Open Journal of Rheumatology and Autoimmune Diseases
Vol.3 No.1(2013), Article ID:27948,7 pages DOI:10.4236/ojra.2013.31007
Molecular Mimicry, the Hygiene Hypothesis, Stealth Infections and Other Examples of Disconnect between Medical Research and the Practice of Clinical Medicine in Autoimmune Disease
![]()
University of Bridgeport, Division of Health Sciences, Bridgeport, CT, USA
Email: dbrady@bridgeport.edu
Received November 12th, 2012; revised December 20th, 2012; accepted December 29th, 2012
Keywords: Autoimmunity; Inflammation; Arthritis; Mimicry; Hygiene
ABSTRACT
Autoimmune disorders have been on a steep rise in the industrialized countries over the past several decades and while research has been starting to develop a detailed understanding of pathophysiology and many of the underlying mechanisms, any meaningful incorporation of this information into clinical medicine has been painfully slow. Concepts of molecular mimicry, the hygiene hypothesis, intestinal hyper-permeability (leaky gut syndrome) and aggressive use of predictive antibody testing are explored in this article with examples given on how emerging information on these phenomena may aid the clinician in a new, more proactive, approach to management of these conditions.
1. Introduction
There is simply no doubt that the incidence of autoimmune disorders has been rising sharply over the past several decades in the Western industrialized countries, particularly the United States (see Figure 1) [1]. A broad array of disorders considered immune-dysregulatory and autoimmune in nature, encompassing both those classically categorized as Th1 and Th2-dominant, are included in this phenomenon. The question is why has there been such a sharp rise in the incidence of these disorders? The answers may very well be found in the current medical research, but you would probably never know it by visiting a doctor. This may be because this situation serves as an example of the giant chasm that often exists between medical research, which is often outstanding, and the practice of clinical medicine, which often leaves quite a bit to be desired when it comes to the management of chronic disorders with high morbidity but low mortality.
The typical allopathic clinical approach to autoimmune disorders focuses on the management of symptoms with various anti-inflammatory medications and often the use of chemotherapeutics, and very potent immuno suppressive agents with nasty potential side-effects like leukemia and lymphoma [2]. While these approaches admittedly can provide substantial symptomatic relief to the patient, they do not really get to the cause of these conditions and some research suggests that these approaches may result in a furthering of the pathological process. However, modern research into autoimmune phenomenon suggest radically different approaches may be required to reverse the above cited trends, including a strong emphasis on very early detection with predictive auto-antibodies, a focus on optimizing gastrointestinal
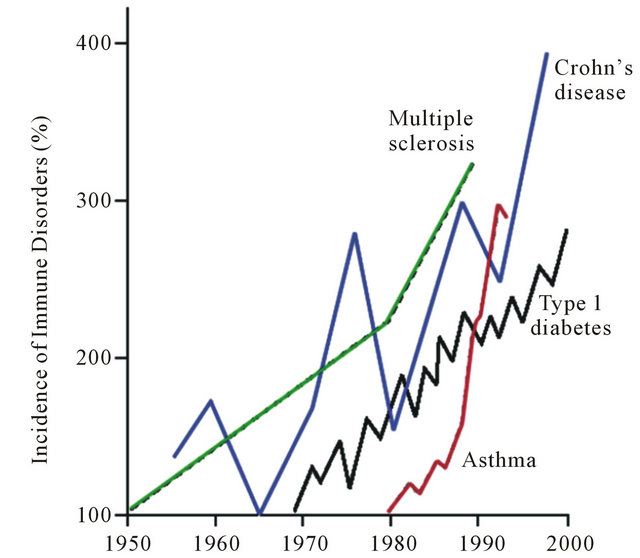
Figure 1. Rising incidence of autoimmune disorders [1].
mucosal immune function and the microbiome, eradication of infectious agent triggers with antimicrobial therapy, and even the seemingly bizarre use of parasitic agents therapeutically. Some of these concepts have a long history in naturopathic and functional models of medicine, but now are emerging as hot areas of emphasis in mainstream medical research journals and investigative communities in immunology.
2. Molecular Mimicry
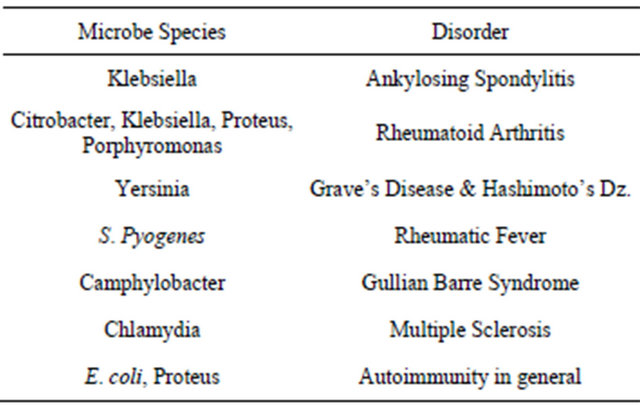
The concept of molecular mimicry is really a simple one, and it is an area attracting considerable research related to the genesis of autoimmune disorders. Simply stated, environmental exposure to specific antigens (including dietary peptides and those expressed by microbes), can in genetically susceptible individuals induce cross-reactions with structurally similar amino acid motifs associated with specific host tissues. There are now multitudes of associations that have been firmly established between immune incompatibility with specific dietary-derived antigens, as well as the overgrowth of certain opportunistic and pathogenic gastrointestinal bacteria, and the presence of specific autoimmune disorders (see Table 1) [3]. While some of these associations have been known for quite some time, mechanisms of causality are rapidly being established in the research. However, patients suffering from disorders like rheumatoid arthritis (RA), ankylosing spondylitis (AS), and autoimmune thyroiditis (i.e., Hashimoto’s or Grave’s disease) who visit a rheumatologist or endocrinologist do not routinely have stool analysis of their GI microbiota or food sensitivity testing performed. This is ironic, particularly in the case of opportunistic microbial overgrowth in the gut, as the conventional medical paradigm typically assumes an infectious cause, doesn’t it? Perhaps this is just another example of resistance to significant change in clinical approach within medicine, even in the face of compelling evidence to do so, as it would then require a least a passive admission that something so seemingly simple was missed for so long.
Pishak et al. have demonstrated that the mucous membrane of healthy people is colonized by Bifidobacteria, Lactobacilli, Bacteroides, Escherichia and Enterococci; as contrasted with the mucous membrane in RA subjects which is mainly colonized by aerobic opportunistic conventionally pathogenic enterobacteria (i.e., enteropathogenic Escherichia, Citrobacter, Enterobacter, Klebsiella, etc.), Staphylococci, Enterococci and other anaerobic bacteria (Bacteroides, Peptococci, Peptostreptococci, etc.) [4]. They have also reported the observed phenomenon that as RA exacerbates and then enters remission, which is a common occurrence across the spectrum of autoimmune disorders, the composition of the subject’s GI miTable 1. Selected associations of microbial overgrowth and autoimmune disorders [3].
crobiota correspondingly also changes between the aberrant pattern detailed above and the one typical of normal subjects. The data of Tiwana et al. suggests an increased immune response to Klebsiella in patients with AS, ulcerative colitis (UC), and Crohn’s disease (CD) and to Proteus in patients with RA [5]. Alan Ebringer and his group in the United Kingdom have established over the course of many years that a substantial percentage of patients diagnosed with RA have chronic stealth infection with Proteus mirabilis in the upper urinary tract [6]. His group has also established the specific amino acid motifs of cross-reaction between the Proteus hemolysin and the RA-associated HLA-DR molecules, as well as those between the Proteus urease enzyme and hyaline cartilage, containing type XI collagen, the type only found in the small joints affected in RA. His successful treatment protocol includes antibiotic therapy, such as ciprofloxacin (sometimes in combination with NSAIDs, DMARDS, and immunosuppressive agents as needed), with the added use of natural blocking agents such as cranberry juice, vitamin C for urine acidification, and plenty of fluids [7].
Oral bacterial infection with Porphyromonas gingivalis, the primary cause of periodontal disease, may also play a role in peptide citrullination, theorized to be involved in the loss of self-tolerance and development of autoimmunity in RA, according to Liao et al. [8]. Clearly one of the challenges to the acceptance of bacterial and viral agents as the cause of these autoimmune diseases has been that there is no universally observed association or one specific universally-causative agent. This issue is addressed head-on in research by Harkiolaki et al. using a mouse model of multiple sclerosis (MS) when he states: “We show that a microbial peptide, common to several major classes of bacteria, can induce MS-like disease in humanized mice by cross-reacting with a T cell receptor (TCR) that also recognizes a peptide from myelin basic protein, a candidate MS auto-antigen. Structural analysis demonstrates this cross-reactivity is due to structural mimicry of a binding hotspot shared by self and microbial antigens, rather than to a degenerate TCR recognition. Thus, these data suggest a possible explanation for the difficulty in incriminating individual infections in the development of MS.” [9]. A similar phenomenon is also likely in play across a multitude of autoimmune disorders, and is not something unique to MS.
Researchers have now gone beyond establishing mere associations between the presence of various microbes and autoimmune disorders. Some have actually experimentally induced autoimmune disease by infecting animals with specific pathogens. Mazmanian et al. inoculated a wild-type mouse with the bacterium Helicobacter hepaticus to create an experimental mouse version of the autoimmune disorder inflammatory bowel disease (IBD) [10]. H. hepaticus activates Th17 cells which release cytokines associated with inflammation, such as IL-17, which cause symptoms of the disease. They then introduced Bacteroides fragilis expressing the polysaccharide A (PSA) to the gut of the animals where the PSA molecule was taken up by dentritic cells and presented on their surface, activating CD4 T cells and regulatory T cells (Tregs). The Tregs release IL-10 which suppresses the inflammatory action of IL-17, alleviating the IBD in mice. In summary, the researches induced autoimmune disease by introducing specific bacteria to the gut, and resolved it by introducing another, making a compelling argument for a causal relationship between the GI microbiota and autoimmune activity.
Autoimmune thyroid disorders also have been linked to bacterial and viral infections, mainly GI overgrowth of the opportunistic organism Yersinia enterocolitica. Petru et al state; “Yersinia shows on its surface saturable binding sites for TSH. TSH receptor antibodies could be produced in selected individuals having been infected with bacteria showing TSH receptors. It may, therefore, be assumed that the gram-negative bacterium Yersinia enterocolitica may have an active part in triggering immunogenic thyroid diseases” [11]. Other researchers have shown a much higher prevalence of Yesinia serum antibodies in patients with thyroid disease versus controls. However, once again, there is no universal causality established, as autoimmune phenomena is a complex issue and seems to be potentially fueled by a multitude of potential antecedents, triggers, and mediators. For example, dietary antigens have also been linked to autoimmune thyroid disease. Celiac patients have approximately 10 times the rate of auto-immune thyroid diseases (such as Hashimoto’s thyroiditis and Grave’s disease) as nonceliac individuals, reflective of the affinity of gluten-gliadin antigen-antibody complexes for thyroid tissue [12]. It may be no coincidence that the emergence of an apparent epidemic of autoimmune diseases has corresponded with the ever-increasing consumption of poor-quality modern processed foods known to both negatively alter the GI microbiome and to contain a constant (often hidden) stream of offending dietary antigens, including gluten-containing grains.
While all of these associations may be interesting to researchers, what does this really mean to a clinician? Some critics would argue that there is a lack of interventional data to suggest eradication of these associated organisms and/or avoidance of these dietary antigens positively affects patient outcomes. This may be true in some instances, but it has been well established, for instance, by Ebringer that successful treatment of Proteus clinically helps those with RA [7], and dietary elimination of gluten-containing grains is entirely accepted as the most viable intervention in Celiac disease. One potential issue in play is that by the time a patient is diagnosed with autoimmune disease there is often already substantial host-tissue damage. Perhaps the horse has already left the barn? However, what if potential triggers were routinely screened for and removed by health care providers, particularly in those with a family history of autoimmune disorders? The entire course of the disorder might be favorably altered, and many of these disorders might potentially never emerge clinically. In the naturopathic and functional medicine models, there is a strong emphasis on both early detection and interventions that target the underlying pathophysiologic basis and underlying dysfunction of a disease process. Therefore, in these models the goal is to take clinical actions to reduce the potential for the disease process to progress. This also seems to intuitively make sense even in those who already have established disease; even though you may not be able to undo the damage already done, you can likely—if nothing else, slow down the train. This is particularly true since the interventions required pose little or no risk and are also relatively inexpensive; including probiotics, antimicrobial botanicals and volatile oils, mucosal-supporting nutrients and botanicals, and dietary modulation. Substantially improved molecular methods to assess the GI microbiota, utilizing PCR-DNA analysis, are also now available to clinicians at relatively low cost with rapid turn-around time [13].
3. The Hygiene Hypothesis
The concept of the hygiene hypothesis is also one that is quite simple, with the complexity being in the details. The thought that we have induced dysregulation into our immune system’s by virtue of living in too clean of an environment and the over eradication of infection is not new (see Figure 1), but it has gained favor with researchers who have begun to work out exactly why this may be the case. Some of these concepts were elegantly addressed by Weiss in an editorial in the New England Journal of Medicine entitled Eat Dirt-The Hygiene Hypothesis and Allergic Disease [14]. While there is no doubt that modern public health measures, such as adequate sewage systems, water treatment, the use of antibiotic agents, and various other aspects of modern hygiene have lessened deadly infectious outbreaks and have prevented unnecessary deaths. However, as with most things, there is a yin and yang. This “new clean world” has likely resulted in a lack of adequate sampling of our environment, including a lack of exposure to all of the microbes that we share our planet with, particularly while we are young and our immune systems are developing the delicate balance between adequate defense and tolerance. In a 2010 paper in Nature Reviews-Immunology entitled Farm Living-Effects of Childhood Asthma and Allergy authors Mutius and Vercelli state: “Numerous epidemiological studies have shown that children who grow up on traditional farms are protected from asthma, hay fever and allergic sensitization. Early-life contact with livestock and their fodder, and consumption of unprocessed cow’s milk have been identified as the most protective exposures. Studies of the immunobiology of farm living point to activation and modulation of innate and adaptive immune responses by intense microbial exposures and possibly xenogenic signals delivered before or soon after birth” [15]. Does this mean that our children who are: 1) growing up in more urban and suburban environments; 2) living in comparatively sterile homes; 3) drinking chlorinated water; 4) being bathed and scrubbed daily with anti-bacterial soap; 5) not being allowed to play in the dirt; 6) being given antibiotics every time they have a sniffle… are actually being harmed from an immunologic perspective and will carry this dysfunction with them throughout their entire lives? This is likely the case, and one of the reasons why, as parents of two young boys, my wife and I constantly try and balance the need for cleanliness with allowing them to be children and dig in the dirt, play in the stream in our backyard, and otherwise sample their living environment.
4. The Role of Parasites
As reported by David Gutierrez in Natural News, researchers in a study conducted at the University of Nottingham, point out that humans and gastrointestinal parasites might have co-evolved in a way that the parasites actually help regulate the human immune system to prevent allergies [16]. They believe that over the course of millions of years, gastrointestinal parasites have evolved the ability to suppress the human immune system as a survival mechanism. Because parasitic infestation has been so common throughout human evolutionary history, the human immune system has in turn evolved to compensate for this effect. This means that if the parasites are removed, the immune system may actually function too strongly, resulting in maladaptive immune responses such as asthma, allergies, and eczema. To test this concept the researchers studied over 1500 children in rural villages in Vietnam where parasitic infestation with hookworm is extremely common and allergies are not. Eradication of parasitic infection resulted in skyrocketing incidence of allergy, including dust mite sensitivity, supporting the hypothesis that parasites were modeling their immune response.
With issues such as the hygiene hypothesis, and the role of parasites in immune function in mind, gastroenterologist and researcher Dr. Joel Weinstock, originally at the University of Iowa, and now Tufts University, has performed novel work with subjects with inflammatory bowel disease (IBD) [17]. IBD was unheard of before the 20th century. Beginning of 20th century incidence is thought to be about 1:10,000 and is now 1:250. Similar data exists with the incidences of asthma, hay fever, DM, MS, etc. Weinstock conducted various studies of IBD patients and treated them with the therapeutic parasite Trichuris suis, a porcine whipworm, which was an ideal choice as it only remains viable in the human GI tract for a short time and must be continually administered. The organism, when introduced into patients with IBD; 1) induced changes in regulatory T cell function; 2) blocked T cell proliferation; 3) altered cytokine production and expression of innate immunity; 4) altered the intestinal flora; and 5) generally produced a lessening of symptoms and severity of disease. Pharmaceutical agents are now being developed along these lines to treat IBD.
5. Intestinal Hyper-Permeability (Aka: “Leaky Gut Syndrome”)
Leaky gut syndrome for much of the past twenty years seemed something that just functional medicine doctors talked about. Not any longer! Prestigious researchers such as Alessio Fasano at the University of Maryland, have been researching the role of intestinal permeability in the pathogenesis of autoimmune disorders and bringing this concept full-speed to the conventional medical research community through his publications in top-tier immunology and gastroenterology journals [18]. In a 2009 article in Scientific American he eloquently brought the topic to the lay audience with his article Surprises from Celiac Disease, where he described that his theory that leaky gut contributes to Celiac disease and autoimmunity was initially greeted with skepticism by his colleagues [19]. Fasano has proposed that in order for autoimmune disease to manifest there must be three factors present, and he equates these to a triangle, or three-legged stool, where if any are not present the disease cannot exist. These three factors include; 1) an environmental trigger (i.e., antigen), 2) genetic susceptibility (i.e., an HLA pattern that is particularly efficient at presenting the antigen to the immune cells, such as the presence of the HLA-DQ2 and HLA-DQ8 pattern in celiac disease), and 3) intestinal hyper-permeability (i.e., “leaky gut syndrome”). He goes on to opine that by far the easiest of these three factors to alter clinically is intestinal permeability. Much of his work involves the study, and future therapeutic manipulation, of a protein which alters intestinal permeability by the name of zonulin.
Sapone et al., from a paper in Diabetes in 2006, expands on the role of zonulin and leaky gut in autoimmune disorders saying, “Zonulin, a protein that modulates intestinal permeability, is upregulated in several autoimmune diseases and is involved in the pathogenesis of autoimmune diabetes. Zonulin upregulation seems to precede the onset of the disease, providing a possible link between increased intestinal permeability, environmental exposure to non-self antigens, and the development of autoimmunity in genetically susceptible individuals [20]”. Fasano summarizes the role of intestinal mucosal health and hyper-permeability in autoimmunity best in a 2005 paper when he states, “Together with the gut-associated lymphoid tissue and the neuroendocrine network, the intestinal epithelial barrier, with its intercellular tight junctions, controls the equilibrium between tolerance and immunity to nonself-antigens. When the finely tuned trafficking of macromolecules is dysregulated in genetically susceptible individuals, both intestinal and extraintestinal autoimmune disorders can occur [18].”
Functional medicine and naturopathic physicians, and other nutritionally-minded providers, have been addressing the issue of leaky gut for a long time with effective natural agents, including; L-glutamine, N-acetyl-glucosamine, anti-inflammatory botanicals and bioflavonoids, mucilaginous herbs, zinc-carnosine, omega-3-fatty acids and more. However, one popular nutrient that is used frequently as an immune modulator in autoimmune conditions is vitamin D. However, most clinicians are not aware of the role vitamin D plays directly in intestinal permeability. According to Kong et al. in their 2008 paper entitled Novel Role of the Vitamin D Receptor in Maintaining the Integrity of the Gastrointestinal Barrier, “In vitro experiments demonstrate that the VDR mediates the activity of 1,25(OH)2D3 that induces junction protein expression and strengthens the tight junction complex. These data are consistent with, and explain at least in part, the observation reported in the literature that vitamin D deficiency is linked to increased incidence of IBD in human population [21].”
Another possible role for vitamin D in the treatment of autoimmune disease, including MS, involves antimicrobial action. In addition to the previously cited findings by Harkiolaki et al. regarding molecular mimicry induced by various GI bacteria in MS [9], researchers like Dr. Charles Strattonat Vanderbilt University have made clear associations between MS and Chlamydia pneumonia [22], and others, including Dr. Donald Gilden, have implicated various viral triggers in MS [23]. It has also been shown that the human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25 dihydroxy vitamin D3 [24]. Meaning, as vitamin D levels rise, so does the production of this endogenous antimicrobial peptide in the body, and this may account for some of the clinical benefit observed with vitamin D therapy in MS and other autoimmune disorders.
6. Predictive Autoantibody Testing (A True Application of Preventive Medicine?)
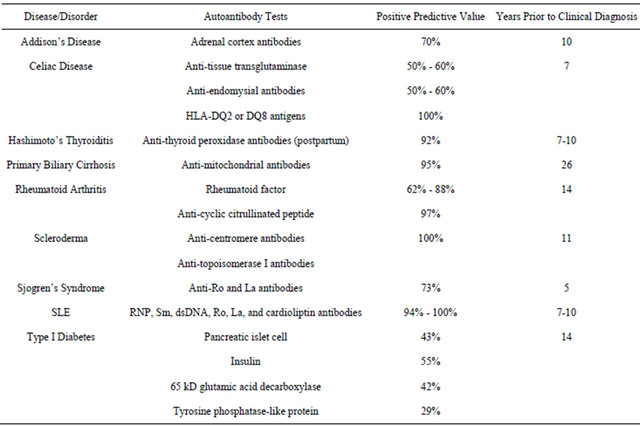
In a 2007 Scientific American article entitled New Predictors of Disease, Abner Louis Notkins stated “Molecules called predictive autoantibodies appear in blood years before people show symptoms of various disorders. Tests that detect these molecules could warn of the need to take preventive action [25].” While some of these tests have been used for many years in a very selective manner, often simply to confirm the presence of a disease strongly suspected by clinical presentation and examination. However, the development and availability of lowcost autoantibody arrays has ushered in the possibility to use autoantibody testing in a much more proactive screening strategy to predict the future emergence of autoimmune disorders so that preventive action can be initiated early to short-circuit the disease process [26]. Table 2 outlines some of the available predictive autoantibody tests, their positive predictive value (PPV), and the years before clinical diagnosis that they generally appear in the blood of subjects with specific disorders [27-29].
According to Aristo Vojdani, Ph.D. [30], autoantibodies could:
• Predict the risk of falling ill.
• Project the probability of contracting a particular disease so that the potential patient could consider preventive therapy:
1) Primary prevention: Remove environmental factors that trigger disease.
2) Secondary prevention: Modulate the destructive process before onset of clinical symptoms.
• Anticipate the timing of a disorder, revealing how soon a disease is likely to cause symptoms.
Table 2. Selected predictive autoantibody tests.
• Project the course of a disease.
Predict the severity and probable rate of progression of a disease
• Classify the disease.
In a patient with an established disease, autoantibodies can help define the nature of the condition as autoimmune or non-autoimmune.
As inexpensive tests for predictive autoantibodies continue to be developed, they could become part of a routine check-up, particularly by preventive providers such as naturopathic and functional medicine physicians.
7. Summary
It is hoped that this article will help the physician to develop a comprehensive conceptual framework from which to view autoimmune disease and to institute a new proactive clinical model from which to evaluate patients. Physicians should look for immune dysregulatory conditions with a strong emphasis on: 1) very early detection with predictive auto-antibodies; 2) a focus on optimizing gastrointestinal mucosal immune function and the microbiome; 3) the eradication of infectious triggers with antimicrobial therapy; 4) the detection and elimination of food sensitivities; and 5) the promotion of an anti-inflammatory lifestyle.
REFERENCES
- J. F. Bach, “The Effect of Infections on Susceptibility to Autoimmune and Allergic Diseases,” The New England Journal of Medicine, Vol. 347, No. 12, 2002, pp. 911- 920. doi:10.1056/NEJMra020100
- M. Inaba, S. Ushijim, N. Hirata, et al., “MethotrexateRelated Lyphomatoid Granulomatosis in a Patient with Rheumatoid Arthritis,” Nihon Kokyuki Gakkai Zasshi, Vol. 49, No. 8, 2011, pp. 597-601.
- M. D. Mayes, “Epidemiologic Studies of Environmental Agents and Systemic Autoimmune Diseases,” Environmental Health Perspectives, Vol. 107, Supplement 5, 1999, pp. 743-748.
- O. V. Pishak, “Bukovian State Medical Academy, Public Health Ministry of Ukraine,” Mikrobiolohichnyĭ Zhurnal, Vol. 61, No. 5, 1999, pp. 41-47.
- H. Tiwana, C. Wilson, R. S. Walmsley, et al., “Antibody Responses to Gut Bacteria in Ankylosing Spondylitis, Rheumatoid Arthritis, Crohn’s Disease and Ulcerative Colitis,” Rheumatology International, Vol. 17, No. 1, 1997, pp. 11-16. doi:10.1007/PL00006845
- A. Ebringer and T. Rahid, “Rheumatoid Arthritis Is an Autoimmune Disease Triggered by Proteus Urinary Tract Infection,” Clinical and Developmental Immunology, Vol. 13, No. 1, 2006, pp. 41-48. doi:10.1080/17402520600576578
- A. Ebringer, T. Rahid and C. Wilson, “Rheumatoid Arthritis: Proposal for the Use of Anti-Microbial Therapy in Early Cases,” Scandinavian Journal of Rheumatology, Vol. 32, No. 1, 2003, pp. 2-11. doi:10.1080/03009740310000337
- F. Liao, Z. Li, Y. Wang, et al., “Porphyromonas Gingivalis May Play an Important Role in the Pathogenesis of Periodontitis-Associated Rheumatoid Arthritis,” Medical Hypotheses, Vol. 72, No. 6, 2009, pp. 732-735.
- M. Harkiolaki, S. L. Holmes, P. Svendsen, et al., “T-CellMediated Autoimmune Disease Due to Low-Affinity Cross Reactivity to Common Microbial Peptides,” Immunity, Vol. 30, No. 3, 2009, pp. 348-357. doi:10.1016/j.immuni.2009.01.009
- S. K. Mazmanian, J. L. Round and D. L. Kasper, “A Microbial Symbiosis Factor Prevents Intestinal Inflammatory Disease,” Nature, Vol. 453, No. 7195, 2008, pp. 620- 625. doi:10.1038/nature07008
- G. Petru, D. Stunzner, P. Lind, et al., “Antibodies to Yersinia Enterocolitica in Immunogenic Thyroid Diseases,” Acta Medica Austriaca, Vol. 14, No. 1, 1987, pp. 11-14.
- N. Anasaldi, T. Palmas, A. Corrias, et al., “Autoimmune Thyroid Disease and Celiac Disease in Children,” Journal of Pediatric Gastroenterology and Nutrition, Vol. 37, No. 1, 2003, pp. 63-66. doi:10.1097/00005176-200307000-00010
- D. Brady, “Novel Options in GI Diagnostics: DNA Detection of Gut Microbiota,” Complementary Medicine, 2008, pp. 28-31.
- S. T. Weiss, “Eat Dirt—The Hygiene Hypothesis and Allergic Disease (Editorial),” The New England Journal of Medicine, Vol. 347, No. 12, 2002, pp. 930-931. doi:10.1056/NEJMe020092
- E. von Mutius and D. Vercelli, “Farm Living: Effects on Childhood Asthma and Allergy,” Nature Reviews Immunology, Vol. 10, No. 12, 2010, pp. 861-868. doi:10.1038/nri2871
- D. Gutierrez, “Parasites in Your Gut Actually Help Protect You from Allergies,” NaturalNews, 2011. http://www.naturalnews.com/028141_parasites_allergies.html.
- R. W. Summers, D. E. Elliott, J. V. Weinstock, et al., “Trichuris Suis Seems to Be Safe and Possibly Effective in the Treatment of Inflammatory Bowel Disease,” The American Journal of Gastroenterology, Vol. 98, No. 9, 2003, pp. 2034-2041. doi:10.1111/j.1572-0241.2003.07660.x
- A Fasano and T. Shea-Donohue, “Mechanisms of Disease: The Role of Intestinal Barrier Function in the Pathogenesis of Gastrointestinal Autoimmune Diseases,” Nature Clinical Practice Gastroenterology & Hepatology, Vol. 2, No. 9, 2005, pp. 416-422. doi:10.1038/ncpgasthep0259
- A. Fasano, “Surprises from Celiac Disease,” Scientific American, Vol. 301, No. 2, 2009, pp. 54-61. doi:10.1038/scientificamerican0809-54
- A. Sapone, L. de Magistris and M. Pietzak, “Zonulin Upregulation Is Associated with Increased Gut Permeability in Subjects with Type I Diabetes and Their Relatives,” Diabetes, Vol. 55, No. 5, 2006, pp. 1443-1449. doi:10.2337/db05-1593
- J. Kong, Z. Zhang, M. W. Musch, et al., “Novel Role of the Vitamin D Receptor in Maintaining the Integrity of the Intestinal Mucosal Barrier,” American Journal of Physiology—Gastrointestinal and Liver Physiology, Vol. 294, No. 1, 2008, pp. G208-G216. doi:10.1152/ajpgi.00398.2007
- S. Y. Yao, C. W. Stratton and W. M. Mitchell, “CSF Oligoclonal Bands in MS Include Antibodies against Chlamydophilia Antigens,” Neurology, Vol. 56, No. 9, 2001, pp. 1168-1176. doi:10.1212/WNL.56.9.1168
- D. H. Gilden, “Infectious Causes of Multiple Sclerosis,” The Lancet Neurology, Vol. 4, No. 3, 2005, pp. 195-202.
- A. F. Gombart, N. Borregaard and H. P. Koeffler, “Human Cathelicidin Antimicrobial Peptide (Camp) Gene Is a Direct Target of the Vitamin D Receptor and Is Strongly Up-Regulated in Myeloid Cells by 1,25-Hihydroxyvitamin D3,” Future Microbiology, Vol. 4, No. 9, 2009, pp. 1151-1165. doi:10.2217/fmb.09.87
- A. L. Notkins, “New Predictors of Disease,” Scientific American, Vol. 296, No. 3, 2007, pp. 72-79. doi:10.1038/scientificamerican0307-72
- D. Leslie, P. Lipsky and A. L. Notkins, “Autoantibodies as Predictors of Disease,” Journal of Clinical Investigation, Vol. 108, No. 10, 2001, pp. 1417-1422.
- T. O’Bryan, “American College for Advancement in Medicine Annual Symposium Presentation 2009”.
- Y. Shoenfeld, M. Blank, M. Abu-Shakra, et al., “The Mosaic of Autoimmunity: Prediction, Autoantibodies, and Therapy in Autoimmune Disease,” IMAJ, Vol. 10, No. 1, 2008, pp. 13-19.
- B. Lindberg, S. A. Iverson, et al., “Islet Autoantibodies in Cord Blood in Children Who Develop Type I (InsulinDependent) Diabetes Mellitus before 15 Years of Age,” Diabeteologia, Vol. 42, No. 2, 1999, pp. 181-187. doi:10.1007/s001250051137
- A. Vojdani, “Antibodies as Predictors of Complex Autoimmune Diseases and Cancer,” International Journal of Immunopathology and Pharmacology, Vol. 21, No. 3, 2008, pp. 553-566.

