Open Journal of Modern Neurosurgery
Vol.4 No.1(2014), Article ID:41708,5 pages DOI:10.4236/ojmn.2014.41007
Central Neurocytoma and Epidermoid Tumor Occurring as Collision Tumors: A Rare Association
Peter Y. M. Woo1, Ho Hung Cheung1, Calvin H. K. Mak1, Siu Ki Chan2, Kar Ming Leung1, Kwong Yau Chan1
1Department of Neurosurgery, Kwong Wah Hospital, Hong Kong, China
2Department of Pathology, Kwong Wah Hospital, Hong Kong, China
Email: peterymwoo@gmail.com
Copyright © 2014 Peter Y. M. Woo et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Peter Y. M. Woo et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
Received December 1, 2013; revised January 1, 2014; accepted January 7, 2014
ABSTRACT
Different brain tumors of distinct histology can co-exist in the setting of phakomatoses or as a complication of radiotherapy. In the absence of these predisposing factors, this phenomenon is uncommon. When the lesions are in close proximity they are described as collision tumors and are extremely rare. A 58-year-old woman presented with persistent headache and cognitive decline for three months. Magnetic resonance imaging revealed a tumor arising from the atrium of the left lateral ventricle with heterogeneous contrast enhancement. This intraventricular lesion was adjacent to another extensive infiltrating tumor of the basal cisterns. Operative findings revealed a vascular ventricular tumor and gross total resection was achieved. An adjacent avascular basal cistern tumor with a pearly white sheen was encountered and partial excision was performed. The histopathological diagnosis was central neurocytoma and epidermoid tumor. There is only one documented description of a central neurocytoma co-existing with a tumor of different pathology. To our knowledge, this is the first reported collision tumor case involving central neurocytoma. Since the incidence of both lesions co-existing juxtaposed is extremely low, a chronic oncogenetic inflammatory process stimulated by the epidermoid tumor to the subventricular region is suggested. Other mechanisms for tumor collision are discussed and we suggest a classification system for this rare association to reflect their pathogenesis.
Keywords: Collision Tumors; Epidermoid Tumor; Central Neurocytoma
1. Introduction
Collision brain tumors refer to the condition when two or more synchronous lesions of distinct histology are in close proximity or in direct contact with each other [1,2]. This phenomenon is rare and provides a unique opportunity to understand the pathogenesis of such tumors. We report a patient with a concurrent central neurocytoma (CNC) adjacent to a diffuse basal cisternal epidermoid tumor that was initially believed to be a single lesion. Such a combination of collision brain tumors has not been described earlier. The radiological, intra-operative and pathological findings as well as a theory for their occurrence are discussed.
2. Case Report
A 58-year-old woman presented with persistent headache for three months and recent memory loss. Neurological examination detected right upper limb numbness and a cognitive deficit of 17/30 according to the Montreal Cognitive Assessment. Non-enhanced computed tomography (CT) scans showed a left intraventricular lesion with calcifications entrapping the left temporal horn and extending into the basal cisterns (Figure 1(a)). Magnetic resonance (MR) imaging revealed the lesion arising from the atrium of the left lateral ventricle and was believed to infiltrate through the choroidal fissure of the temporal horn into the extra-ventricular cisternal space. The intra-

Figure 1. Non-contrast enhanced CT image (a, axial) showing a left isodense intraventricular tumor with calcifications (white arrow) and entrapment of the left temporal horn of the lateral ventricle. There is also an extra-axial hypodense tumor of the interpeduncular cistern adjacent to the ventricular lesion. T1W MR images (b, c, axial) revealing an isointense, non-gadolium contrast enhancing basal cisternal lesion colliding with a ventricular tumor of heterogeneous enhancement. T2W MRI images (d, e axial; f, coronal) depict an extensive hyperintense cisternal tumor involving the interpeduncular, ambient, prepontine and bilateral cerebellopontine cisterns (arrow heads). The ventricular lesion was initially believed to have infiltrated the basal cisterns via the choroidal fissure of the temporal horn. DWI sequence (g, h, axial) showing restricted diffusion of the cisternal lesion, a characteristic of epidermoid tumors.
ventricular component of the tumor was of heterogeneous signal intensity with contrast enhancement on T1- weighted imaging (Figures 1(b) and (c)). The extra-ventricular component of the tumor extended into the ambient, interpeduncular, prepontine and cerebellopontine cisterns. There was markedly hypointensity on T1- and hyperintensity on T2-weighted sequences (Figures 1(d)-(f)). Diffusion weighted imaging (DWI) showed restricted diffusion (Figures 1(g) and (h)). Unlike the ventricular portion of the tumor no contrast enhancement was observed. The initial impression was a supratentorial ependymoma with extensive infiltration of the basal cisterns.
Excision of the ventricular portion of the tumor was performed adopting a lateral trans-temporal approach through the inferior temporal sulcus. A vascular intraventricular lesion was encountered and gross total resection was achieved (Figures 2(a) and (b)). The basal cisterns were entered via the medial wall of the temporal horn and a discrete extra-axial a vascular tumor of pearly white appearance was confronted with partial excision performed (Figures 2(c) and (d)).
Histopathology of the intraventricular tumor showed uniform round cells arranged in nests and perivascular pseudorosettes within areas of nucleus-free neuropil (Figures 3(a) and (b)). Cells possessed small round nuclei with finely speckled chromatin and mitoses were inconspicuous (Figure 3(c)). There was scant cytoplasm with a perinuclear halo. Occasional calcifications were also identified, but there was neither evidence of microvascular proliferation nor necrosis. Immunohistochemistry showed diffuse positivity for synaptophysin and S- 100 protein characteristic of central neurocytoma (Figures 4(a) and (b)). There were only small clusters of cells expressing glial fibrillary acidic protein (GFAP). The strong positivity for synaptophysin and relative poor reactivity for GFAP ruled out the main histological differential diagnoses of oligodendroglioma or ependymoma (Figure 4(c)). Cells were also negative for epithelial membrane antigen (EMA) that effectively excluded meningioma and adenocarcinoma (Figure 4(d)). The MIB-1 proliferative index was 2% and the final diagnosis was CNC (Figure 3(d)). The extra-ventricular tumor was composed of stratified squamous epithelium with lamellated keratinous material, features characteristic of an epidermoid tumor (Figures 5(a) and (b)).
3. Discussion
The synchronous occurrence of multiple primary brain tumors of distinct histopathology is rare with the reported incidence of 0.3% [3]. Meningiomas are the most frequently reported to co-exist with another tumor of different cell type and the majority does not collide [3,4]. Even more rare are collision brain tumors an ill-defined description where histologically discrete lesions are in such close proximity with each other that no discernible boundary exists between them on conventional imaging. When two tumors are juxtaposed to one another epidermoid tumors have been identified as the commonest col-

Figure 2. Intraoperative photograph showing a vascular intraventricular tumor arising from the atrium of the left lateral ventricle (a) and dissected away from the underlying ependyma (b, asterisk, tumor; triangle, ependyma). Entry into the ambient cistern revealed an extra-axial avascular pearly white tumor (c). During excision the previously encased basilar artery was exposed (d, white arrow).

Figure 3. Intraventricular tumor. Photomicrograph depicting isomorphous small cells within an arborizing vascular network and fibrillary background (a, H&E ×100). Cells arranged in nests with occasional perivascular pseudorosettes (white circle, b, H&E ×200). Round to oval nuclei with finely speckled chromatin and small nucleoli (c, H&E ×400). MIB-1 proliferative index of this section was 2% (d, ×200).
lision partner and is often associated with vestibular Schwannomas [2,3,5-9]. Only seven epidermoid tumor collision cases have been described previously and to our knowledge the concomitant occurrence with central neurocytoma has never been reported. This is also the second documented case where CNC is seen to co-exist with another tumor.
Multiple primary brain tumors of entirely different cell origins are known to be associated with phakomatoses or previous irradiation [3,5]. In the absence of these risk
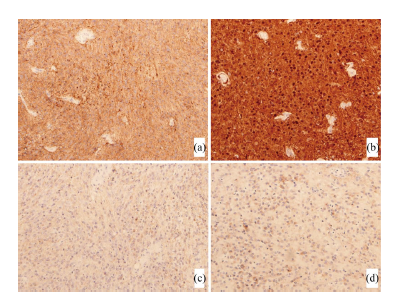
Figure 4. Intraventricular tumor. Diffuse reactivity to synaptophysin (a, ×100) and S100 protein (b, ×200) suggesting a neuronal tumor. Negative expression of GFAP (c, ×200) and EMA (d, ×200) effectively excludes glial tumors and meningiomas.

Figure 5. Cisternal tumor. Cyst lesion lined with stratified squamous epithelium and supported by dense fibrous tissue (a, H&E ×100). Lamellated keratinous material is present (b, H&E ×200).
factors and in the setting of collision tumors the phenomenon becomes extremely rare raising interesting questions on their pathogenesis. Epidermoid tumors, like cholesteatomas, arise in-utero when squamous epithelium are included in the neural tube when the neuroectoderm separates from the cutaneous ectoderm during the third to fifth week of gestation. They account for one per cent of primary brain tumors [2]. They are slow growing usually spreading along the natural cleavage planes of the subarachnoid space becoming symptomatic from 20 to 40 years of age [2]. In contrast central neurocytomas are World Health Organisation grade II tumors that develop postnatally [10,11]. They follow a benign clinical course with most patients presenting between the ages of 20 and 40 years. From large surgical series they account for 0.25% of brain tumors [10,11]. The relative rarity of these lesions renders the prospect of their co-existence by chance alone extremely low. Russell and Rubinstein were the first to propose that the presence of a tumor could provide the stimulus for adjacent brain parenchyma or meninges to provoke neoplasia of a different cell lineage [12]. Chronic irritation from repeated epidermoid cyst rupture of its the keratin-cholesterol rich contents could induce contiguous tissue oncogenesis. The close proximity of the ambient cistern to the atrium and temporal horn of the lateral ventricle via the choroidal fissure lays ground for this hypothesis [13]. Evidence suggests that central neurocytomas originate from adult bipotential progenitor cells of the subventricular zone (SVZ), otherwise known as the subependymal plate, located at the floor of the lateral ventricle [14]. Neural stem cells have been isolated from the SVZ, dentate gyrus, hippocampus and subcortical white matter [15-17]. These precursor cells are capable of dual differentiation to neurons or glia, but are strongly biased towards proneuronal transformation [10,14]. This explains the avid immuno-reactivity to synaptophysin, a reliable neuronal diagnostic biomarker although occasional small islands of GFAP positive cells can be observed reflecting astroglial differentiation of these precursor cells. It has long been suggested that the SVZ, the largest germinal region, is a possible source of gliomas [15,18,19] and CNC could represent the neurogenic manifestation of its oncologic potential [20].
We propose that the indolent inflammatory nature and the extensive cisternal infiltration of the epidermoid tumor could have driven neuroglial progenitor cells of the SVZ to oncogenesis. This hypothesis is supported by the frequent occurrence of epidermoid tumors in collision with other primary brain tumors including malignant brainstem gliomas [1,2,16]. The exact molecular pathogenesis of central neurocytomas remains enigmatic. Epidermal growth factor receptor (EGFR) amplification is rarely seen in CNC contrary to its frequent association with numerous gliomas [21]. There is increasing evidence that over activation of the signaling pathways involving insulin-like growth factor-2 (IGF-2), fibroblast growth factor- 2 (FGF-2) and platelet derived growth factor (PDGF) is crucial for CNC oncogenesis [11,14]. These cytokines are similarly over expressed during chronic oxidative stress in epidermoid epithelium allowing for hyperproliferation and promoting its ability to migrate [22]. The common reliance of these mitogens forms a preliminary biologic basis for our theory that epidermoid tumor-mediated irritation or paracrine stimulation of SVZ progenitor cells could result in CNC development. A similar reverse proposition was described whereby vestibular Schwannomas secreting paracrine factors, EGF and neuregulin, could stimulate dormant epithelial cell rests to form an epidermoid tumor [5].
Another form of brain tumor collision that falls under the same definition is metastasis from a primary malignancy to the site of a different tumor. This well-recognized phenomenon, although uncommon, is the prevalent mode of collision with more than eighty documented cases described [23]. Otherwise known as tumor-to-tumor metastasis meningiomas are implicated as the most frequently colonized brain tumor from malignancies such as bronchogenic or breast carcinoma [4,23]. Diagnostic criteria include: a) the existence of at least two primary tumors; b) the metastatic focus must be at least partially enclosed by a rim of histologically distinct host tumor tissue with evidence of established growth within it; c) the confirmation of a primary carcinoma with compatible metastasis [4]. Several theories, the high incidence and slow growth rate of meningiomas not withstanding, support the unique biologic nature of meningothelial cells to interact with metastatic tumor cells [23]. The high expression of e-cadherin, a cell adhesion molecule, in addition to the up regulation of estrogen receptors in meningiomas and breast adenocarcinomas are likely to play an important role [24,25].
An alternative collision mechanism is when two distinct primary tumors both spread distally usually hematogenously, to the same anatomical location. Palka et al first coined the term “collision metastasis” to describe this condition in a patient with bronchogenic small cell carcinoma that metastasized to a left frontal lobe melanoma secondary [26]. Due to the benign nature of CNC and epidermoid tumors it is unlikely that metastatic spread was accountable for this patient’s condition. In summary we propose a classification system for collision tumors that may reflect differing pathogenesis mechanisms into the following: juxtapositional collision, where epidermoid tumors predominate as in our case; tumor-totumor collision, where meningiomas are commonly involved as the recipient tumor and finally collision metastasis, which is the rarest manifestation.
4. Conclusion
This case is an example of the possible oncogenic potential of epidermoid tumors to induce central neurocytomas. The frequency of epidermoid tumors as a common juxtapositional collision partner, the relative rarity of central neurocytomas and the possible mechanism of chemical irritation to explain its etiology support this theory rather than co-incidental casual occurrence. We also suggest a new system for classifying collision tumors of the central nervous system to account for various collision pathogenesis processes.
[1] REFERENCES
[2] Shah, A., Goel, A. and Goel, N. (2010) A case of cerebellopontine angle epidermoid tumor and brainstem squamous cell carcinoma presenting as collision tumor. Acta Neurochir (Wien), 152, 1087-1088. http://dx.doi.org/10.1007/s00701-010-0606-9
[3] Muzumdar, D.P., Goel, A. and Desai, K.I. (2001) Pontine glioma and cerebellopontine angle epidermoid tumour occurring as collision tumours. British Journal of Neurosurgery, 15, 68-71. http://dx.doi.org/10.1080/026886901300004157
[4] Singh, D.K., Singh, N., Parihar, A., et al. (2013) Craniopharyngioma and epidermoid tumour in same child: A rare association. BMJ Case Reports.
[5] Takei, H. and Powell, S.Z. (2009) Tumor-to-tumor metastasis to the central nervous system. Neuropathology, 29, 303-308. http://dx.doi.org/10.1111/j.1440-1789.2008.00952.x
[6] Zhao, A.S., Lee, H.J. and Jyung, R.W. (2010) Concomitant, contralateral vestibular schwannoma and epidermoid cyst. Laryngoscope, 120, S220. http://dx.doi.org/10.1002/lary.21687
[7] Kleinpeter, G., Matula, C. and Koos, W. (1994) Another case of acoustic schwannoma and epidermoid cyst occurring as a single cerebellopontine angle mass: Possibly not so rare? Surgical Neurology, 41, 310-312. http://dx.doi.org/10.1016/0090-3019(94)90180-5
[8] Goodman, R.R., Torres, R.A. and McMurtry, J.G. (1991) Acoustic schwannoma and epidermoid cyst occurring as a single cerebellopontine angle mass. Neurosurgery, 28, 433-436. http://dx.doi.org/10.1227/00006123-199103000-00017
[9] Saito, A., Sugawara, T., Watanabe, R., et al. (2009) Evolution of vestibular schwannoma after removal of epidermoid cyst of the same location: Case report. Neurologia Medico-Chirurgica (Tokyo), 49, 495-498. http://dx.doi.org/10.2176/nmc.49.495
[10] Kitaoka, K., Abe, H., Tashiro, K., et al. (1986) The coexistence of basal epidermoid tumor and trigeminal neurinoma within the posterior fossa. No Shinkei Geka, 14, 1243- 1248.
[11] Figarella-Branger, D., Soylemezoglu, F. and Burger, P.C. (2007) Central neurocytoma and extraventricular neurocytoma. In: Louis, D.N., Ohgaki, H., Wiestler, O.D., et al., Eds., WHO Classification of Tumours of the Central Nervous System, 3rd Edition, World Health Organization Press IARC, Lyon, 106-109.
[12] Schmidt, R.F. and Liu, J.K. (2013) Update on the diagnosis, pathogenesis, and treatment strategies for central neurocytoma. Journal of Clinical Neuroscience, 20, 1193- 1199. http://dx.doi.org/10.1016/j.jocn.2013.01.001
[13] Russell, D.S. and Rubinstein, L.J. (1971) Pathology of tumors of the nervous system. 3rd Edition, Williams & Wilkins, Baltimore, 1971.
[14] Nagata, S., Rhoton Jr., A.L. and Barry, M. (1988) Microsurgical anatomy of the choroidal fissure. Surgical Neurology, 30, 3-59. http://dx.doi.org/10.1016/0090-3019(88)90180-2
[15] Sim, F.J., Keyoung, H.M., Goldman, J.E., et al. (2006) Neurocytoma is a tumor of adult neuronal progenitor cells. Journal of Neuroscience, 26, 12544-12555. http://dx.doi.org/10.1523/JNEUROSCI.0829-06.2006
[16] Sanai, N., Alvarez-Buylla, A. and Berger, M.S. (2005) Neural stem cells and the origin of gliomas. New England Journal of Medicine, 353, 811-822. http://dx.doi.org/10.1056/NEJMra043666
[17] Nunes, M.C., Roy, N.S., Keyoung, H.M., et al. (2003) Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Natural Medicine, 9, 439-447. http://dx.doi.org/10.1038/nm837
[18] Eriksson, P.S., Perfilieva, E., Bjork-Eriksson, T., et al. (1998) Neurogenesis in the adult human hippocampus. Natural Medicine, 4, 1313-1317. http://dx.doi.org/10.1038/3305
[19] Lim, D.A., Cha, S., Mayo, M.C., et al. (2007) Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Journal of Neuro-Oncology, 9, 424-429. http://dx.doi.org/10.1215/15228517-2007-023
[20] Waters, D., Newman, B. and Levy, M.L. (2010) Stem cell origin of brain tumors. In: Jandial, R., Ed., Frontiers in Brain Repair, Springer, New York, 58-65. http://dx.doi.org/10.1007/978-1-4419-5819-8_5
[21] Horoupian, D.S., Shuster, D.L., Kaarsoo-Herrick, M., et al. (1997) Central neurocytoma: One associated with a fourth ventricular PNET/medulloblastoma and the second mixed with adipose tissue. Human Pathology, 28, 1111- 1114. http://dx.doi.org/10.1016/S0046-8177(97)90066-6
[22] Tong, C.Y., Ng, H.K., Pang, J.C., et al. (2000) Central neurocytomas are genetically distinct from oligodendrogliomas and neuroblastomas. Histopathology, 37, 160-165. http://dx.doi.org/10.1046/j.1365-2559.2000.00977.x
[23] Albino, A.P. and Parisier, S.C. (1999) The enigmatic biology of human cholesteatoma. In: Ars, B., Ed., Pathogenesis in Cholesteatoma, Kugler Publications, The Hague, 37-65.
[24] Moody, P., Murtagh, K., Piduru, S., et al. (2012) Tumorto-tumor metastasis: pathology and neuroimaging considerations. International Journal of Clinical and Experimental Pathology, 5, 367-373.
[25] Watanabe, T., Fujisawa, H., Hasegawa, M., et al. (2002) Metastasis of breast cancer to intracranial meningioma: Case report. American Journal of Clinical Oncology, 25, 414-417. http://dx.doi.org/10.1097/00000421-200208000-00019
[26] Caroli, E., Salvati, M., Giangaspero, F., et al. (2006) Intrameningioma metastasis as first clinical manifestation of occult primary breast carcinoma. Neurosurgical Reviews, 29, 49-54. http://dx.doi.org/10.1007/s10143-005-0395-4
[27] Palka, K.T., Lebow, R.L., Weaver, K.D., et al. (2008) Intracranial collision metastases of small-cell lung cancer and malignant melanoma. Journal of Clinical Oncology, 26, 2042-2046. http://dx.doi.org/10.1200/JCO.2007.14.5540

