016/j.jaci.2005.02.003
- A. J. Frew, J. St-Pierre and L. M. Teran, “Cellular and Mediator Responses Twenty-Four Hours after Local Endobronchial Allergen Challenge of Asthmatic Airways,” Journal of Allergy and Clinical Immunology, Vol. 98, No. 1, 1996, pp. 133-143. doi:10.1016/S0091-6749(96)70235-X
- L. Bancalari, F. L. Dente, S. Cianchetti, C. Prontera, M. Taccola, E. Bacci, A. Carletti, A. Di Franco, D. Giannini, B. Vagaggini, M. Ferdeghiani and P. L. Paggiare, “Blood Markers of Early and Late Airway Responses to Allergen in Asthmatic Subjects. Relationship with Functional Findings,” Allergy, Vol. 52, No. 1, 1997, pp. 32-40. doi:10.1111/j.1398-9995.1997.tb02543.x
- B. Pedersen, R. Dahl, B. B. Larsen and P. Venge, “The Effects of Salmeterol on the Earlyand Late-Phase Reaction to Bronchial Allergen and Postallergen Variation in Bronchial Reactivity, Blood Eosinophils, Serum Eosinophil Cationic Protein, and Serum Eosinophil Protein X,” Allergy, Vol. 48, No. 5, 1993, pp. 377-382. doi:10.1111/j.1398-9995.1993.tb02410.x
- Z. Pelikan and M. Pelikan-Filipek, “The Late Asthmatic Response to Allergen Challenge—Part I and II,” Annals of Allergy, Vol. 56, No. 5, 1986, pp. 414-420,421-435.
- Z. Pelikan, “Delayed Type of Asthmatic Response to Allergen Challenge. I. Clinical Features,” Annals of Allergy, Asthma & Immunology, Vol. 104, No. 5, 2010, pp. 394-404. doi:10.1016/j.anai.2010.03.014
- Z. Pelikan, “Delayed Asthmatic Response to Bronchial Challenge with Allergen and Mediators, Eicosanoids, Eosinophil and Neutrophil Constituents in Blood and Serum,” Respiration, Vol. 82, No. 3, 2011, pp. 225-236.
- M. Pelikan-Filipek, Y. A. Trimbach, M. Van Oosten, S. Fouchier and Z. Pelikan, “The Changes in the Count of Particular Cell Types in Blood during the Immediate/ Early Asthmatic Response [IAR/EAR] Due to the Allergen Challenge,” Allergy, Vol. 51, Suppl. 31, 1996, p. 29.
- Z. Pelikan and M. Pelikan-Filipek, “The Counts of Particular Cell Types and Their Changes in Blood Accompanying the Late Asthmatic Response [LAR] Due to the Allergen Challenge,” Allergy, Vol. 51, Suppl. 31, 1996, p. 118.
- T. J. H. Clark, C. B. Cagnani, J. Bousquet, et al., “Global Initiative for Asthma (GINA): Global Strategy for Asthma Management and Prevention. NHLBI/WHO Workshop Report,” NHI Publication No. 02-3659, Bethesda, Geneve, 2002.
- G. Melillo, S. Bonini, G. Cocco, R. J. Davies, R. G. De Monchy, L. Frolund and Z. Pelikan, “Provocation Tests with Allergens,” Allergy, Vol. 52, Suppl. 35, 1997, pp. 5-36.
- P. J. Sterk, L. M. Fabbri, P. H. Quanjer, D. W. Cockcroft, P. M. O’Byrne, S. D. Anderson, E. F. Juniper and J. L. Malo, “Airway Responsiveness. Standardized Challenge Testing with Pharmacological, Physical and Sensitizing Stimuli in Adults,” European Respiratory Journal, Vol. 16, 1993, pp. 53-83.
- D. J. Houd and C. C. Taylor, “Multivariate Analysis of Variance and Repeated Measures,” Chapman & Hall, London, 1987.
- S. Finotto, L. M. Fabbri, V. Rado, C. E. Mapp and P. Maestrelli, “Increase in Numbers of CD8 Positive Lymphocytes and Eosinophils in Peripheral Blood of Subjects with Late Asthmatic Reaction Induced by Toluene Diisocyanate,” British Journal of Industrial Medicine, Vol. 48, No. 2, 1991, pp. 116-121.
- W. O. Cookson, C. F. Craddock, M. K. Benson and S. R. Durham, “Falls in Peripheral Eosinophil Counts Parallel the Late Asthmatic Response,” American Journal of Respiratory and Critical Care Medicine, Vol. 139, No. 2, 1989, pp. 458-462. doi:10.1164/ajrccm/139.2.458
- W. J. Metzger, K. Nugent, H. B. Richerson, P. Moseley, R. Lakin and D. Zavala, “Methods for Bronchoalveolar Lavage in Asthmatic Patients Following Bronchoprovocation and Local Allergen Challenge,” Chest, Vol. 87, Suppl. 1, 1985, pp. 16S-19S. doi:10.1378/chest.87.1_Supplement.16S
- R. Dahl, P. Venge and I. Olsson, “Variations of Blood Eosinophils and Eosinophil Cationic Protein in Serum in Patients with Bronchial Asthma,” Allergy, Vol. 33, No. 4, 1978, pp. 211-215. doi:10.1111/j.1398-9995.1978.tb01536.x
- I. M. Ndukwu, E. T. Naureckas, C. Maxwell, M. Waldman and A. R. Leff, “Relationship of Cellular Transmigration and Airway Response after Allergen Challenge,” American Journal of Respiratory and Critical Care Medicine, Vol. 160, No. 5, 1999, pp. 1516-1524.
- B. Asman, V. Strand, G. Bylin and K. Bergström, “Peripheral Neutrophils after Allergic Asthmatic Reaction,” International Journal of Clinical & Laboratory Research, Vol. 27, No. 2-4, 1997, pp. 185-188. doi:10.1007/BF02912455
- T. Nabe, N. Shinoda, K. Yamashita, H. Yamamura and S. Kohno, “Leukocyte Kinesis in Blood, Bronchoalveoli and Nasal Cavities during Late Asthmatic Responses in Guinea-Pigs,” European Respiratory Journal, Vol. 11, No. 3, 1998, pp. 636-642.
- C. Fornhem, M. Kumlin, J. M. Lundberg and K. Alving, “Allergen-Induced Late-Phase Airways Obstruction in the Pig: Mediator Release and Eosinophil Recruitment,” European Respiratory Journal, Vol. 8, No. 7, 1995, pp. 1100-1109. doi:10.1183/09031936.95.08071100
- Q. Hamid, M. K. Tulic, M. C. Liu and R. Moqbel, “Inflammatory Cells in Asthma: Mechanisms and Implications for Therapy,” Journal of Allergy and Clinical Immunology, Vol. 111, No. 1, 2003, pp. S5-S17. doi:10.1067/mai.2003.22
- B. M. Saraiva-Romanholo, V. Barnabe, A. L. Carvalho, M. A. Martins, P. H. Saldiva and P. Nunes Mdo, “Comparison of Three Methods for Differential Cell Count in Induced Sputum,” Chest, Vol. 124, No. 3, 2003, pp. 1060-1066. doi:10.1378/chest.124.3.1060
- P. Maestrelli, M. Saetta, A. Di Stefano, P. G. Calcagni, G. Turato, M. P. Ruggieri, A. Roggeri, C. E. Mapp and L. M. Fabbri, “Comparison of Leukocyte Counts in Sputum, Bronchial Biopsies, and Bronchoalveolar Lavage,” American Journal of Respiratory and Critical Care Medicine, Vol. 152, No. 6, 1995, pp. 1926-1931.
- L. W. Poulter, A. Norris, C. Power, A. Condez, B. Schmekel and C. Burke, “T-Cell Dominated Inflammatory Reactions in the Bronchi of Asthmatics Are Not Reflected in Matched Bronchoalveolar Lavage Specimens,” European Respiratory Journal, Vol. 5, 1992, pp. 182-18.
- N. N. Jarjour, W. J. Calhoun, E. A. Kelly, G. J. Gleich, L. B. Schwartz and W. W. Busse, “The Immediate and Late Allergic Response to Segmental Bronchopulmonary Provocation in Asthma,” American Journal of Respiratory and Critical Care Medicine, Vol. 155, 1997, pp. 1515-1521.
- R. P. Baughman, “Technical Aspects of Bronchoalveolar Lavage: Recommendations for a Standard Procedure,” Seminars in Respiratory and Critical Care Medicine, Vol. 28, No. 5, 2007, pp. 475-485. doi:10.1055/s-2007-991520
- H. Imaoka, G. M. Gauvreau, R. M. Watson, T. Strinich, G. L. Obminski, K. Howie, K. J. Killian and P. M. O’Byrne, “Sputum Inflammatory Cells and Allergen-Induced Airway Responses in Allergic Asthmatic Subjects,” Allergy, Vol. 66, No. 8, 2011, pp. 1075-1080. doi:10.1111/j.1398-9995.2011.02588.x
- Z. Pelikan, “Delayed Type of Asthmatic Response to Allergen Challenge and Cytokines in the Peripheral Blood,” Respiration, Vol. 84, No. 5, 2012, pp. 385-395. doi:10.1159/000335258
- Z. Pelikan, “Chemokine Profiles in Blood Associated with Delayed Asthmatic Response to Allergen Challenge,” Respiratory Medicine, 2012. doi:10.1016/j.rmed.2012.09.013

